Abstract
Clinical results with NK cells in investigational tumor immunotherapy protocols have at best resulted in partial responses only. The inability of ex vivo expanded NK cells to proliferate in vivo, as well as to home to and be retained in the tumor micro-environment, likely plays a role in their limited efficacy to date.
Clinical grade NK cells expanded using EBV-LCL feeder cells (FC) have recently been evaluated in the clinic (NHLBI) for hematological malignancies and metastatic cancers with only minor responses being observed to date. We observed that CD62L expression was down-regulated on EBV-LCL NK cells compared to fresh NK cells, potentially limiting their clinical activity. Since NAM up-regulates CD62L on NK cells cultured in feeder-free (FF) conditions, we compared the ability of FF NK cells with EBV-LCL NK cells to home and persist in vivo following adoptive transfer into NSG mice.
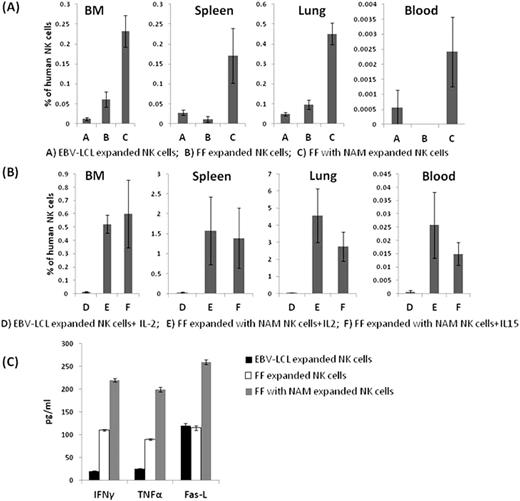
FF NK cultures were initiated with CD3 depleted PB TNC while EBV-LCL NK cell cultures were initiated by co-culturing 100 cGy-irradiated SMI-EBV-LCL FC with CD3 depleted, CD56 enriched cells. NSG mice received 200 cGy of TBI followed 24 hours later by 10 million IV NK cells. Three cohorts were studied: a) NK cells expanded using EBV-LCL FC; b) FF expanded NK cells without NAM and c) FF expanded NK cells with NAM (5mM). All NK cell cohorts were expanded from the same human donor. Cells were harvested from the blood, lungs, spleen, and BM of mice 4 days after infusion (n=5). NK cells expanded with NAM in FF conditions were detectable in all mouse organs including the PB at significantly higher levels than NK cells expanded in FF conditions without NAM or using EBV-LCL FC (Fig.1a). Subsequent experiments transferring CFSE-labeled NK cells expanded with NAM into irradiated NSG mice showed a marked reduction in CFSE intensity and the presence of multiple peaks after 4 days, indicative of in vivo proliferation. We next evaluated the impact of daily exogenous IL-2 or IL-15 administration on the homing potential of expanded NK cells 4 days post-infusion into irradiated NSG mice (n=5). We observed that both IL-2 and IL-15 enhanced homing of NK cells expanded with NAM in FF conditions, but failed to enhance the homing of NK cells expanded with EBV-LCL FC (Fig. 1b). With the exception of CD62L, no consistent differences in the phenotype of NK cells between the various expansion methods were observed. NK cells expanded in all groups maintained similar levels of cytotoxicity against multiple different targets including K562 cells, myeloma and renal cell carcinoma tumor cell lines. The number of NK cells expanded using FF conditions was lower than NK cells expanded with EBV-LCL. Nevertheless, NK cells cultured in NAM without feeder cells still expanded a median 50 (37-87) fold, yielding a total of 140x108 NK cells (purity >98%) from a single aphaeresis collection. Further, cytokine levels measured from the supernatants of NK cells cultured with tumor targets showed significantly higher levels of IFNγ, TNFα and FAS-L secretion from FF NK cells expanded with NAM in comparison to the other two groups (Fig1c).
These data show human NK cells expanded ex vivo in NAM utilizing FF conditions substantially up-regulate CD62L, have enhanced inflammatory cytokine secretion against tumors, and have improved in vivo proliferation and homing to multiple organs including the bone marrow compared to EBV-LCL expanded NK cells. These differences suggest NAM expanded NK cells could have superior clinical efficacy compared to EBV-LCL expanded NK cells following adoptive transfer into patients with hematological malignancies and metastatic cancers.
Frei:Gamida Cell: Employment. Peled:Gamida Cell: Employment. Persi:Gamida Cell: Employment. Lador:Gamida Cell: Employment. Peled:Gamida Cell: Consultancy.
Nicotinamide (NAM) is a small molecule form of Vitamin B3 and a potent inhibitor of enzymes that use NAD for their activity and thus is involved in the control of redox-sensitive enzymes, mitochondrial functions, cell metabolism and production of energy and cell motility. NAM, when used as an epigenetic modulator has been shown to increase the homing and engraftment efficacy to the BM of ex vivo expanded CD34+ cells.
Recently, we found (Gamida-Cell) that NAM also enhances the in-vivo homing and retention of peripheral blood (PB) derived NK cells expanded over two weeks in feeder-free culture conditions stimulated with IL-2 or IL-15. Immunophenotype studies demonstrated NAM-treated cultures had a substantial increase in CD62L (L-selectin), pivotal for NK cell trafficking and homeostatic proliferation.
Author notes
Asterisk with author names denotes non-ASH members.

