Key Points
Platelet-derived ERp57 plays an important role in physiologic platelet function and thrombosis.
ERp57 directly interacts with αIIbβ3 in regulating its function.
Abstract
The platelet protein disulfide isomerase called ERp57 mediates platelet aggregation, but its role in thrombus formation is unknown. To determine the specific role of platelet-derived ERp57 in hemostasis and thrombosis, we generated a megakaryocyte/platelet-specific knockout. Despite normal platelet counts and platelet glycoprotein expression, mice with ERp57-deficient platelets had prolonged tail-bleeding times and thrombus occlusion times with FeCl3-induced carotid artery injury. Using a mesenteric artery thrombosis model, we found decreased incorporation of ERp57-deficient platelets into a growing thrombus. Platelets lacking ERp57 have defective activation of the αIIbβ3 integrin and platelet aggregation. The defect in aggregation was corrected by the addition of exogenous ERp57, implicating surface ERp57 in platelet aggregation. Using mutants of ERp57, we demonstrate the second active site targets a platelet surface substrate to potentiate platelet aggregation. Binding of Alexa 488−labeled ERp57 to thrombin-activated and Mn2+-treated platelets lacking β3 was decreased substantially, suggesting a direct interaction of ERp57 with αIIbβ3. Surface expression of ERp57 protein and activity in human platelets increased with platelet activation, with protein expression occurring in a physiologically relevant time frame. In conclusion, platelet-derived ERp57 directly interacts with αIIbβ3 during activation of this receptor and is required for incorporation of platelets into a growing thrombus.
Introduction
Protein disulfide isomerase (PDI) is the prototypic member of the PDI family of enzymes, best known in forming disulfide bonds in endoplasmic reticulum proteins.1 Several members of this family have a role in platelet function and thrombosis, including PDI, ERp5, and ERp57.2-12 Platelet-derived PDI mediates platelet aggregation, secretion,3,5 and adhesion.4,6 However, platelet-derived PDI was reported to not be required for thrombosis and specifically for fibrin deposition after laser-induced injury in vivo.13 The role of platelet PDI in platelet accumulation could not be assessed in this study because platelets were inhibited.13 A recent study in which the authors used platelet-specific PDI knockout mice clarified this question by demonstrating an important role for platelet PDI in platelet accumulation.14
ERp57 is a glycoprotein-specific member of the PDI family that has substantial homology with PDI with 50% amino acid identity in the catalytic a and a′ domains and ∼20% amino acid identity in the substrate binding b and b′ domains.15 The catalytic domains of PDI and ERp57 contain 2 CGHC active-site sequences that catalyze the reversible oxidation of thiols to disulfides and the isomerization of disulfide bonds. We and Holbrook et al reported that ERp57 was required for platelet function and thrombosis.11,12 To inhibit thrombosis in vivo, both studies infused inhibitory antibodies to ERp57. An antibody infused into mice would inhibit all sources of extracellular ERp57 found at the site of vascular injury. The goal of the current study was to determine specifically whether platelet-derived ERp57 has a role in thrombosis and platelet accumulation in vivo and to characterize this role. The best way to do this is with the use of targeted ERp57 knockout mice.
Although many of the mechanisms of platelet activation have been elucidated, relatively little is known about extracellular mechanisms required for the terminal steps in activation of αIIbβ3. Platelet adhesion, the structure and function of primary agonist receptors, and cytoplasmic signaling pathways of platelet activation have been studied extensively.16,17 The final intracellular steps in activation of αIIbβ3 involving talin and kindlin interactions with the β3 subunit are a subject of active investigation.18 In contrast to intracellular platelet activation events, the final extracellular mechanisms involved in activation of αIIbβ3 remain obscure. In the present study we found that platelet surface ERp57 targeting αIIbβ3 is required for platelet incorporation into a developing thrombus. Using the targeted knockout, we provide evidence for a role of ERp57 in platelet function that is distinct from the role of PDI or other members of this family of enzymes.
Materials and methods
Materials
Phycoerythrin (PE)-labeled JON/A antibody, antimouse αIIbβ3 complex, and GpIbα and GpVI were from Emfret. PE-conjugated anti–P-selectin antibody was from eBioscience. Anti-PDI antibody (RL90) was from Abcam. Polyclonal rabbit anti-ERp5 antibody was from Santa Cruz Biotechnology, Inc., and polyclonal rabbit anti-ERp72 antibody was from Stressgen. A polyclonal and 2 monoclonal antibodies to ERp57 have been previously described.12 Anti-CD41 Ag-binding F(ab′)2 fragment (BD Bioscience) was conjugated with Alexa 488 (Invitrogen).
Generation and genotyping of mice with platelets deficient in ERp57
Mice homozygous for the ERp57 floxed allele (loxP sites flanking exons 2 and 3) on a C57BL/6 background19 were crossed with Pf4-Cre mice (The Jackson Laboratory) on a C57BL/6 background.20 To generate platelet-specific ERp57-knockout mice and experimental control mice, Pf4-Cre/ERp57fl/fl mice were mated with ERp57fl/fl mice, which produced Pf4-Cre/ERp57fl/fl mice and ERp57fl/fl littermate controls. Expression of platelet ERp57 was assessed by reverse-transcription polymerase chain reaction of total platelet messenger RNA. The primers for ERp57 were sense 5′-tatgatgggcctaggactgc-3′ and antisense 5′-tgctggctgcttttaggaat-3′; the primers for Cre-recombinase were sense 5′-cccatacagcacaccttttg-3′ and antisense 5′-tgcacagtcagcaggtt-3′; and the primers for mouse β-actin were sense 5′-gtccctcaccctcccaaaag-3′ and antisense 5′-gctgcctcaacacctcaaccc-3′. Experiments with mice were performed in accordance with Temple University and Soochow University institutional guidelines and approval of the Animal Care Committees. Pentobarbital (50 mg/kg ) was administered intraperitoneally as anesthesia.12 Complete blood cells counts, white blood cell differential counts, and red blood cell and platelet indices were measured with a Sysmex Coulter Counter (XT2000-iV).
β3 integrin−null mice
Previously described β3 integrin−null mice on a mixed C57BL/6 and 129SV background21 were backcrossed over 10 generations onto a C57BL/6 background. Control mice were matched C57BL/6 wild-type mice.
Bleeding time analysis
The bleeding time assay was performed as described with the use of a razor blade to transect the mouse tail 3 mm from the tip.12 When necessary, bleeding was stopped manually at the 15-minute time point.
FeCl3-induced thrombosis of the carotid artery
Carotid artery thrombosis was induced in mice ages 6-8 weeks by treating the exposed artery with filter paper soaked in 5% FeCl3 for 2 minutes, and blood flow through the carotid artery was monitored with the use of a small animal transducer as described.12 An occlusion was considered stable when flow completely stopped for at least 5 minutes. The operator was blinded to the mouse genotype while performing experiments.
FeCl3- induced platelet accumulation and thrombosis of the mesenteric artery
To follow in vivo accumulation of platelets, Alexa 488−labeled antimouse CD41 Ag-binding fragment antibody was injected into mice through the tail vein. The mesenteric artery was exteriorized through a midline abdominal incision. A single arteriole was visualized under a Zeiss Scope A fluorescent microscope. Vessel injury was generated by placing a 1 × 2-mm patch of No.1 Whatman filter paper soaked in 5% FeCl3 above the exposed artery for 1 minute. The filter paper was removed, and platelet accumulation observed and photographed every minute for 15 minutes. The fluorescent intensity (FI) of the platelet accumulation and thrombus formation was analyzed by Image J software. The FI divided by the area analyzed provided the relative FI for each injury and is indicated by FI/µm2. The area analyzed was obtained from the length analyzed multiplied by the diameter of each injured vessel. The length of the thrombi was from the beginning of the upstream edge of the thrombi to 1 mm downstream of this site. The background FI of the uninjured vessel was negligible.
Immunostaining of endothelial cells
The presence of endothelial cells at the site of vascular injury was determined as described elsewhere22 with some modifications (legend of supplemental Figure 1; see the Blood Web site).
Flow cytometry and aggregation studies of mouse platelets
Flow cytometry studies on mouse platelets were performed with the use of platelet-rich plasma prepared and diluted into Tyrode’s buffer as described.12 The platelet-rich plasma was prepared from blood obtained into acid-citrate dextrose solution through the inferior vena cava.12 Aggregation studies were performed with washed platelets prepared as described.23 Platelet concentration was measured and adjusted to 200 000/μL using the Sysmex Coulter Counter.
Flow cytometry and aggregation studies of human platelets
All studies on human platelets were performed after approval by the Temple University or Soochow University Institution Review Boards with informed consent in accordance with the Declaration of Helsinki. Flow cytometry studies of surface ERp57 were performed with the use of platelet-rich plasma prepared and diluted into Tyrode’s buffer as described.12 The data were combined to obtain the mean FI after the background binding of control immunoglobulin G antibody to platelets was subtracted. Aggregation studies were performed with the use of washed platelets prepared and suspended in modified Tyrode’s buffer, to which calcium (1 mmol/L) was added as described.24 The percent aggregation was calculated from the amplitude of the tracings and normalized to the response of the untreated control within an individual experiment.
Preparation of catalytically inactive first or second active sites of ERp57
Two different mutant ERp57 enzymes were prepared (QuikChange Site-Directed Mutagenesis Kit) with both cysteine residues of one active site mutated to serine residues (C57S with C60S, or C406S with C409S). The mutants are designated as ERp57 (oo-ss, representing the Cys/SH change to Ser/OH in the first CGHC active site) and ERp57 (ss-oo, representing the Cys/SH change to Ser/OH in the second CGHC active site). DNA sequencing confirmed the correct base substitutions. Also prepared were wild-type ERp57 (ss-ss) and completely inactivated ERp57 (oo-oo) as previously described.12 The wild-type ERp57 was previously shown to potentiate platelet aggregation, whereas the catalytically inactive ERp57 (oo-oo) inhibited aggregation.12
Procedure to measure platelet surface ERp57 activity
Statistics
The statistics for the in vivo experiments were performed as described.12 The Student t test was used for 2-group comparisons. GraphPad Prism software analysis of variance was used for multiple group comparisons (GraphPad Software, San Diego, CA). P values of < .05 were considered as statistically significant.
Results
Characterization of Pf4-Cre/ERp57fl/fl mouse platelets
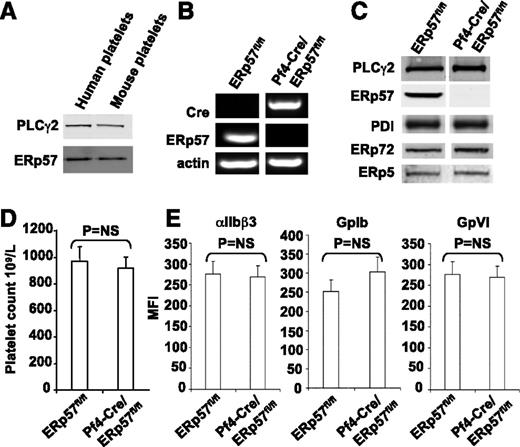
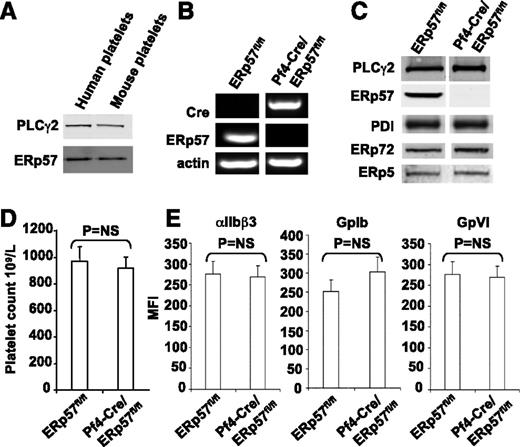
Human and mouse ERp57 have 93% identity and are the same size on western blots (Figure 1A). Because global deletion of ERp57 is embryonically lethal in mice,15 to determine whether platelet-derived ERp57 had a role in thrombus formation, we generated a platelet-specific knockout. The messenger RNA for ERp57 was absent from platelets of Pf4-Cre/ERp57fl/fl mice (Figure 1B). ERp57 was absent in western blots of platelet lysate prepared from mice homozygous for the floxed alleles confirming successful targeting of the enzyme (Figure 1C). No changes in PDI, ERp72, or ERp5 were detected on western blotting in ERp57-deficient platelets.
Characterization of the platelets of Pf4-Cre/ERp57fl/flmice. (A) The monoclonal anti-ERp57 antibody (Mab1) reacts with mouse ERp57 by western blot (with PLCγ2 loading control; 30 μg of protein). (B) Platelet reverse-transcription polymerase chain reaction products of ERp57fl/fl and Pf4-Cre/ERp57fl/fl mice. The primers for ERp57 gave the predicted 186-bp product in wild-type but not ERp57-deficient platelets. The primers for Cre-recombinase gave the predicted 450-bp product in Cre-positive ERp57-deficient platelets but not wild-type platelets. The primers for mouse β-actin yielded the predicted 265-bp product. (C) Western blot of lysates using the anti-ERp57 antibody (PLCγ2 control). Blotting results for PDI, ERp72, and ERp5 are shown. In panels B-C, the lanes for a specific RNA or protein were run on the same gel but were noncontiguous. (D) Platelet counts in ERp57fl/fl and Pf4-Cre/ERp57fl/fl mice ± SE (n = 10). (E) Normal glycoprotein expression in platelets from Pf4-Cre/ERp57fl/fl mice compared with ERp57fl/fl littermate controls ± SE (n = 8). Mouse platelets were incubated with antibodies to αIIbβ3, GPIb, or GPVI and analyzed by flow cytometry.
Characterization of the platelets of Pf4-Cre/ERp57fl/flmice. (A) The monoclonal anti-ERp57 antibody (Mab1) reacts with mouse ERp57 by western blot (with PLCγ2 loading control; 30 μg of protein). (B) Platelet reverse-transcription polymerase chain reaction products of ERp57fl/fl and Pf4-Cre/ERp57fl/fl mice. The primers for ERp57 gave the predicted 186-bp product in wild-type but not ERp57-deficient platelets. The primers for Cre-recombinase gave the predicted 450-bp product in Cre-positive ERp57-deficient platelets but not wild-type platelets. The primers for mouse β-actin yielded the predicted 265-bp product. (C) Western blot of lysates using the anti-ERp57 antibody (PLCγ2 control). Blotting results for PDI, ERp72, and ERp5 are shown. In panels B-C, the lanes for a specific RNA or protein were run on the same gel but were noncontiguous. (D) Platelet counts in ERp57fl/fl and Pf4-Cre/ERp57fl/fl mice ± SE (n = 10). (E) Normal glycoprotein expression in platelets from Pf4-Cre/ERp57fl/fl mice compared with ERp57fl/fl littermate controls ± SE (n = 8). Mouse platelets were incubated with antibodies to αIIbβ3, GPIb, or GPVI and analyzed by flow cytometry.
Complete blood counts in Pf4-Cre/ERp57fl/fl mice revealed no abnormalities. Platelet counts (Figure 1D) and size (mean platelet volume = 6.6 ± 0.01 fL) were comparable with those of Cre-negative littermates (6.4 ± 0.01; P = NS, n = 10). There were no differences in expression of the major platelet surface glycoproteins, αIIbβ3, GpIbα, and GpVI (Figure 1E).
Mice with platelets lacking ERp57 have prolonged tail bleeding times and times to occlusion
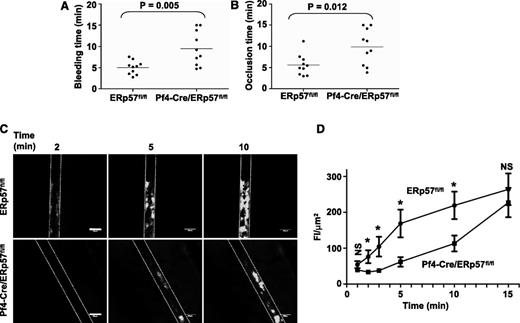
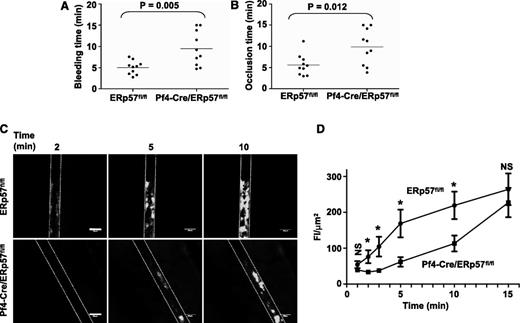
The tail bleeding times were approximately double in mice with ERp57-deficient platelets (Figure 2A). The times to occlusive thrombosis after FeCl3-induced injury of the carotid artery also were approximately double (Figure 2B), which indicates that platelet-derived ERp57 is required for hemostasis and thrombosis.
Platelet-derived ERp57 is required for hemostasis, thrombosis, and platelet accumulation into a growing thrombus. (A) Tail bleeding times and (B) time to occlusion of FeCl3-induced carotid artery thrombosis in Pf4-Cre/ERp57fl/fl mice compared with ERp57fl/fl littermate controls. (C) Incorporation of platelets from ERp57fl/fl or Pf4-Cre/ERp57fl/fl mice into the growing thrombus. (D) Composite of FI/µm2 in ERp57fl/fl, • (n = 18) and Pf4-Cre/ERp57fl/fl, ■ (n = 16) mice ± SE; *P < .05. Mean vessel diameters; ERp57fl/fl 114.8 ± 5.7; Pf4-Cre/ERp57fl/fl: 111.1 ± 5.4 (P = NS). Dotted lines mark the vessel wall. White bar = 100 µm. Images are ×100 magnification.
Platelet-derived ERp57 is required for hemostasis, thrombosis, and platelet accumulation into a growing thrombus. (A) Tail bleeding times and (B) time to occlusion of FeCl3-induced carotid artery thrombosis in Pf4-Cre/ERp57fl/fl mice compared with ERp57fl/fl littermate controls. (C) Incorporation of platelets from ERp57fl/fl or Pf4-Cre/ERp57fl/fl mice into the growing thrombus. (D) Composite of FI/µm2 in ERp57fl/fl, • (n = 18) and Pf4-Cre/ERp57fl/fl, ■ (n = 16) mice ± SE; *P < .05. Mean vessel diameters; ERp57fl/fl 114.8 ± 5.7; Pf4-Cre/ERp57fl/fl: 111.1 ± 5.4 (P = NS). Dotted lines mark the vessel wall. White bar = 100 µm. Images are ×100 magnification.
Incorporation of ERp57-deficient platelets into a growing thrombus is decreased
We directly examined incorporation of ERp57-deficient platelets into a developing thrombus by using a mesenteric artery thrombosis model. A relatively low dose of FeCl3 (5%) was applied to the vessel for 1 minute.27,28 This injury is not expected to cause endothelial cell denudation.27 The integrity of the endothelial cell layer was preserved after FeCl3 injury in our model as shown by the staining pattern for platelet endothelial cell adhesion molecule-1/CD31,22 a constitutively expressed endothelial cell adhesion molecule (supplemental Figure 1). Incorporation of ERp57-deficient platelets into the thrombus was substantially decreased (Figure 2C), with the decrease being statistically significant over the 2- to 10-minute time interval (Figure 2D). Platelet incorporation into the thrombus was almost completely abolished in β3-null mice or with infusion of eptifibatide (to block fibrinogen binding to platelet αIIbβ3) into wild-type mice (supplemental Figure 2), demonstrating dependence of platelet accumulation on platelet αIIbβ3. These results indicate that in this model platelet-derived ERp57 has an important role in platelet accumulation.
ERp57-deficient platelets have decreased activation of αIIbβ3 and decreased aggregation
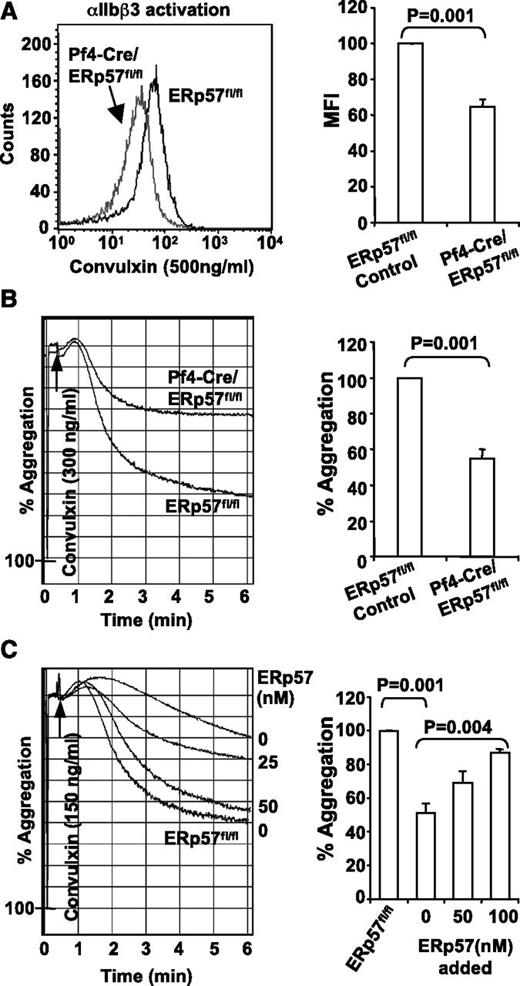
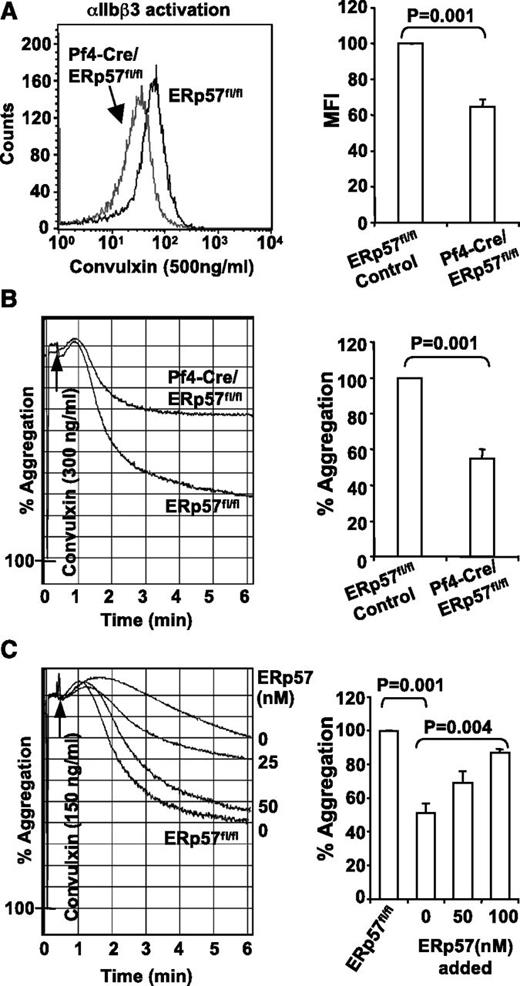
We characterized the role of platelet-derived ERp57 in platelet aggregation. Convulxin-induced activation of αIIbβ3 (Figure 3A) and platelet aggregation (Figure 3B) were decreased in platelets lacking ERp57. The aggregation defect in ERp57-deficient platelets was corrected by addition of exogenous ERp57 to the platelets in a dose-dependent manner (Figure 3C), which indicates the important site of functional ERp57 is the platelet surface.
Deficiency of platelet ERp57 impairs activation of αIIbβ3 and platelet aggregation. (A) ERp57-deficient platelets have a defect in activation of αIIbβ3 (detected by the JON/A activation dependent antibody). Depicted is a representative sample and the mean fluorescent intensity (MFI) of cumulative data ± SE (n = 5). (B) Representative aggregation tracing and combined results (n = 6) showing the aggregation defect in ERp57-deficient platelets using convulxin (300 ng/mL). (C) Correction of the aggregation defect of Pf4-Cre/ERp57fl/fl platelets by addition of ERp57 relative to ERp57fl/fl control platelets. Additional experiments show correction of the aggregation defect in ERp57-deficient platelets by addition of 50 or 100 nM ERp57 (n = 3 for each condition). Recombinant ERp57 was added for 5 minutes before aggregation was induced by convulxin (150 ng/mL).
Deficiency of platelet ERp57 impairs activation of αIIbβ3 and platelet aggregation. (A) ERp57-deficient platelets have a defect in activation of αIIbβ3 (detected by the JON/A activation dependent antibody). Depicted is a representative sample and the mean fluorescent intensity (MFI) of cumulative data ± SE (n = 5). (B) Representative aggregation tracing and combined results (n = 6) showing the aggregation defect in ERp57-deficient platelets using convulxin (300 ng/mL). (C) Correction of the aggregation defect of Pf4-Cre/ERp57fl/fl platelets by addition of ERp57 relative to ERp57fl/fl control platelets. Additional experiments show correction of the aggregation defect in ERp57-deficient platelets by addition of 50 or 100 nM ERp57 (n = 3 for each condition). Recombinant ERp57 was added for 5 minutes before aggregation was induced by convulxin (150 ng/mL).
The catalytic activity of the second active site of ERp57 is required for platelet aggregation
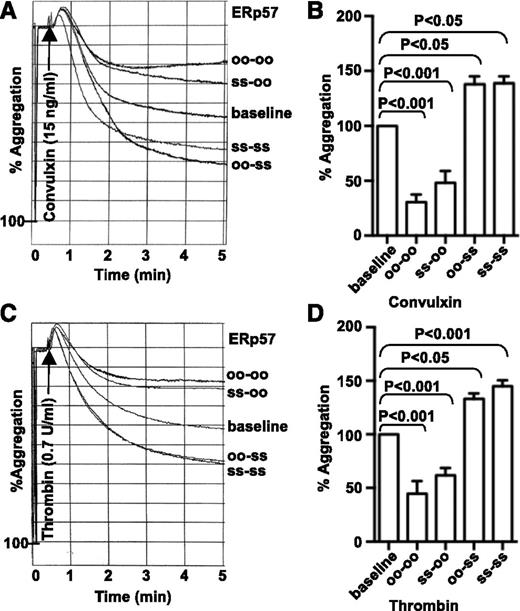
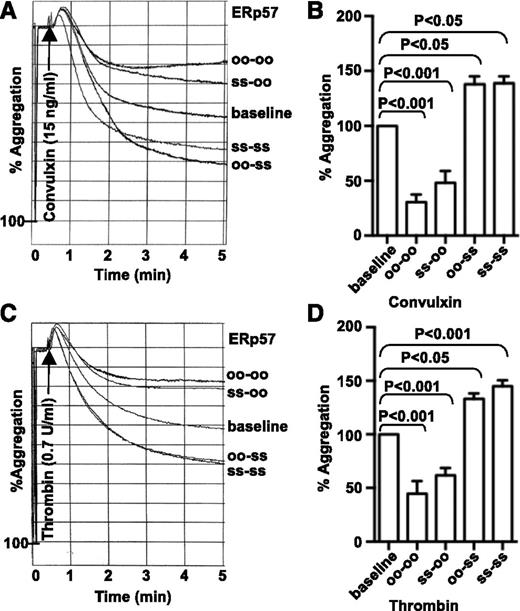
To begin to elucidate the mechanisms by which ERp57 stimulates platelet aggregation, we tested the effect of 2 mutants of the enzyme on aggregation of human platelets. One mutant had the first active site inactivated (oo-ss); the other mutant had the second active site inactivated (ss-oo). The mutant that retained the second active site potentiated convulxin and thrombin-induced aggregation to a similar degree as wild-type ERp57 (ss-ss; Figure 4A-D). In contrast, the mutant with the inactive second active site inhibited aggregation, similar to an ERp57 mutant with both active sites rendered catalytically inactive (oo-oo).12 These results indicate that the second active site of ERp57 is the important active site for platelet aggregation.
The second active site of ERp57 is critical for platelet aggregation. (A-D) Effect of preincubating human platelets with the ERp57 mutants (ss-oo or oo-ss). Wild-type (ss-ss) or completely inactivated ERp57 (oo-oo) were used as controls. Submaximal aggregation (baseline) was stimulated with convulxin (A) or thrombin (C). Representative tracings from a single experiment show the effect of adding ERp57 (2 µM) 10 minutes before the addition of the agonist. Panels B,D are the corresponding cumulative data ± SE (n = 3). There were no statistically significant differences between oo-oo and ss-oo or between oo-ss and ss-ss for convulxin or thrombin.
The second active site of ERp57 is critical for platelet aggregation. (A-D) Effect of preincubating human platelets with the ERp57 mutants (ss-oo or oo-ss). Wild-type (ss-ss) or completely inactivated ERp57 (oo-oo) were used as controls. Submaximal aggregation (baseline) was stimulated with convulxin (A) or thrombin (C). Representative tracings from a single experiment show the effect of adding ERp57 (2 µM) 10 minutes before the addition of the agonist. Panels B,D are the corresponding cumulative data ± SE (n = 3). There were no statistically significant differences between oo-oo and ss-oo or between oo-ss and ss-ss for convulxin or thrombin.
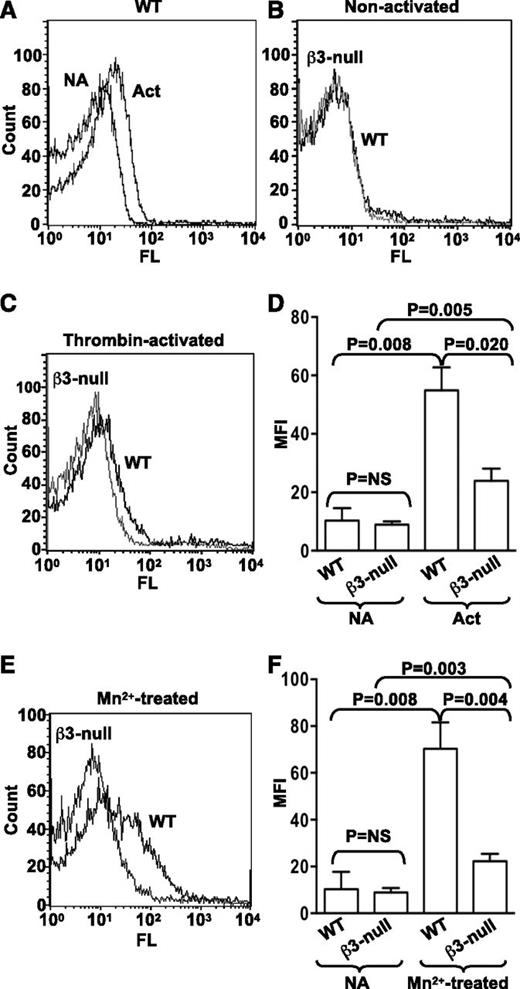
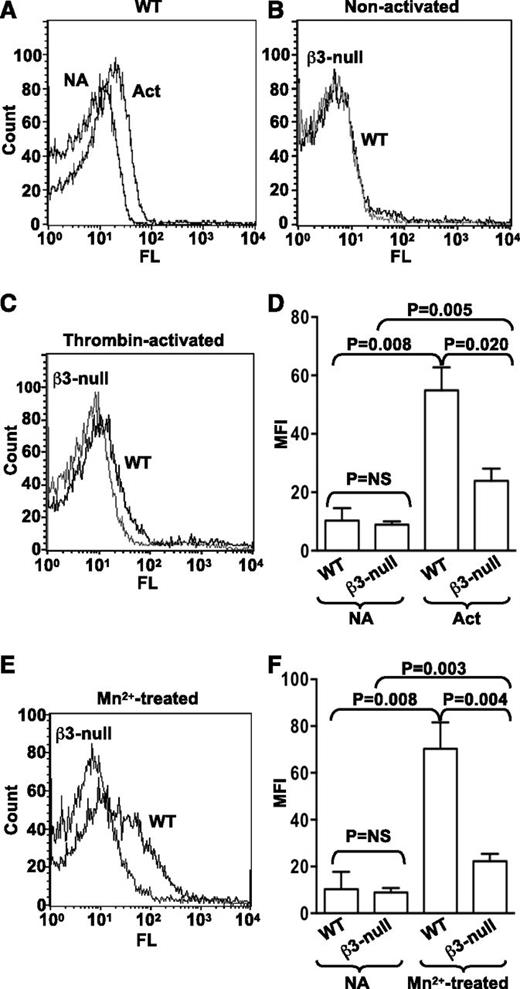
Binding of Alexa 488−labeled ERp57 to activated β3-null platelets is decreased
Binding of purified recombinant ERp57 conjugated with Alexa 488 to wild-type mouse platelets increases with thrombin activation of the platelets (Figure 5A). We next sought to determine whether ERp57 was interacting with αIIbβ3 on platelets. Alexa 488-ERp57 was incubated with mouse platelets lacking β3-integrins. Although we did not detect decreased binding of ERp57 to nonactivated β3-null platelets (Figure 5B,D), there was a major decrease in binding of ERp57 to thrombin-activated β3-null platelets compared with binding to activated wild-type platelets (Figure 5C-D). Moreover, binding to Mn2+-treated β3-null platelets was similarly decreased compared with binding to wild-type platelets (Figure 5E-F). The increase in binding of ERp57 to activated or Mn2+-treated β3-null platelets was relatively small (Figure 5D,F).
ERp57 interacts with β3 integrins on mouse platelets. (A) Binding of Alexa 488-ERp57 to nonactivated (NA) and thrombin-activated (Act) wild-type (WT) mouse platelets. (B) Binding of ERp57 to nonactivated WT and β3-null platelets showing superimposable curves. (C) Binding of ERp57 to thrombin-activated WT and β3-null platelets. (D) Cumulative data for ERp57 binding to thrombin-activated platelets ± SE (n = 3). (E) Binding to Mn2+-treated platelets. (F) Cumulative data of ERp57 binding to Mn2+-treated platelets ± SE (n = 3). Washed mouse platelets (3 × 108/mL) were preincubated with Alexa 488-ERp57 (30 µg/mL) for 10 minutes at 37°C and then activated by thrombin (0.3 U/mL) for 5 minutes or treated with Mn2+ (5 mM) for 10 minutes at 37°C. Surface binding of Alexa 488-ERp57 was detected by flow cytometry.
ERp57 interacts with β3 integrins on mouse platelets. (A) Binding of Alexa 488-ERp57 to nonactivated (NA) and thrombin-activated (Act) wild-type (WT) mouse platelets. (B) Binding of ERp57 to nonactivated WT and β3-null platelets showing superimposable curves. (C) Binding of ERp57 to thrombin-activated WT and β3-null platelets. (D) Cumulative data for ERp57 binding to thrombin-activated platelets ± SE (n = 3). (E) Binding to Mn2+-treated platelets. (F) Cumulative data of ERp57 binding to Mn2+-treated platelets ± SE (n = 3). Washed mouse platelets (3 × 108/mL) were preincubated with Alexa 488-ERp57 (30 µg/mL) for 10 minutes at 37°C and then activated by thrombin (0.3 U/mL) for 5 minutes or treated with Mn2+ (5 mM) for 10 minutes at 37°C. Surface binding of Alexa 488-ERp57 was detected by flow cytometry.
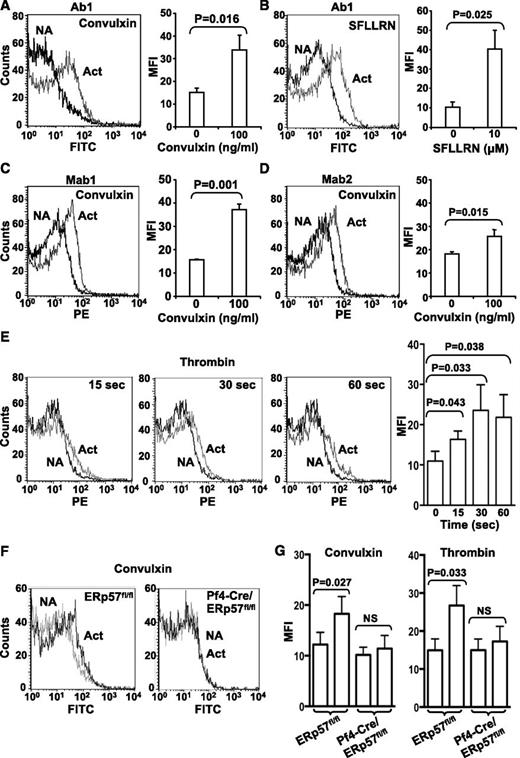
ERp57 protein and activity increase on the platelet surface with platelet activation
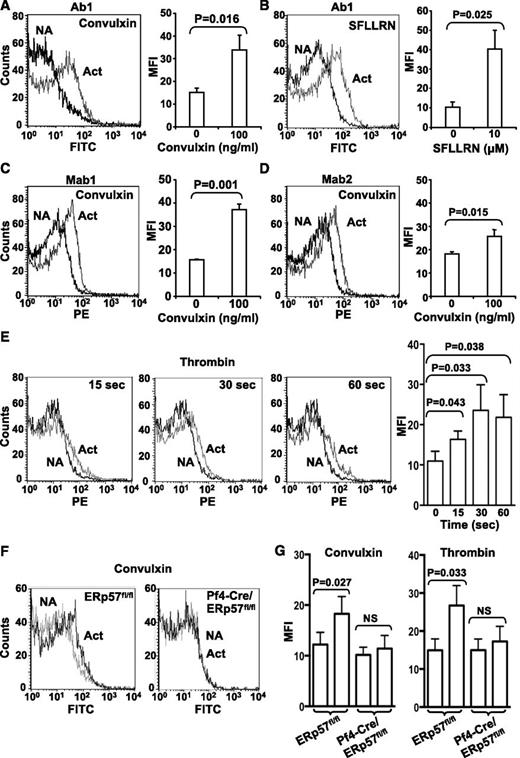
Using a polyclonal antibody specific to ERp57 (Ab1), we reported low levels of ERp57 on the surface of resting gel-filtered platelets.12 Using previously characterized monoclonal antibodies to ERp57 (Mab1 and Mab2), we did not detect surface ERp57 on resting human platelets (not shown). The polyclonal antibody likely recognizes more epitopes on ERp57, allowing easier detection of the enzyme. Using Ab1, we detected increased surface expression of ERp57 after convulxin and SFLLRN−induced platelet activation (Figure 6A-B). Mab1 and Mab2 also detected increased surface expression with convulxin-induced activation (Figure 6C-D). Low binding of control antibodies to activated platelets compared with specific antibodies confirmed the specificity of the detection antibodies (supplemental Figure 3). The increased surface expression was detected by 15 seconds after activation (Figure 6E). Surface expression of ERp57 was similarly increased with activation of wild-type mouse platelets (Figure 6F-G). In contrast, no expression of ERp57 was found in ERp57-deficient platelets with convulxin or thrombin-induced platelet activation (Figure 6F-G).
Platelet surface ERp57 protein increases with platelet activation. Surface expression was detected with the polyclonal anti-ERp57 antibody (Ab1) in response to convulxin (A) and SFLLRN (B). Surface expression was also detected by the monoclonal anti-ERp57 antibodies Mab1 (C) or Mab2 (D) using convulxin. Left panels show a representative result with non-activated (NA) and activated (Act) platelets; right panels show the MFI from several experiments ± SE (A, n = 8; B, n = 4; C, n = 5; D, n = 5). The platelets were incubated with convulxin or SFLLRN for 5 minutes before the analysis. When primary monoclonal antibodies were used, secondary PE-labeled antimouse antibodies were used. (E) Time course of surface expression of ERp57 in platelets using thrombin (3 U/mL) ± SE (n = 3). Left panels, representative flow histograms; right panel MFI ± SE (n = 3). (F) Mab1 binds to ERp57fl/fl platelets but not to ERp57-deficient platelets. Shown is binding of Mab1 to nonactivated platelets and activated platelets that express (ERp57fl/fl) or lack ERp57 (Pf4-Cre/ERp57fl/fl). The platelets were activated with convulxin (600 ng/mL). The curves with nonactivated and activated ERp57-deficient platelets are superimposable. (G) Cumulative data showing binding of Mab1 to mouse platelets activated with convulxin (1 µg/mL) or thrombin (3 U/mL) ± SE (n = 3).
Platelet surface ERp57 protein increases with platelet activation. Surface expression was detected with the polyclonal anti-ERp57 antibody (Ab1) in response to convulxin (A) and SFLLRN (B). Surface expression was also detected by the monoclonal anti-ERp57 antibodies Mab1 (C) or Mab2 (D) using convulxin. Left panels show a representative result with non-activated (NA) and activated (Act) platelets; right panels show the MFI from several experiments ± SE (A, n = 8; B, n = 4; C, n = 5; D, n = 5). The platelets were incubated with convulxin or SFLLRN for 5 minutes before the analysis. When primary monoclonal antibodies were used, secondary PE-labeled antimouse antibodies were used. (E) Time course of surface expression of ERp57 in platelets using thrombin (3 U/mL) ± SE (n = 3). Left panels, representative flow histograms; right panel MFI ± SE (n = 3). (F) Mab1 binds to ERp57fl/fl platelets but not to ERp57-deficient platelets. Shown is binding of Mab1 to nonactivated platelets and activated platelets that express (ERp57fl/fl) or lack ERp57 (Pf4-Cre/ERp57fl/fl). The platelets were activated with convulxin (600 ng/mL). The curves with nonactivated and activated ERp57-deficient platelets are superimposable. (G) Cumulative data showing binding of Mab1 to mouse platelets activated with convulxin (1 µg/mL) or thrombin (3 U/mL) ± SE (n = 3).
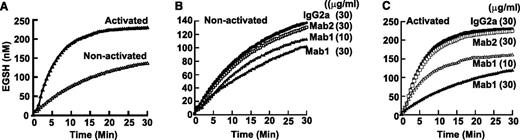
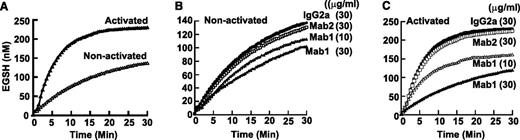
To characterize the activity of surface ERp57 in human platelets, we used the disulfide reductase assay that measures the fluorescent eosin-glutathione (EGSH) product of Di-E-GSSG.12,26 Resting platelets converted Di-E-GSSG to EGSH (Figure 7A) at an initial velocity (Vo) of ∼9-10 nM EGSH/min. Activation of the platelets with thrombin resulted in a dramatic increase of conversion of Di-E-GSSG to EGSH with an initial velocity of 44 nM EGSH/min. To specifically inhibit ERp57 on platelets, we used the inhibitory monoclonal antibody Mab1 that does not inhibit PDI (or ERp5).12 The demonstration that Mab1 does not react with ERp57-deficient platelets (Figure 6F-G) further confirms the specificity of this antibody for ERp57. When this antibody was used, a fraction of the initial velocity (32%) with gel-filtered resting platelets was attributable to ERp57 (Figure 7B). With platelet activation, there was a striking increase in the initial velocity (82%) of reductase activity attributable specifically to ERp57 (Figure 7C). The isotype specific Mab2 that does not inhibit soluble ERp5712 did not inhibit ERp57 activity (Figure 7B-C).
Platelet surface ERp57 activity increases with platelet activation. (A) Conversion of Di-E-GSSG to EGSH increases with platelet activation. Effect of the inhibitory Mab1 and non-inhibitory Mab2 on nonactivated platelets (B) and activated platelets (C). The curves are from the combined data from 3 different experiments. Gel-filtered platelets (4 × 108/mL) were activated with α-thrombin (0.5 U/mL) for 10 minutes. The antibodies were then added for 10 minutes at the concentrations indicated. Di-E-GSSG with DTT was added to give a final platelet concentration of 4 × 107/mL and Di-E-GSSG and DTT concentration of 150 nM and 5 µM, respectively. Conversion of Di-E-GSSG to EGSH was monitored over time in a Spectra Max M2 fluorimeter (Molecular Devices) as previously described12 with the platelets present.26
Platelet surface ERp57 activity increases with platelet activation. (A) Conversion of Di-E-GSSG to EGSH increases with platelet activation. Effect of the inhibitory Mab1 and non-inhibitory Mab2 on nonactivated platelets (B) and activated platelets (C). The curves are from the combined data from 3 different experiments. Gel-filtered platelets (4 × 108/mL) were activated with α-thrombin (0.5 U/mL) for 10 minutes. The antibodies were then added for 10 minutes at the concentrations indicated. Di-E-GSSG with DTT was added to give a final platelet concentration of 4 × 107/mL and Di-E-GSSG and DTT concentration of 150 nM and 5 µM, respectively. Conversion of Di-E-GSSG to EGSH was monitored over time in a Spectra Max M2 fluorimeter (Molecular Devices) as previously described12 with the platelets present.26
Discussion
Megakaryocyte/platelet-specific knockouts via the use of Pf4-Cre mice have been used to study platelet production and function.29-32 In this study, using Pf4-Cre/ERp57fl/fl mice we demonstrated that platelet-derived ERp57 is required for hemostasis, thrombosis, and platelet accumulation into a growing thrombus. A requirement for ERp57 in platelet production or expression of major platelet surface glycoproteins would hinder studies on the role of platelet-derived ERp57 in hemostasis and thrombosis. We documented that platelets counts and platelet glycoprotein expression in Pf4-Cre/ERp57fl/fl mice were similar to those of control mice. Moreover, the mean platelet volume of ERp57-deficient platelets were similar to that of littermate controls, indicating that ERp57 deficiency does not change platelet size. Either ERp57 does not have a significant role in platelet production or biogenesis of the major glycoproteins, or other members of the PDI family compensate for the loss of ERp57 as found in other cells.1,33
Deficiency of ERp57 in platelets prolongs the tail bleeding times and times to occlusion of the carotid artery. These results provide the first direct evidence that platelet-derived ERp57 has an important role in hemostasis and thrombosis. Moreover, in a mesenteric artery thrombosis model, we visually observed a major decrease in accumulation of ERp57-deficient platelets for up to 10 minutes after vessel injury. We did not find a significant difference at the 15-minute time point, which raises the possibility that nonplatelet sources of ERp57 such as endothelial cells begin to compensate for platelet-derived ERp57 at this time point. Contrary to what is generally believed, a recent study demonstrated that FeCl3-induced injury to the carotid or mesenteric arteries does not result in endothelial cell denudation.27 In that study the authors used greater concentrations of FeCl3 (10%) and longer durations of injury (up to 5 minutes) than we did. Moreover, immunohistochemical staining of mesenteric arteries for platelet endothelial cell adhesion molecule-1 confirmed the presence of endothelial cells in our injury model. Although endothelial cells at the site of injury may contribute to local expression of ERp57,11,34-37 our data indicate that ERp57 from endothelial cells cannot fully compensate for the loss of platelet-derived ERp57. This finding confirms the critical importance of platelet sources of this enzyme at the site of thrombosis. Taken together, our data from 2 different arterial thrombosis models show that platelet-derived ERp57 contributes substantially to thrombus formation. The incorporation of platelets into the thrombus in the mesenteric artery model is αIIbβ3 dependent, raising the possibility that platelet-derived ERp57 regulates αIIbβ3 activation in vivo.
We and others previously showed that inhibitory anti-ERp57 antibodies added to platelets inhibited the activation of αIIbβ3 and platelet aggregation,11,12 suggesting the important location of ERp57 in platelet function is the extracellular platelet surface. In this study we found the aggregation defect in ERp57-deficient platelets was corrected by addition of exogenous ERp57 to the platelets in a dose-dependent manner (Figure 3C). This finding indicates that the important site of functional ERp57 is the platelet surface. These results also imply that αIIbβ3 is functionally preserved in ERp57-deficient platelets but that ERp57 is required for the conformational changes leading to full activation of αIIbβ3.
To further elucidate the mechanisms of ERp57 regulation of αIIbβ3 activation, we examined the effect of 2 mutants of ERp57 on platelet aggregation. ERp57 with an inactive first active site but intact second active site, ERp57 (oo-ss), potentiated aggregation (Figure 4). In contrast, ERp57 with an inactive second active site, ERp57 (ss-oo), inhibited platelet aggregation. These findings imply that the second active site of ERp57 interacts with a platelet surface substrate of ERp57 to potentiate platelet aggregation. In contrast, ERp57 with an inactive second active site competes with normal platelet surface ERp57, inhibiting aggregation. These data indicate (1) the catalytic activity of ERp57 is required for ERp57 function in platelets; (2) the 2 active sites are not functionally equivalent; and (3) the second CGHC active site is critical for platelet function.
We found a substantial increase in the binding of soluble Alexa 488-ERp57 to activated wild-type or Mn2+-treated platelets that was dependent on the presence of αIIbβ3 (Figure 5C-F). There was little binding of soluble ERp57 to resting wild-type platelets (Figure 5B,D,F), suggesting that the increase of ERp57 binding with platelet activation or Mn2+ treatment is from binding to αIIbβ3 as it undergoes conformational changes. Although Mn2+ is not a physiologic agonist, it causes conformational changes in purified αIIbβ3 that induce this receptor to bind fibrinogen.38 Because Mn2+ directly induces conformational changes in αIIbβ3, it is likely that ERp57 directly interacts with αIIbβ3 on the platelet surface. Together with the aggregation results, these data suggest that ERp57 directly mediates activation of αIIbβ3 to its high affinity binding conformation.
These considerations are in line with our model of the activation of αIIbβ3 by PDI.2 A structural model for activation of αIIbβ3 involves transitions from a bent over inactive conformation to an extended intermediate affinity (closed) conformation, to a high-affinity (open) conformation.39 Because ERp57 does not bind to αIIbβ3 on nonactivated platelets, we propose that ERp57 plays a role in the transition from an intermediate-affinity conformation of αIIbβ3 to a high-affinity conformation. An ERp57-catalyzed reaction involving αIIbβ3 could stabilize the new integrin conformation, preventing it from reverting to a resting state, or cause the formation of the high affinity conformation. This reaction could affect surrounding integrins in the vicinity, and proximity to neighboring nonactivated αIIbβ3 molecules may result in their conversion into an activated form. Moreover, it is possible that ERp57 regulates ligand-binding avidity of αIIbβ3.
We propose that the important functional site of ERp57 is the platelet surface. In support of this concept, increased surface expression of ERp57 on human platelets was detected after platelet activation by the use of 3 different antibodies to ERp57 (Figure 6). These data with a previous report using thrombin activation40 provide strong evidence for the recruitment of ERp57 to the platelet surface. Increased surface expression of ERp57 is seen within 15 seconds of platelet activation when inside-out signaling begins to induce conformational changes in αIIbβ3.41
On resting platelets, a relatively small fraction of disulfide reductase activity was attributable to ERp57 (Figure 7B). This finding is consistent with previous studies in which authors used the same assay and reported that the majority of surface reductase activity on nonstimulated platelets was attributable to PDI.42,43 However, ERp57 surface reductase activity increased dramatically with thrombin-induced platelet activation (Figure 7C), with ERp57 now contributing the large majority (83%) of the disulfide reductase activity on activated platelets. The large increase of platelet surface reductase activity with platelet activation attributable to ERp57 indicates that active sites of the enzyme are in the free thiol form required to cleave the S-S bond in GSSG, the form of the active sites that also catalyze thiol-disulfide exchange reactions.2 ERp57 could activate αIIbβ3 by catalyzing a thiol-disulfide exchange reaction or by direct cleavage of a disulfide bond.2 Both reactions involve the breaking of a disulfide bond.2 Earlier studies implicated thiol-disulfide exchange in αIIbβ3 in the activation of this receptor,25,44,45 and αIIbβ3 can be activated by disruption of disulfide bonds in the cysteine-rich epidermal growth factor domains of β3.46-48
In this study we found a defect in both platelet aggregation and in vivo thrombosis resulting from deficiency of ERp57 in platelets with normal PDI levels. This finding indicates that PDI can’t compensate for the absence of ERp57 and is consistent with previous studies showing that antibodies that inhibit ERp57, but not PDI, inhibit platelet aggregation.11,12 This provides strong evidence for distinct roles of ERp57 and PDI in platelet function. It is possible that ERp57 and PDI catalyze different reactions (eg, disulfide cleavage versus thiol-disulfide exchange) or target different sites within αIIbβ3. The residual binding to β3-null platelets (Figure 5D,F) likely represents binding to non-β3 integrin substrates (as α2β1) or nonintegrin substrates. Substrate differences may contribute to the inability of the enzymes to compensate for each other.
In conclusion, our study with a targeted ERp57 knockout establishes a requirement for platelet-derived ERp57 in hemostasis, thrombosis, and platelet accumulation in a developing thrombus. The aggregation defect in ERp57-deficient platelets represents a major functional consequence of deleting this enzyme: correction of this defect by soluble ERp57 highlights the importance of surface exposed ERp57. The second CGHC active site of ERp57 targets a platelet surface substrate and αIIbβ3 appears to be the major target of this enzyme. An updated working model of the extracellular events involved in activation of αIIbβ3 would incorporate an ERp57-catalyzed reaction in αIIbβ3.2 Upon platelet activation substantial expression of ERp57 occurs in a physiologically relevant time frame. Together, these data suggest a major role for platelet-derived ERp57 in activation of αIIbβ3 and platelet incorporation into a growing thrombus. Characterizing ERp57-dependent mechanisms of activation of αIIbβ3 and determining ways to inhibit the second active site of ERp57 could lead to novel inhibitors of platelet function and thrombosis.
Presented in abstract form at the 53rd annual meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr A. K. Rao for a critical review of the manuscript.
This work was supported by startup funds from Temple University School of Medicine, the Priority Academic Program Development of Jiangsu Higher Education Institutions, Natural Science Foundation of China (81270592, 30971491), a Grant in Aid from the American Heart Association (Great Rivers Affiliate), and a National Heart, Lung, and Blood Institute, National Institutes of Health research grant (1R01HL118526).
Authorship
Contribution: D.W.E. designed the project, supervised the research, analyzed the data and wrote the manuscript; Y.W. helped in the design, supervised experiments, and helped revise the manuscript; L.W., Y.W., J.Z., and S.S.A. performed research and collected and analyzed data; B.M. helped set up the Di-E-GSSG assay; N.G. and G.H. generated the ERp57 loxp mice; and J.L. provided the β3-null mice and helped with the mesenteric artery experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Wu, The Cyrus Tang Hematology Center, Jiangsu Institute of Hematology, First Affiliated Hospital, Soochow University, Suzhou, 215123, China; e-mail: yiwu99@gmail.com; and David W. Essex, Temple University School of Medicine, Room 300C OMS, 3400 North Broad St, Philadelphia, PA 19140; e-mail: david.essex@temple.edu.
References
Author notes
L.W. and Y.W. contributed equally to this study.