Abstract
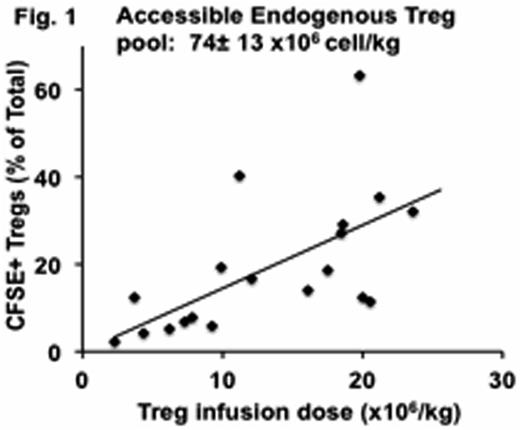
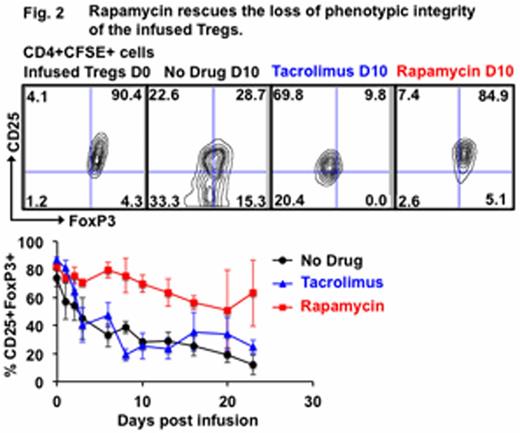
Ex-vivo expanded natural regulatory T cells (nTregs) are emerging as promising cellular therapeutics for the prevention of allograft rejection and graft-versus host disease. However, their widespread translation to the clinic has not yet occurred, in part because of the lack of a clear understanding of their in-vivo quantitative and functional dynamics. Here we have used our translational non-human primate model to answer some of the critical questions related to the survival, stability and immunologic compatibility of Tregs with other immunosuppressive therapies. By infusing escalating doses of CFSE-labeled, autologous CD4+CD25++CD127-/lowFoxP3+ Tregs, we determined, for the first time, that in primates, the accessible, peripheral Treg pool is quite large: ∼74± 13 x106 cell/kg: > 20-times larger than the number of Tregs that have previously been infused into patients (Figure 1). Tregs infused in the absence of concomitant immunosuppression had a surprisingly short in-vivo half-life, even when transferred into autologous recipients (T1/2=2.1± 0.3 days): Tregs infused at dose of 20x106 cell /kg were undetectable in either the blood, bone marrow or lymph nodes within 2 weeks after their infusion, despite their initial trafficking to all three sites early after infusion. In addition to their short half-life, infused Tregs quickly underwent rapid phenotypic alteration, with significant loss of both CD25 and FoxP3 expression within 6 days of transfer, suggestive of a potential concomitant loss of suppressive function (Figure 2). By Day +10, only 28.0± 2.7% of infused CD4+CFSE+ cells were CD25+FoxP3+ compared to 74.2± 6.6% on the day of infusion (p< 0.01).
While treatment with tacrolimus was able to rescue the loss of CD25 expression, it did not improve the rapid loss of FoxP3 expression that occurred in the infused cells, resulting in a half-life that was not improved compared to Tregs infused without immunosuppression (2.6± 0.2 days, p = ns compared to no immunosuppression) as well as a similarly rapid loss of FoxP3 expression (25± 9 % FoxP3 expression on day +10 post-infusion).
In striking contrast, rapamycin extended the persistence of the infused Tregs beyond day 35 (T1/2 = 3.9± 0.8 days, p< 0.01 compared to no immunosuppression) and significantly improved the CD25+FoxP3+ phenotype of the adoptively transferred cells, with 70± 6% retaining expression of both CD25 and FoxP3 at day +10, p= 0.01 compared to no immunosuppression) (Figure 2). In addition, compared to animals receiving Tregs either without immunosuppression or in the presence of tacrolimus, rapamycin treatment resulted in significantly more infused Tregs in the bone marrow and lymph nodes when measured one week after transfer (in the bone marrow, 6± 0.7 % vs 1± 0.6 % without immunosuppression and 2.4± 0.4 % on tacrolimus, p< 0.01 compared to either treatment).
These results provide the first quantitative evaluation of the availability of the accessible Treg pool in primates, and suggest that Tregs undergo a significant and potentially deleterious phenotypic degradation after transfer. While tacrolimus was unable to fully rescue this degradation, rapamycin significantly improved both the survival and phenotypic stability of these cells. These results suggest that even natural Tregs may be phenotypically and functionally unstable after transfer, and that adjunctive strategies to stabilize function and half-life, including mTOR inhibition, should be strongly considered when using these cells as an adoptive immunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

