Abstract
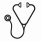
The pediatric-inspired intensified GRAALL protocol yielded a marked improvement in the outcome of adults with Ph-negative acute lymphoblastic leukemia (ALL) (Huguet et al,JCO 2009), raising the issue of the place of allogeneic HSCT in this new context. We report here the results associated with HSCT in first complete remission (CR) in younger adults treated in the GRAALL-2003/2005 trials.
In these trials, HSCT was offered in first CR to patients aged 15-55 years with ALL at higher risk of relapse. High-risk factors included white blood count >30 x 109/L for B-lineage ALL, central nervous system (CNS) involvement, t(4;11)/MLL anomalies, t(1;19), low hypodiploidy/near triploidy, complex karyotype, early resistance to the steroid prephase (CsR), early resistance to chemotherapy (ChR) as assessed by poor bone marrow blast clearance at day 8, and late CR. Per protocol, HSC donor should be a matched sibling or an unrelated donor (10/10 or eventually 9/10 HLA matched) and conditioning regimen should include single fraction 10-Gy or fractionated 12-Gy total body irradiation (TBI) and high-dose cyclophosphamide. The role of HSCT was evaluated by HSCT versus no-HSCT cohort comparison (Mantel-Byar time-dependent analysis).
Among the 522 high-risk patients eligible, 283 (54%) actually received HSCT in first CR (185 B-lineage and 98 T-lineage ALL; median age, 31 years). There were no significant differences in baseline characteristics and early response between HSCT and no-HSCT cohorts, except more HSCT patients with t(4;11)/MLL anomalies. Among the 283 HSCT patients, 46 had t(4;11)/MLL anomalies, 13 had CNS disease, 116 had CsR and 176 had ChR ALL. Origin of HSC was 140 sibling and 143 unrelated donors, including 47 unrelated donors with 1 HLA mismatch. The following HSCT protocol violations were observed: a) conditioning did not include TBI in 17 patients; b) 10 patients received reduced-intensity conditioning (RIC); and c) 13 patients received cord blood HSCT. These patients remained in the HSCT cohort for the analysis. With a median post-transplant follow-up of 3.5 years, post-HSCT cumulative incidence of relapse (CIR), non-relapse mortality (NRM) and relapse-free survival (RFS) were estimated respectively at 19% (95% CI, 15-25), 16% (95% CI, 12-21) and 64% (95% CI, 58-70) at 3 years, without any RFS difference between sibling and unrelated donors. A marked trend for a higher NRM was observed in patients aged 45 years or more (26% versus 13% at 3 years; HR, 1.6; p=0.059), while post-transplant CIR was similar in older and younger patients. Mantel-Byar RFS estimations showed no significant difference between both HSCT and no-HSCT cohorts (HR, 0.80; p=0.13), meaning that the risk factors used in these protocols failed to identify patients who could significantly benefit from HSCT in first CR. As minimal residual disease (MRD) had a major prognostic impact, we retrospectively analyzed the effect of HSCT in the 278/522 patients evaluated for 6-week Ig/TCR MRD level (154 HSCT and 124 no-HSCT patients, including 92 and 62 patients with MRD >=10-4, respectively). Mantel-Byar RFS estimations showed that HSCT did not benefit to patients with MRD <10-4 (HR, 1.2; p=0.67) as opposed to those with MRD >=10-4 (HR, 0.6; p=0.02), with a positive HSCT-by-MRD interaction (p=0.04) (Figure 1).
In adult patients with high-risk ALL in first CR treated in the GRAALL-2003/2005 trials, myeloablative allogeneic HSCT from sibling and unrelated donors was associated with a 64% RFS at 3 years. The 26% NRM observed at 3 years in patients aged 45 years or more suggests considering RIC-HSCT in these patients. MRD response appears to be a powerful tool to select patients who might benefit from HSCT. This selection tool will be prospectively evaluated in the next GRAALL-2013 trial.
ND and AH contributed equally to the work.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract