Key Points
PCR negativity is a strong outcome predictor after rituximab-intensive immunochemotherapy at multiple posttreatment times.
PCR is predictive even when maintenance is delivered, and accumulation of PCR-negative results further reduces the likelihood of relapse.
Abstract
We assessed the prognostic value of minimal residual disease (MRD) within the ML17638 phase 3 trial from the Fondazione Italiana Linfomi, investigating the role of rituximab maintenance in elderly follicular lymphoma (FL) patients after a brief first-line chemoimmunotherapy. MRD for the bcl-2/IgH translocation was determined on bone marrow cells in a centralized laboratory belonging to the Euro-MRD consortium, using qualitative and quantitative polymerase chain reactions (PCRs). Of 234 enrolled patients, 227 (97%) were screened at diagnosis. A molecular marker (MM) was found in 51%. Patients with an MM were monitored at 8 subsequent times. Of the 675 expected follow-up samples, 83% were analyzed. Conversion to PCR negativity predicted better progression-free survival (PFS) at all post-treatment times (eg, end of therapy: 3-year PFS, 72% vs 39%; P < .007). MRD was predictive in both maintenance (83% vs 60%; P < .007) and observation (71% vs 50%; P < .001) groups. PCR positivity at the end of induction was an independent adverse predictor (hazard ratio, 3.1; 95% confidence interval, 1.36-7.07). MRD is a powerful independent outcome predictor in FL patients who receive rituximab-intensive programs, suggesting a need to investigate its value for decision-making. This trial was registered at www.clinicaltrial.gov as #NCT01144364.
Introduction
Treatment of follicular lymphoma (FL) has advanced in recent years. Because of rituximab-supplemented chemotherapy, most patients currently achieve complete remission (CR), and overall survival (OS) rates have improved since the 1990s. However, most patients still relapse, and a proportion die of the disease.1-3 The risk for recurrence is more pronounced among patients older than 60 years, as they often receive less-intense treatments.4
Considerable evidence indicates that the persistence of polymerase chain reaction (PCR)-detectable residual tumor cells in the bone marrow (BM) and, to a lesser extent, peripheral blood is an independent predictor of relapse in FL5-24 ; nevertheless, a few studies have failed to confirm this observation.25-28 Concerns about the value of minimal residual disease (MRD) detection as an effective prognostic tool have been raised, particularly when applied to rituximab-containing chemotherapy regimens, which are characterized by multiple rituximab administrations (with or without maintenance) and are not autotransplant-based. Moreover, the majority of previous studies have some limitations, including a retrospective nature, small sample size, mixed tissue sources (peripheral blood vs BM), and lack of prospective planning for MRD time points.
The ML17638 study from the Fondazione Italiana Linfomi is a randomized prospective phase 3 trial investigating the value of shortened rituximab maintenance after brief first-line chemoimmunotherapy (rituximab, fludarabine, mitoxantrone, dexamethasone [R-FND]) followed by rituximab consolidation in patients with advanced FL, aged 60 to 75 years. The ML17638 study included an extensive centralized MRD monitoring program that used both qualitative nested-PCR (N-PCR) and real-time quantitative PCR (RQ-PCR). MRD was determined for BM samples taken at the time of study entry and at 8 subsequent fixed times. The results of the MRD analysis in the ML17638 trial are the subject of this article.
Patients and methods
Study population and treatment modalities
Between January 2004 and December 2007, this randomized, multicenter, open-label, phase 3 study enrolled 242 treatment-naive patients aged 60 to 75 years with a confirmed diagnosis of B-cell, CD20-positive FL (grade 1, 2, or 3a) requiring treatment. The inclusion criteria and exclusion criteria were described previously.29 In accordance with the Declaration of Helsinki, written informed consent included evaluation of MRD. The protocol was approved by the ethics committees of all participating institutions.
The treatment procedures were described previously.29 Briefly, patients received 4 monthly courses of the R-FND regimen, consisting of 375 mg/m2 rituximab (day 1), 25 mg/m2 fludarabine (days 2-4), 10 mg/m2 mitoxantrone (day 2), and 10 mg dexamethasone (days 2-4), followed by 4 weekly infusions of 375 mg/m2 rituximab as consolidation treatment. Patients achieving CR or a partial response (PR) at month 8 (M8) were randomly assigned in a 1:1 ratio to receive either a shortened maintenance with 375 mg/m2 rituximab once every 2 months (total of 4 doses, group A) or no further therapy (group B). A full response assessment, including physical examination and a computed tomography scan of the neck, thorax, and abdomen, was performed after 4 cycles of R-FND (M5), 1 to 2 months after completion of rituximab consolidation (M8), and at M12, M18, M24, M30, M36, and M42 from study entry, according to published criteria.30 BM biopsy was performed at M5, M8, M18, M30, and M42 if results were abnormal at baseline. Progression-free survival (PFS) and OS were calculated as previously reported.30
MRD monitoring
Times
At enrollment, the patients were screened on BM cells for a molecular marker based on the bcl-2/IgH major breakpoint region (MBR) or minor cluster region (mcr). Patients with a molecular marker at diagnosis were then tested by N-PCR on BM cells at 8 fixed points: after induction (M5), at the end of consolidation (M8), during maintenance/observation, and at follow-up (M12, M18, M24, M30, M36, and M42) or until relapse, death for any cause, or study withdrawal. RQ-PCR analysis was performed in MBR-positive cases if an adequate amount of DNA was available after N-PCR (supplemental Table 2, available on the Blood Web site).
N-PCR and RQ-PCR for molecular markers
N-PCR was performed using the bcl-2/IgH rearrangement, as described previously.31 The sensitivity of N-PCR was 3.3 × 10−6 (ie, 1 neoplastic rearrangement in 300 000 normal cells), as expected, based on the amount of DNA used, as the amplification was able to detect a single copy of rearranged DNA. RQ-PCR for bcl-2/IgH was carried out as described previously.32,33 Standard curves were prepared according to Euro-MRD guidelines, using translocation-positive cell lines.34 DNA to be used as “no template DNA control” and for standard curve dilutions was obtained from chemo-treated patients without lymphoma to avoid false-positives35,36 as recommended by Euro-MRD. Reactions were performed in an AbiPrism 7900HT sequence detector system (PE Applied Biosystems). The conditions and reference standard genes for DNA quality and normalization were reported previously.37,38 For all cases, the calculation of MRD was based on comparative cycle threshold analysis between follow-up samples and standards according to Euro-MRD criteria.39 On the basis of these criteria, the reaction was able to reach a sensitivity of 1.0 × 10−5 (ie, 1 neoplastic rearrangement in 100 000 normal cells) and a quantitative range of 5 × 10−5. For quantitative analyses, “positive not quantifiable” results (defined based on MRD criteria) were conventionally scored to an MRD level of 1.0 × 10−5.39
Direct sequencing of the bcl-2/IgH rearrangement at diagnosis and in PCR-positive follow-up samples was performed in 20% of cases with at least a single PCR-positive follow-up sample. The randomly chosen samples confirmed the identity of the rearrangement detected at diagnosis and after treatment (100% identity).
Statistical analysis
The prognostic role of MRD in PFS was investigated. PFS was calculated from enrollment to the date of disease progression, relapse, or death from any cause. Landmark analyses were also performed according to intermediate evaluations of MRD (M5, M8, M12, M18, M24) during follow-up. PFS functions were estimated using the Kaplan-Meier method and compared between groups, deriving from the MRD evaluations, by log-rank test. Landmark analysis at M8 to compare positive and negative N-PCR patients was performed using a Cox proportional hazard model, adjusting for FL International Prognostic Index (FLIPI), age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), clinical response, and randomization group (maintenance, observation).
Finally, to evaluate the effect of N-PCR negativity during follow-up on PFS, we considered the whole follow-up period starting from M5, including all available MRD evaluations, as a time-varying covariate calculated in a cumulative manner (0, 1, 2, 3, or more consecutive N-PCR-negative times). This analysis was performed using a Cox model, adjusting for the baseline covariates FLIPI, age, ECOG PS, and sex, and the time-varying covariates complete clinical response after induction phase M8 and randomization to the maintenance group. For this final analysis, which included all available evaluations of MRD, missing N-PCR values were multiple-imputed, using the method of chained equations.40 In the logit model used for the imputation of N-PCR missing values, in addition to baseline and time-varying covariates mentioned earlier, we included the 2 additional covariates of the N-PCR status (0 = negative, 1 = positive, 2 = missing) both immediately preceding and after each N-PCR evaluation. Combined estimates41 were obtained from 20 imputed datasets.
Statistical analyses were performed using STATA (version 11.1) and the ice command for multiple imputations.
Results
Patient characteristics and clinical outcomes
Patient demographics and clinical characteristics, consort diagram, and clinical outcomes were reported elsewhere.29 Briefly, 242 previously untreated elderly (age, 60-75 years) patients with FL were enrolled in the study. A total of 234 patients with a confirmed diagnosis of FL were included in the present analysis. The CR/CR unconfirmed and PR rates were 55% and 37%, respectively, at M5, and 69% and 17%, respectively, at the end of treatment (M8). Overall, 202 responding patients were randomly assigned to maintenance treatment (group A) or observation (group B). With a median follow-up of 42 months after randomization, 3-year PFS and OS were 66% (95% confidence interval [CI], 59%-72%) and 89% (95% CI, 85%-93%), respectively.29 After randomization, 2-year PFS was 81% for group A and 69% for group B (stratified hazard ratio [HR], 0.74; 95% CI, 0.45-1.21; P = .226), as previously reported.29
MRD study: sample flow and baseline bcl-2/IgH status
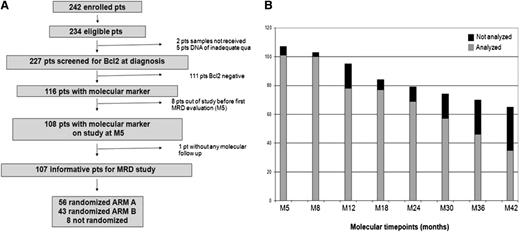
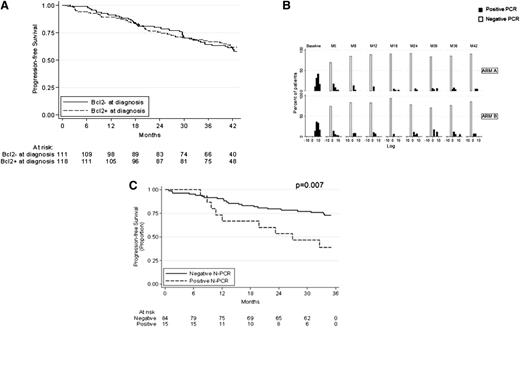
A total of 227 patients among the 234 eligible patients (97%) were successfully screened for a molecular marker at the time of study entry (Figure 1A). Five cases were missed as a result of inadequate DNA quality or nondelivery. A molecular marker was found in 116 (51%) of the 227 patients. These 116 bcl-2/IgH-positive patients were a representative sample of the whole trial population, with the exception of an expected increase in BM invasion and an excess number of males and patients randomly assigned to maintenance (supplemental Table 1). Eight of the 116 patients were excluded from further MRD analyses because of study withdrawal before M5 (7%). Four patients withdrew because of progression, 3 withdrew because of toxicity, and 1 patient was lost to follow-up (Figure 1A). On the basis of the clinical outcomes of the study population, 675 follow-up samples were expected; 559 (83%) samples were analyzed. Sample availability was excellent at early times, although a decline of compliance was noted at late times (Figure 1B). Seventy-nine percent of patients were evaluable for more than 75% of planned times, 17% for from 50% to 75% of times, 2% for from 25% to 50% of times, and 1% for from 1% to 25% of times (data not shown). No follow-up samples were available for 1 patient. Patients with and without a marker at diagnosis had identical PFS (61% at M42 for both; Figure 2A). On the basis of previous observations, we evaluated the prognostic role of baseline molecular tumor burden as assessed by RQ-PCR in bcl-2/IgH-positive patients.18 Patients with low, intermediate, and high molecular tumor burden at diagnosis had a PFS of 80%, 75%, and 66%, respectively (HR for high tumor burden, 1.7; 95% CI, 0.64-4.54). Molecular tumor burden by RQ-PCR did not correlate with FLIPI (P = .402, data not shown), although a correlation with BM invasion was observed (P = .047, data not shown). However, molecular tumor burden at diagnosis by RQ-PCR did not emerge as an independent predictor in multivariate analysis (supplemental Table 3).
Study plan for MRD analysis. (A) Flowchart of patients for molecular screening and follow-up. (B) Sample availability was excellent at early times, although a decline of compliance was noted at late times.
Study plan for MRD analysis. (A) Flowchart of patients for molecular screening and follow-up. (B) Sample availability was excellent at early times, although a decline of compliance was noted at late times.
Descriptive results of bcl-2/IgH PCR analysis. (A) PFS based on the presence or absence of a molecular marker. (B) Kinetics of MRD as assessed by RQ-PCR. (C) PFS based on N-PCR status at M8.
Descriptive results of bcl-2/IgH PCR analysis. (A) PFS based on the presence or absence of a molecular marker. (B) Kinetics of MRD as assessed by RQ-PCR. (C) PFS based on N-PCR status at M8.
MRD kinetics
On the basis of N-PCR, 70% of patients achieved PCR negativity at M5, and 84% at M8. At M8, 69% of patients were both in CR and were PCR-negative, 11% were in CR and were PCR-positive, 15% were in PR and were PCR-negative, and 5% achieved neither CR nor PCR negativity. MRD status at subsequent times for the whole population and according to treatment group is shown in supplemental Table 2. As expected, at times evaluated before randomization (M5, M8), the proportion of PCR positivity was similar in the 2 groups (group A, 20%; group B, 22%; P = .134). In contrast, after randomization (M12-M42), the rate of PCR positivity was greater in patients randomly assigned to observation than in those assigned to maintenance (group A 10% vs group B 18%; P < .001). Figure 2B shows the MRD kinetics as detected by RQ-PCR in both treatment groups.
Prognostic role of posttreatment MRD evaluation
Table 1 shows the PFS according to both N-PCR and RQ-PCR at various assessed times. At the preconsolidation point (M5), PCR negativity was not significantly associated with a superior outcome. In contrast, at times M8, M12, M18, M24, and M30, both N-PCR and RQ-PCR exhibited a strong predictive value (Table 1). For example, at the end of therapy (M8), PCR-negative patients according to N-PCR had a 34-month PFS of 72% compared with 39% for PCR-positive patients (P = .007; Table 1; Figure 2C). PCR-positive patients according to RQ-PCR had a median PFS of 12 months, whereas 75% of PCR-negative patients were still free of progression at 36 months (P < .001; Table 1).
Because N-PCR is slightly more sensitive than RQ-PCR, we identified 37 of 559 samples (7%) with a very low burden of residual disease and that were PCR-positive, based on N-PCR, and that were PCR-negative, based on RQ-PCR. These cases did not cluster at a specific time (data not shown). In contrast, we never found cases positive by RQ-PCR and negative by N-PCR. At M8 for PFS, we recorded 8 cases scoring PCR+ by N-PCR and PCR− by RQ-PCR. Interestingly, these patients had a PFS of 60%, which is between double-negative cases (PFS, 75%) and double-positive cases (median PFS, 12 months; P < .001, supplemental Figure 2).
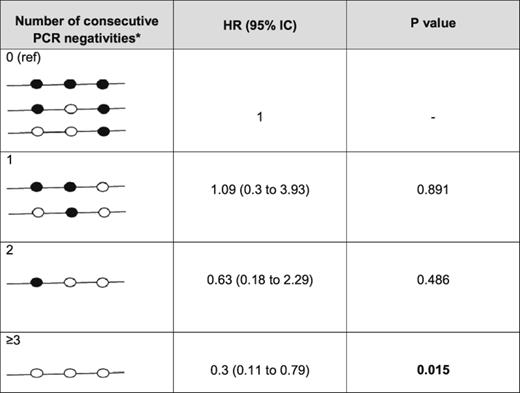
Interestingly, achieving double PCR negativity at M8 to M12 or triple molecular negativity at M8 to M12 to M18 was associated with an increase in PFS (82% vs 46% for M8-M12 [P = .001, data not shown] and 87% vs 53% for M8-M12-M18 [P < .001]; supplemental Figure 2). This finding was supported by the development of a time-varying covariate model including the accumulation of PCR-negative findings, FLIPI, age, sex, ECOG PS, clinical response at M8, and treatment group. This model revealed that a greater number of repeated PCR-negative results leads to a lower likelihood of relapse in patients, with a stratified HR of 1.09 to 0.3 for PCR-negative findings from 1 to 3 and more (Table 2).
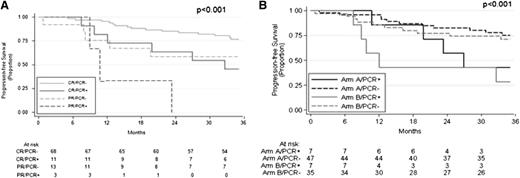
Next, we explored the combined effect of MRD negativity and CR achievement in our series: 3-year PFS for N-PCR-negative patients in CR at M8 was 77% compared with 59% for N-PCR-negative patients in PR (Figure 3A). N-PCR-positive patients achieving CR had a PFS of 45%, whereas the 3 PCR-positive cases in PR relapsed after 8, 11, and 23 months, respectively (Figure 3A). We then assessed the effect of MRD negativity by treatment group. It should be noted that no stratification was made on the basis of the presence of a molecular marker at diagnosis, resulting in a slight excess of bcl-2/IgH-positive patients randomly assigned to group A (56 vs 43). Among patients randomly assigned to maintenance (group A), the PFS was 83% for PCR-negative patients and 60% for PCR-positive patients (P = .007), whereas the PFS was 71% and 50% among PCR-negative and PCR-positive patients randomly assigned to observation (group B), respectively (P < .001; Figure 3B).
Effect of MRD by response status and treatment group. (A) PFS based on N-PCR and clinical remission at M8. (B) PFS based on N-PCR at M8 in the maintenance group (arm A) and observation group (arm B).
Effect of MRD by response status and treatment group. (A) PFS based on N-PCR and clinical remission at M8. (B) PFS based on N-PCR at M8 in the maintenance group (arm A) and observation group (arm B).
Next, a multivariate model was performed including the following covariates: age, FLIPI, ECOG PS, treatment group, CR achievement, and PCR status at M8 (Table 3). After adjusting for all other covariates, PCR status (HR, 3.1; 95% CI, 1.36-7.07), clinical remission (HR, 2.69; 95% CI, 1.1-6.56), and FLIPI (HR, 2.8; 95% CI, 1.23-6.36) emerged as strong predictors of outcome.
We also assessed the effect of MRD on OS. In none of the times did MRD appear predictive for outcome (ie, OS at M8 was 94% for both PCR-positive and -negative patients [P = .789]; data not shown). However, this analysis is merely exploratory, given the short follow-up and the limited number of events observed so far (7 events among patients with a molecular marker at diagnosis).
Discussion
The results for MRD within the ML17638 trial indicate that the conversion to PCR negativity (based on either N-PCR or RQ-PCR) in patients with a documented bcl-2/IgH MBR or mcr positivity at diagnosis is associated with better PFS at any posttreatment time, and its predictive value is strong even when maintenance is delivered; the accumulation of PCR-negative results reduces the likelihood of relapse; a shortened chemotherapy program with rituximab supplementation induces high rates of PCR negativity and major decreases in tumor burden; MRD analysis by both N-PCR and RQ-PCR is feasible with minimal loss of samples in the context of a large, multicenter, phase 3 trial; and PCR negativity is a strong independent prognosticator in the context of rituximab-intensive programs.
The clinical results of the ML17638 trial demonstrate the high efficacy of a brief chemoimmunotherapy program followed by rituximab consolidation in patients with advanced FL, although agents other than fludarabine might perform even better in the context of a similar brief rituximab-intensive therapeutic strategy.42 The MRD results strengthen this observation. The 74% and 80% PCR negativity at M5 and M8 indicates excellent antitumor activity, a result that is comparable to the one observed with more prolonged chemotherapy delivery.14,22 Moreover, the rate of CR and PCR negativity increased after the 4-weekly rituximab consolidation course, suggesting that intensive rituximab delivery is effective in improving the quality of response, which was also shown by RQ-PCR-based MRD kinetics (Figure 1B).
To the best of our knowledge, this is the first study that included an extensive molecular follow-up during rituximab maintenance, using exclusively BM cells. Several studies have investigated the prognostic role of MRD detection in FL. Most studies showed that MRD negativity is associated with a superior outcome and acts as an independent predictor.5-24 However, a few studies failed to confirm this observation.25-28 In particular, a recent report by van Oers et al28 failed to demonstrate any benefit of achieving postinduction PCR-negative status. This study raised concerns regarding the predictive value of MRD detection in the context of rituximab-intensive therapeutic programs. In contrast, our results clearly indicate that MRD is an independent predictor of outcome in rituximab-intensive chemoimmunotherapy. These studies are difficult to reconcile. One important difference is the treatment setting (ie, frontline vs relapse), although previous studies proved the predictive value of MRD also at relapse. Importantly, our analysis included only cases with a documented molecular marker at the time of study entry. Moreover, only BM samples were used in the study, and the analysis was carried out in a laboratory that performs routine quality control in the context of an international consortium such as Euro-MRD.
Our study has several strengths, most notably the inclusion of 97% of enrolled bcl-2/IgH-positive patients in the analysis, the use of 2 different MRD detection tools (N-PCR and RQ-PCR), and multiple fixed times. Importantly, in cases undergoing confirmatory sequencing, we found 100% identity between the rearrangements observed at diagnosis and after treatment, demonstrating that posttreatment MRD persistence is associated with persistence of the malignant clone and not with unrelated rearrangements, which are indeed known to be extremely rare after chemotherapy.35 Moreover, the demonstration of excellent predictive value at multiple times and the progressive reduction in the likelihood of relapse with the accumulation of repeated PCR-negative results, as well as the independent value of PCR in multivariate analysis, are all strong indicators supporting the high reliability of MRD in FL. Finally, the good predictivity of MRD monitoring in the ML17638 trial, addressing treatments and patient populations different from those for which it was originally established and most frequently employed, demonstrates the value of this biomarker over a broad range of antilymphoma treatments.
In this study, only patients with a PCR-detectable bcl-2/IgH translocation at diagnosis were considered in the analysis. Alternatively, all patients could have been followed-up at every point, regardless of baseline t(14;18) MBR or mcr status. This strategy might allow us to pick a minority of cases in which a t(14;18) MBR- or mcr-positive lymphoma was present in the lymph nodes but lacking in the diagnostic BM and later colonize the BM during follow-up. However, the vast majority of cases lacking a PCR-amplifiable t(14;18) MBR or mcr translocation at diagnosis are cases lacking a t(14;18) or displaying a t(14;18) occurring at minor breakpoints. For these cases, the t(14;18) MBR or mcr clearly represents an inadequate marker of tumor persistence, and their inclusion would have introduced a significant bias in the analysis. Indeed the choice of including only patients with a documented tumor marker at diagnosis is in accordance with most previous MRD studies, regardless of the nature of disease and type of marker employed, and specifically in FL studies targeting the bcl2/IgH translocation.5,14,16,17,22
The main limitation of MRD detection is that only 50% to 60% of patients with FL can be evaluated by this approach. The MRD detection methods used in this study were developed in the early years of the past decade. However, tools for MRD detection have been improved during the last couple of years. Novel PCR assays targeting minor bcl-2 rearrangements are being developed.43 Six- and 8-color flow cytometry has been established as a powerful MRD tool in myeloma and chronic lymphocytic leukemia and may potentially be useful in FL.44-46 Finally, next-generation sequencing has been shown to represent an excellent tool for MRD detection in precursor and mature B-cell tumors.47,48 Thus, in the near future, the vast majority of FL cases will be able to undergo effective MRD monitoring. Unfortunately, implementation of MRD results by novel techniques was not possible in the present study because of the planned interruption of molecular follow-up in cases without a molecular marker.
The predictive value of positron emission tomography (PET) at the end of first-line treatment was recently demonstrated in FL.49,50 Thus far, no head-to-head comparison of PET and PCR has been performed to verify whether they identify the same or different subgroups of high-risk patients. On the basis of the fact that PET optimally explores the nodal compartment and PCR optimally explores the BM, the 2 methods should be able to identify different nonoverlapping subgroups of high-risk patients, further refining our ability to predict outcomes in FL. From a more practical point of view, MRD has the limitation of requiring BM sampling and centralized analysis, whereas PET is associated with a nonnegligible radiation exposure.51 Additional studies are required to define the relative value of these approaches in the management of patients with FL.
The ultimate aim of any prognostic tool is to allow the development of “tailored treatments” for patients carrying specific risk factors. This aim implies not only having an effective prognosticator but also proving the clinical value of treatment intensification or deintensification on the basis of the presence of a given predictor. MRD is an effective decision-making tool, particularly in acute lymphoblastic leukemia.52 Studies using MRD as a decision-making tool are ongoing or in preparation for patients with mantle cell lymphoma. In advanced FL, the Fondazione Italiana Linfomi is currently running a phase 3 randomized study that includes MRD-based decision making. This ongoing randomized trial (FOLL-12, NCT 003170-60) will compare a standard maintenance program with a tailored maintenance/consolidation program based on MRD and PET. This study will hopefully prove the benefits of a risk-adapted therapeutic approach in FL.
The results of this study were the subject of an oral presentation at the 2012 American Society of Hematology annual meeting.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Antonella Fiorillo and Franca Trotto Gatta for excellent secretarial support, and to Simone Ferrero for the revision of the manuscript.
This research was funded by Roche Italy SPA. This work was also supported by Progetto di Rilevante Interesse Nazionale (PRIN 2009) from Ministero Italiano dell'Università e della Ricerca (code: 7.07.02.60 AE01), Progetti di Ricerca Finalizzata 2008 (head unit: Istituto di Ricovero e Cura a Carattere Scientifico Centro di Riferimento Oncologico della Basilicata, Rionero in Vulture [Potenza]; code: 7.07.08.60 P49), Progetto di Ricerca Sanitaria Finalizzata 2008 (head unit: Divisione di Ematologia, A.O.S. Maurizio, Bolzano/Bozen; code: 7.07.08.60 P51), Progetto di Ricerca Sanitaria Finalizzata 2009 (head unit: Divisione di Ematologia, A.O.S. Maurizio, Bolzano/Bozen; code: RF-2009-1469205), Progetto di Ricerca Sanitaria Finalizzata 2010 (head unit: Divisione di Ematologia, A.O.S. Maurizio, Bolzano/Bozen; code: RF-2010-2307262), Fondi di Ricerca Locale, Università degli Studi di Torino, and Fondazione Neoplasie del Sangue, Torino.
Authorship
Contribution: M.L., C.L.-B., and U.V. designed and performed the research, analyzed data, and wrote the manuscript; B.M., C.B., E. Genuardi, and A.C. contributed to the experimental design and contributed significantly to preparation of the manuscript; M.C. and A.E. performed statistical analysis and contributed to preparation of the manuscript; and L.B., G.R., A. Pulsoni, F.D.R., L.R., A. Pinto, S.G., A.B., D.R.-S., A.F., F.Z., A.G., F.G.R., G.S., P.M., and E. Gamba performed the research and contributed to preparation of manuscript.
Conflict-of-interest disclosure: M.L. has received honoraria from Celgene, Janssen, Amgen, Roche, and Mundipharma, and research funding from Celgene, Amgen, Pfizer, Italfarmaco, Mundipharma, and Roche; L.B. has received honoraria from Celgene, Roche, and Mundipharma; G.R. has received honoraria from Roche; A. Pinto has received honoraria from Celgene, Mundipharma, and Roche; F.Z. has received honoraria, consultancy, research funding, and other remuneration from Roche; P.M. has received honoraria from Roche, Celgene, and Janssen, as well as consultancy from Celgene and research funding from Roche, Celgene, and Janssen; E. Gamba is an employee and stockholder at Roche; and U.V. has received a consultancy from Roche. The other authors declare no competing financial interests.
Correspondence: Marco Ladetto, Cattedra di Ematologia, via Genova 3, 10126 Torino, Italy; e-mail: marco.ladetto@unito.it.