Key Points
Platelet-VWF exists as a distinct natural glycoform.
Platelet-VWF is resistant to ADAMTS13 proteolysis.
Abstract
Platelet-von Willebrand factor (VWF) is stored within α-granules and accounts for ∼20% of total VWF in platelet-rich plasma. This platelet-VWF pool is distinct from plasma-VWF and is enriched in high molecular weight multimers (HMWM). Previous studies have described significant functional discrepancies between platelet-VWF and plasma-VWF; however, the molecular basis of these differences is not well understood. We have characterized terminal glycan expression on platelet-VWF. Our findings demonstrate that platelet-VWF exists as a distinct natural glycoform. In particular, N-linked sialylation is markedly reduced (>50%) compared with plasma-VWF. Moreover, unlike plasma-VWF, platelet-VWF does not express AB blood group determinants, although precursor H antigen expression is similar to that on plasma-VWF. Because of this differential glycosylation, platelet-VWF exhibits specific resistance to ADAMTS13 proteolysis. Thus platelet activation at sites of vascular injury results in the release of high local concentrations of HMWM platelet-VWF that is more resistant to ADAMTS13, thereby facilitating platelet-plug formation.
Introduction
Von Willebrand factor (VWF) is a large multimeric sialoglycoprotein that plays critical roles in normal hemostasis. Besides circulating in plasma, significant amounts of VWF are also stored within the α-granules of platelets.1,2 This platelet-VWF is synthesized during megakaryocytopoiesis and accounts for 10% to 20% of the total VWF present in normal platelet-rich plasma.2-4 Importantly, the platelet-VWF pool is distinct from plasma-VWF and is enriched in hemostatically active high molecular weight multimers (HMWM).5,6 Consequently, high local concentrations of HMWM platelet-VWF are released from α-granules at sites of vascular injury following platelet activation. Accumulating data from in vitro and in vivo studies suggest that both plasma-VWF and platelet-VWF play critical roles in primary hemostasis.7-10 Furthermore, reduced platelet-VWF levels have also been implicated in mediating the pathogenesis of bleeding disorders including von Willebrand disease and essential thrombocythemia.3,6
Previous studies have described several important functional differences between platelet-VWF and plasma-VWF. In particular, despite the fact that platelet-VWF is enriched in HMWM, it binds to platelet GpIbα with significantly lower affinity compared with plasma-VWF.11 In contrast, platelet-VWF demonstrates significantly enhanced binding to both GpIIbIIIa and heparin.11 The molecular mechanisms responsible for these differences have not been defined, but are likely to relate to variations in the posttranslational modification of platelet-VWF (synthesized within megakaryocytes) as opposed to plasma-VWF (synthesized within endothelial cells). The N- and O-linked glycosylation profiles of plasma-VWF have been characterized in detail.12,13 The glycan profile of platelet-VWF has not been elucidated. However, preliminary data suggest that these structures may differ significantly from those expressed on plasma-VWF.11,14 In recent studies, we and others have clearly demonstrated the key role played by VWF glycans in regulating proteolysis by ADAMTS13.15-18 Importantly, expression of both ABO blood group antigens and terminal sialic acid on VWF were shown to be of critical importance.15,16,18 In this study, we have characterized terminal carbohydrate expression on platelet-VWF. Our findings demonstrate that platelet-VWF exists as a distinct natural glycoform, with a particular marked reduction in N-linked sialic acid expression compared with plasma-VWF. Moreover, as a result of this difference in posttranslational modification, platelet-VWF exhibits specific resistance to ADAMTS13 proteolysis.
Study design
Isolation and purification of platelet- and plasma-VWF
Platelets were isolated from platelet-rich plasma as previously described.19 Platelets were then lysed by performing repeated snap freeze-thaw cycles in the presence of protease inhibitors (Protease Inhibitor Cocktail I, Calbiochem, Merck, UK) followed by centrifugation at 10 000g to clear cellular debris. Finally, platelet-VWF was purified by immunoaffinity chromatography employing the monoclonal antibody CLB-RAg20 as previously described in detail.20 Plasma-derived VWF was purified by cryoprecipitation and 2BCL gel filtration as before,18 followed by CLB-RAg20 immunoaffinity chromatography.20
ADAMTS13 expression and proteolysis of VWF
Recombinant human ADAMTS13 was purified and quantified following stable expression in HEK293 cells as previously described.16 ADAMTS13-VWF cleavage assays were then performed. In brief, 10 µg/mL of VWF was incubated with 3 nM ADAMTS13 in the presence of 1.5 M urea and 10 mM BaCl2. At specific time points (0, 30, 60, 90, and 120 minutes), subsamples were removed and 10 mM EDTA was added to stop the cleavage reaction. VWF proteolysis was analyzed using VWF collagen-binding assay (VWF:CB) and sodium dodecyl sulfate (SDS) agarose gel electorphoresis.16 In preliminary experiments, we established that plasma- and platelet-VWF bound to type III collagen with similar affinities (data not shown). Chymotrypsin (30 U/mg VWF) and carboxypeptidase Y (19 U/mg VWF) digestion of VWF were performed at 37°C for 90 minutes and the extent of cleavage was assessed using a VWF:CB as before.
Glycan analysis and glycosidase treatment of VWF
Lectin plate-binding assays were used to analyze quantitative ABO(H) expression on plasma- and platelet-VWF.21 In addition, terminal N- and O-linked sialic acid expression on VWF was quantified using high-performance liquid chromatography (HPLC) analysis as before.18 To modify endogenous VWF glycan structures, purified plasma- or platelet-VWF (final concentration, 65 µg/mL) were incubated for 2 hours at 37°C with specific exoglycosidases including α2-3,6,8,9 neuraminidase (2500 U/mg; Calbiochem, Merck Chemicals Ltd, UK); PNGase F (1000 U/mg; New England BioLabs, UK); α2-3 neuraminidase (Sigma, Ireland), and O-glycosidase (5 U/mg; Sigma, Ireland). This methodology has been described in detail elsewhere.18
Data were analyzed using the GraphPad Prism program (GraphPad Prism version 5.0 for Windows; GraphPad Software, San Diego, CA). Experiments were performed in triplicate. All data are expressed as mean ± standard error of the mean (SEM). To assess statistical differences, the data were analyzed using unpaired 2-tailed Student t test. P < .05 was considered significant.
Results and discussion
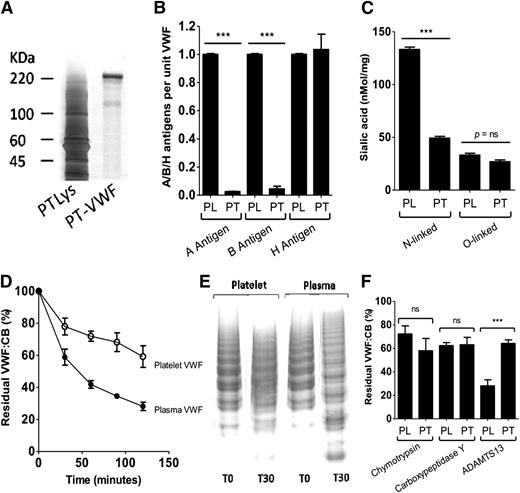
Upon purification, platelet-VWF was visible as a ∼220-kDa band when analyzed by SDS-polyacrylamide gel electrophoresis analysis, similar to that previously observed for plasma-VWF (Figure 1A).14,16 Low-resolution SDS agarose gel electrophoresis confirmed that platelet-VWF was enriched in HMWM compared with plasma-VWF (data not shown). In keeping with previous reports, A and B blood group antigens were strongly expressed on plasma-VWF in a blood group–specific manner (Figure 1B).16,22 In contrast however, A and B antigens were not expressed on platelet-VWF. This finding is interesting given that ABO(H) blood group determinants are abundantly expressed on several different platelet membrane glycoproteins including GpIa, Ib, IIa, and IV.4 H antigen (Fuc α1→2 Gal β1→4 GlcNAc β1→) is the essential carbohydrate acceptor for either α-1,3-N-acetylgalactosaminyltransferase (A transferase) or α-1,3-galactosyltransferase (B transferase).16 Individuals with the rare Bombay phenotype fail to express H antigen and therefore cannot synthesize A or B antigenic structures regardless of their ABO blood group genotype. In spite of the absence of A and B determinant expression on platelet-VWF, we observed strong expression of H antigen on platelet-VWF (Figure 1B). Indeed, quantitative H antigen expression levels on platelet-VWF were strikingly similar to that observed on plasma-VWF.
Glycosylation of platelet-VWF and susceptibility to ADAMTS13 proteolysis. (A) Platelet (PT)-VWF was purified from lysed (Lys) human platelets by immunoaffinity chromatography and analyzed by SDS-polyacrylamide gel electrophoresis and subsequent silver staining. (B) ABO(H) blood group antigen expression on plasma (PL)-VWF and PT-VWF were quantified using lectin plate-binding assays. All experiments were performed in triplicate, and the results shown represent the mean ± SEM (***P < .001). (C) Reverse-phase HPLC analysis was used to quantify sialic acid expression on purified PL-VWF and PT-VWF. Total sialic acid expression on PT-VWF was significantly reduced compared with PL-VWF (76 vs 167 nmol/mg; ***P < .001). To determine relative quantitative sialic acid expression levels on the N-linked glycans of VWF, HPLC analysis of residual VWF-bound sialic acid was performed following digestion with O-glycosidase. To determine quantitative sialic acid expression on the O-linked glycans of VWF, HPLC analysis of residual VWF-bound sialic acid was performed following digestion with PNGase F. (ns, P value is nonsignificant). (D) To investigate whether altered glycosylation on platelet-VWF influences susceptibility to ADAMTS13 proteolysis, PT-VWF and PL-VWF were incubated with 3 nM recombinant human ADAMTS13 in the presence of 1.5 M urea. Rate of cleavage was assessed by determining the reduction in VWF:CB over time. Results (mean of 5 experiments ± SEM) are expressed as the percentage residual collagen-binding activity. In some cases, SEM cannot be seen due to its small size. (E) The susceptibility of PT-VWF and PL-VWF multimers to digestion with recombinant human ADAMTS13 in the presence of 1.5 M urea was further assessed by performing standard nonreducing SDS agarose gel electrophoresis at baseline (T0) and following a 30-minute incubation (T30). (F) To establish whether the altered glycosylation profile of PT-VWF influenced susceptibility to other nonspecific proteases, PL-VWF and PT-VWF were also treated with chymotrypsin (30 U/mg VWF) and carboxypeptidase Y (19 U/mg VWF) at 37°C for 90 minutes. Results are shown as residual VWF:CB at 90 minutes ± SEM.
Glycosylation of platelet-VWF and susceptibility to ADAMTS13 proteolysis. (A) Platelet (PT)-VWF was purified from lysed (Lys) human platelets by immunoaffinity chromatography and analyzed by SDS-polyacrylamide gel electrophoresis and subsequent silver staining. (B) ABO(H) blood group antigen expression on plasma (PL)-VWF and PT-VWF were quantified using lectin plate-binding assays. All experiments were performed in triplicate, and the results shown represent the mean ± SEM (***P < .001). (C) Reverse-phase HPLC analysis was used to quantify sialic acid expression on purified PL-VWF and PT-VWF. Total sialic acid expression on PT-VWF was significantly reduced compared with PL-VWF (76 vs 167 nmol/mg; ***P < .001). To determine relative quantitative sialic acid expression levels on the N-linked glycans of VWF, HPLC analysis of residual VWF-bound sialic acid was performed following digestion with O-glycosidase. To determine quantitative sialic acid expression on the O-linked glycans of VWF, HPLC analysis of residual VWF-bound sialic acid was performed following digestion with PNGase F. (ns, P value is nonsignificant). (D) To investigate whether altered glycosylation on platelet-VWF influences susceptibility to ADAMTS13 proteolysis, PT-VWF and PL-VWF were incubated with 3 nM recombinant human ADAMTS13 in the presence of 1.5 M urea. Rate of cleavage was assessed by determining the reduction in VWF:CB over time. Results (mean of 5 experiments ± SEM) are expressed as the percentage residual collagen-binding activity. In some cases, SEM cannot be seen due to its small size. (E) The susceptibility of PT-VWF and PL-VWF multimers to digestion with recombinant human ADAMTS13 in the presence of 1.5 M urea was further assessed by performing standard nonreducing SDS agarose gel electrophoresis at baseline (T0) and following a 30-minute incubation (T30). (F) To establish whether the altered glycosylation profile of PT-VWF influenced susceptibility to other nonspecific proteases, PL-VWF and PT-VWF were also treated with chymotrypsin (30 U/mg VWF) and carboxypeptidase Y (19 U/mg VWF) at 37°C for 90 minutes. Results are shown as residual VWF:CB at 90 minutes ± SEM.
Plasma-VWF is heavily sialylated.12,13 Moreover, N- and O-linked sialic acid expression plays a critical role in modulating VWF function, proteolysis, and clearance.23-25 Reverse-phase HPLC analysis demonstrated that total sialic acid expression on platelet-VWF was significantly reduced compared with plasma-VWF (76 nmol/mg vs 167 nmol/mg; P < .001). Importantly, this reduction in total sialylation was almost entirely attributable to a specific reduction in sialic acid expression on the N-glycans of platelet-VWF (49.2 nmol/mg vs 134.9 nmol/mg; P < .001) (Figure 1C). In contrast, quantitative O-linked sialic acid expression on platelet-VWF (26.9 nmol/mg) and plasma-VWF (30.2 nmol/mg) were similar.
Terminal sialic acid and ABO(H) determinants expressed on plasma-VWF critically regulate susceptibility to ADAMTS13 proteolysis.15,16,18 Given the marked differences in terminal N-linked glycan expression on platelet-VWF, we investigated platelet-VWF proteolysis by ADAMTS13. Interestingly, ADAMTS13 proteolysis of platelet-VWF was significantly attenuated compared with plasma-VWF (Figure 1D-E). At all time points after 30 minutes, platelet-VWF showed significant increased resistance to ADAMTS13 (P < .05). For example, after incubation with ADAMTS13 (3 nM for 90 minutes) platelet VWF:CB was reduced to 68.2% compared with 34.5% for plasma-VWF (P < .01). In contrast to its ADAMTS13-resistant phenotype, platelet-VWF displayed similar susceptibility to cleavage by other serine and cysteine proteases as plasma-VWF (Figure 1F).
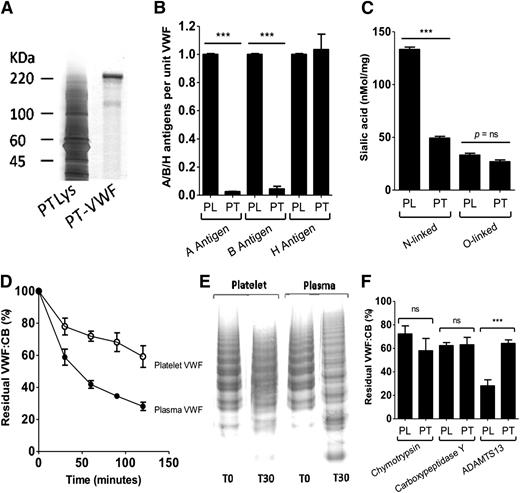
To elucidate the molecular basis underlying this specific ADAMTS13-resistant phenotype, plasma- and platelet-VWF were treated with specific exoglycosidases. In keeping with previous findings, removal of N-linked glycans from plasma-VWF (PNG-PL-VWF) enhanced the rate of proteolysis by ADAMTS13 (Figure 2A).17,18 Similarly, removal of N-linked glycans from platelet-VWF (PNG-PT-VWF) also significantly increased the rate of ADAMTS13 cleavage (P < .05). Nevertheless, PNG-PT-VWF still cleaved significantly more slowly than PNG-PL-VWF, demonstrating that O-linked glycans on platelet-VWF also play a role in modulating ADAMTS13 resistance. This hypothesis was supported by the observation that removal of O-linked glycans from platelet-VWF also enhanced the rate of proteolysis by ADAMTS13 (Figure 2B).
Role of glycans expression in regulating the ADAMTS13-resistant phenotype of PL-VWF. (A) PL-VWF and PT-VWF were incubated with PNGase F to remove N-linked glycans. Subsequently, the effects of N-linked deglycosylation in regulating susceptibility to ADAMTS13 proteolysis were investigated. Removal of N-linked glycans significantly increased PL-VWF cleavage by ADAMTS13 (PL-VWF [□] vs PNG-PL-VWF [▪]; P < .001 at all time points). Treatment of platelet-VWF with PNGase F was also associated with significantly enhanced proteolysis by ADAMTS13 (PT-VWF [○] vs PNG-PT-VWF [●]; P < .001). Nevertheless, PNG-treated PT-VWF remained more resistant to ADAMTS13 proteolysis compared with PNG-treated PL-VWF. All experiments were performed a minimum of 3 times and results are expressed as mean ± SEM at 120 minutes. (B) PL-VWF and PT-VWF were incubated with O-glycosidase (OGly) to remove O-linked glycans. Subsequently, the effects of O-linked deglycosylation in regulating susceptibility to ADAMTS13 proteolysis were investigated. Removal of O-linked glycans from PL-VWF had no significant effect on cleavage by ADAMTS13 (PL-VWF [□] vs OGly-PL-VWF [▪]. In contrast, treatment of PL-VWF with OGly was associated with significantly enhanced proteolysis by ADAMTS13 (PT-VWF [○] vs PNG-PT-VWF [●]; P < .01). (C) PL-VWF and PT-VWF were incubated with α2-3,6,8,9 neuraminidase to remove terminal sialic acid. Subsequently, the effects of desialylation in regulating susceptibility to ADAMTS13 proteolysis were investigated as previously described. Removal of sialic acid from PL-VWF (neuraminidase [Neu]-PL-VWF) significantly attenuated proteolysis by ADAMTS13 (***P < .001). In keeping with its markedly reduced sialic acid expression levels, Neu digestion of PT-VWF demonstrated only a small effect on susceptibility to ADAMTS13 cleavage.
Role of glycans expression in regulating the ADAMTS13-resistant phenotype of PL-VWF. (A) PL-VWF and PT-VWF were incubated with PNGase F to remove N-linked glycans. Subsequently, the effects of N-linked deglycosylation in regulating susceptibility to ADAMTS13 proteolysis were investigated. Removal of N-linked glycans significantly increased PL-VWF cleavage by ADAMTS13 (PL-VWF [□] vs PNG-PL-VWF [▪]; P < .001 at all time points). Treatment of platelet-VWF with PNGase F was also associated with significantly enhanced proteolysis by ADAMTS13 (PT-VWF [○] vs PNG-PT-VWF [●]; P < .001). Nevertheless, PNG-treated PT-VWF remained more resistant to ADAMTS13 proteolysis compared with PNG-treated PL-VWF. All experiments were performed a minimum of 3 times and results are expressed as mean ± SEM at 120 minutes. (B) PL-VWF and PT-VWF were incubated with O-glycosidase (OGly) to remove O-linked glycans. Subsequently, the effects of O-linked deglycosylation in regulating susceptibility to ADAMTS13 proteolysis were investigated. Removal of O-linked glycans from PL-VWF had no significant effect on cleavage by ADAMTS13 (PL-VWF [□] vs OGly-PL-VWF [▪]. In contrast, treatment of PL-VWF with OGly was associated with significantly enhanced proteolysis by ADAMTS13 (PT-VWF [○] vs PNG-PT-VWF [●]; P < .01). (C) PL-VWF and PT-VWF were incubated with α2-3,6,8,9 neuraminidase to remove terminal sialic acid. Subsequently, the effects of desialylation in regulating susceptibility to ADAMTS13 proteolysis were investigated as previously described. Removal of sialic acid from PL-VWF (neuraminidase [Neu]-PL-VWF) significantly attenuated proteolysis by ADAMTS13 (***P < .001). In keeping with its markedly reduced sialic acid expression levels, Neu digestion of PT-VWF demonstrated only a small effect on susceptibility to ADAMTS13 cleavage.
Recent work from our laboratory demonstrated that expression of α2-6–linked sialic acid on plasma-VWF promotes cleavage by ADAMTS13. Consequently, neuraminidase treatment of plasma-VWF significantly attenuates ADAMTS13 proteolysis (P < .01; Figure 2C). In contrast, in keeping with its markedly reduced N-linked sialic acid expression levels, neuraminidase digestion of platelet-VWF had a negligible effect upon susceptibility to ADAMTS13 cleavage (Figure 2C). Given the reduced sialic acid present on platelet-VWF, we hypothesize that the wild-type platelet-VWF glycoform is similar to neuraminidase-treated plasma-VWF, such that further removal of sialic acid from platelet-VWF does not promote resistance to ADAMTS13. However, definitive glycome mapping studies will be required to determine how sialic acid expression varies across the 12 N-linked glycan sites of platelet-VWF.
In conclusion, these novel data demonstrate that platelet-VWF exists as a distinct natural glycoform, with a particularly marked reduction in N-linked sialylation. As a result of this differential glycan profile, platelet-VWF exhibits resistance to ADAMTS13 proteolysis. Thus, at sites of vascular injury, not only will high local concentrations of HMWM platelet-VWF be released, but this VWF will be partially resistant to ADAMTS13 further enhancing platelet plug formation. Further in vivo studies will be needed to elucidate the physiological significance of the ADAMTS13-resistant phenotype of platelet-VWF.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Science Foundation Ireland Principal Investigator Award (11/PI/1066) (J.S.O.).
Authorship
Contribution: R.T.M., M.v.d.B., B.B., J.M.O., and O.R. performed experiments; R.T.M., M.v.d.B., B.B., O.R., R.O., J.V., R.J.S.P., and J.S.O. designed the research and analyzed the data; and all authors were involved in writing and reviewing the paper.
Conflict-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Leo Pharma, and Octapharma; has served on the advisory boards of Baxter, Bayer, Octapharma, and Pfizer; and has received research grant funding awards from Baxter, Bayer, and Novo Nordisk.
Correspondence: James O’Donnell, Haemostasis Research Group, Institute of Molecular Medicine, St James’s Hospital, Trinity College Dublin, Ireland; jodonne@tcd.ie.

![Figure 2. Role of glycans expression in regulating the ADAMTS13-resistant phenotype of PL-VWF. (A) PL-VWF and PT-VWF were incubated with PNGase F to remove N-linked glycans. Subsequently, the effects of N-linked deglycosylation in regulating susceptibility to ADAMTS13 proteolysis were investigated. Removal of N-linked glycans significantly increased PL-VWF cleavage by ADAMTS13 (PL-VWF [□] vs PNG-PL-VWF [▪]; P < .001 at all time points). Treatment of platelet-VWF with PNGase F was also associated with significantly enhanced proteolysis by ADAMTS13 (PT-VWF [○] vs PNG-PT-VWF [●]; P < .001). Nevertheless, PNG-treated PT-VWF remained more resistant to ADAMTS13 proteolysis compared with PNG-treated PL-VWF. All experiments were performed a minimum of 3 times and results are expressed as mean ± SEM at 120 minutes. (B) PL-VWF and PT-VWF were incubated with O-glycosidase (OGly) to remove O-linked glycans. Subsequently, the effects of O-linked deglycosylation in regulating susceptibility to ADAMTS13 proteolysis were investigated. Removal of O-linked glycans from PL-VWF had no significant effect on cleavage by ADAMTS13 (PL-VWF [□] vs OGly-PL-VWF [▪]. In contrast, treatment of PL-VWF with OGly was associated with significantly enhanced proteolysis by ADAMTS13 (PT-VWF [○] vs PNG-PT-VWF [●]; P < .01). (C) PL-VWF and PT-VWF were incubated with α2-3,6,8,9 neuraminidase to remove terminal sialic acid. Subsequently, the effects of desialylation in regulating susceptibility to ADAMTS13 proteolysis were investigated as previously described. Removal of sialic acid from PL-VWF (neuraminidase [Neu]-PL-VWF) significantly attenuated proteolysis by ADAMTS13 (***P < .001). In keeping with its markedly reduced sialic acid expression levels, Neu digestion of PT-VWF demonstrated only a small effect on susceptibility to ADAMTS13 cleavage.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/25/10.1182_blood-2013-04-496851/4/m_4107f2.jpeg?Expires=1770412892&Signature=IF2F6VvYQwVCZe5qt5uGTBrvexIhFaU92wMQRrNUaLzMCnym-qktwUzKc0fBbL8PIAb~IzMJViSsIcNHKhYkIcwQeGGuW7GDB1opkvZL~iSWZJ0ERWusUD~cyLXk1KY0cukEeJhLlgemH09eGQBbVzLOha6BdobsmSkv8FtusyoMGRhwuBnmMg5GBZufrcXDeIj3ZBhMvDTK0sLOBhIRqDxSCnrG4-DrzXy1wpj~nTTU27jhPboQ5mPEY2WWoX2JFQZTAFX81A-742W55FGih6bW4NSgC0X0opZPoxYh8NCmiSSWffMvBWkWM4VA5QKciMr8l9xp0fgF6pbARhZx3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)