Abstract
GVHD (graft-vs-host disease) is one of the major complications after allogeneic hematopoietic stem cell transplantation leading to substantial morbidity and mortality. Several murine models are used to investigate this systemic inflammatory disease in vivo and to identify possible targets for clinical treatment. However, current well-established murine GVHD models have their clinical limitations as they are based on major histocompatibility mismatches and only lethal irradiation as conditioning. Development of new treatment options would be forwarded by the availability of a more clinically relevant murine model.
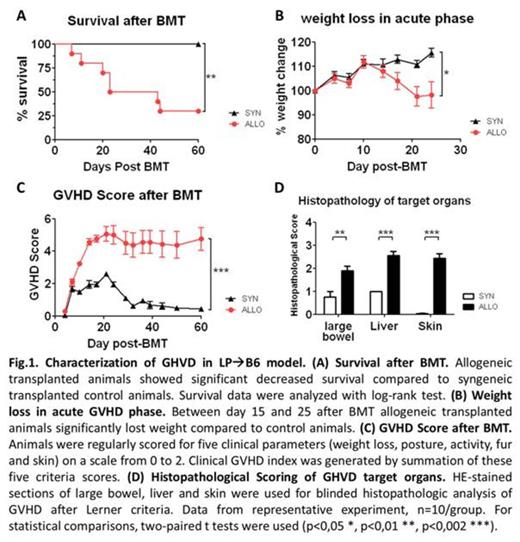
To reflect the clinical situation of transplantation and GVHD, we established a new murine GVHD model which is based on Chemotherapy conditioning and MHC-matched bone marrow transplantation (BMT). In LP/J -> C57BL/6 mouse model, our chemotherapy transplantation protocol resulted in stable full donor chimerism and development of acute GVHD.
C57BL/6 recipients were conditioned with a 7-day busulfan-cyclophosphamide Chemotherapy protocol. Mice received for 5 days 20 mg/kg/day busulfan intraperitoneally (i.p.) and the last three days additionally 100 mg/kg/day cyclophosphamide. After a two-day resting phase, recipients were transplanted with 1.5 x 107 bone marrow and 2 x 106 splenic T cells from LP/J donors. Recipients showed good engraftment with already 80 % engraftment in peripheral blood at day 15 after BMT. 90 % donor chimerism in peripheral blood and bone marrow could be shown already at day 30 after BMT.
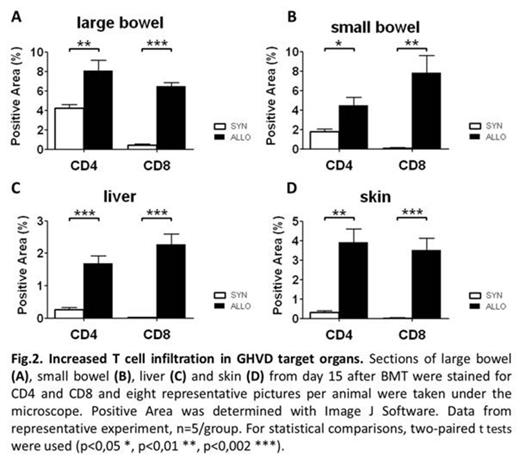
We next characterized systemic inflammation in our model by determining the number of circulating inflammatory cells in peripheral blood at different time points by flow cytometry. CD8+ T cells were significantly increased in GVHD animals in acute phase (day 15 after BMT), whereas CD4+ T cells were elevated in late phase (day 50-60 after BMT). These data suggests, that in our model two inflammatory phases occur, an early acute CD8-driven and a late CD4-driven phase.
In summary, we could establish a novel chemotherapy based minor mismatch GVHD model which shows good engraftment, typical features of acute GVHD and systemic and target organ specific inflammation. Our murine GVHD model closely resembles the clinical situation of MHC-matched stem cell transplantation and may help to better understand pathogenic mechanisms in GVHD and develop new treatment options.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.