Key Points
Donor-derived anti-CD19-CAR T cells cause regressions of refractory malignancies after allogeneic transplantation.
Abstract
New treatments are needed for B-cell malignancies persisting after allogeneic hematopoietic stem cell transplantation (alloHSCT). We conducted a clinical trial of allogeneic T cells genetically modified to express a chimeric antigen receptor (CAR) targeting the B-cell antigen CD19. T cells for genetic modification were obtained from each patient’s alloHSCT donor. All patients had malignancy that persisted after alloHSCT and standard donor lymphocyte infusions (DLIs). Patients did not receive chemotherapy prior to the CAR T-cell infusions and were not lymphocyte depleted at the time of the infusions. The 10 treated patients received a single infusion of allogeneic anti-CD19-CAR T cells. Three patients had regressions of their malignancies. One patient with chronic lymphocytic leukemia (CLL) obtained an ongoing complete remission after treatment with allogeneic anti-CD19-CAR T cells, another CLL patient had tumor lysis syndrome as his leukemia dramatically regressed, and a patient with mantle cell lymphoma obtained an ongoing partial remission. None of the 10 patients developed graft-versus-host disease (GVHD). Toxicities included transient hypotension and fever. We detected cells containing the anti-CD19-CAR gene in the blood of 8 of 10 patients. These results show for the first time that donor-derived allogeneic anti-CD19-CAR T cells can cause regression of B-cell malignancies resistant to standard DLIs without causing GVHD. This trial was registered at www.clinicaltrials.gov as #NCT01087294.
Introduction
Many patients with advanced B-cell malignancies can be cured by allogeneic hematopoietic stem cell transplantation (alloHSCT).1,2 Unfortunately, many patients with B-cell malignancies are not cured after alloHSCT, and the leading cause of death after alloHSCT is progressive malignancy.2-5 Patients with progressive malignancy after alloHSCT are often treated with infusions of unmanipulated donor lymphocytes obtained from the allogeneic transplant donor (donor lymphocyte infusions, DLIs).4,6-8 DLIs can induce complete remissions (CRs), but the percentage of patients obtaining remissions after DLIs depends on the type of B-cell malignancy being treated, and regardless of the type of B-cell malignancy being treated, many patients do not enter sustained CRs.3,4,6-9 In addition, approximately one-third of patients receiving DLIs develop clinically significant graft-versus-host disease (GVHD), which is a potentially fatal complication.4,7 Development of new T-cell therapies that specifically target malignancy-associated antigens would be a major advance for the hematopoietic transplantation field.
Chimeric antigen receptors (CARs) are fusion proteins incorporating an antigen recognition moiety and T-cell activation domains.10-13 T cells can be genetically modified to express CARs and transferred to patients.13-21 CARs targeting the B-cell antigen CD19 have been intensively investigated.13-26 Several groups, including our own, are conducting clinical trials of autologous anti-CD19-CAR T cells, and many patients treated on these clinical trials have obtained sustained CRs,13,15,18,19,21,27 but using donor-derived allogeneic anti-CD19-CAR T cells to treat B-cell malignancies persisting after alloHSCT has not been reported.
Methods
Clinical trial design and protocol eligibility requirements
All enrolled patients gave informed consent in accordance with the Declaration of Helsinki. The protocol (registered at www.clinicaltrials.gov as #NCT01087294) was evaluated and allowed by the Institutional Review Board of the National Cancer Institute. Patients had to have a CD19+ B-cell malignancy that persisted despite alloHSCT and at least 1 standard DLI. Patients were required to have either no GVHD, grade 1 acute GVHD,28 or mild global score chronic GVHD.29 The trial had 2 arms, 1 for recipients of HLA-matched sibling donor (MSD) transplants and 1 for recipients of unrelated-donor (URD) transplants. Treatment responses of chronic lymphocytic leukemia (CLL) or lymphoma were defined according to standard international criteria.30,31
Preparation of anti-CD19-CAR T cells and ex vivo assays
Peripheral blood mononuclear cells (PBMCs) were obtained from the patient’s MSD or URD. URD cells were obtained through the National Marrow Donor Program. The PBMCs were cultured and transduced as described in Figure 1B and the supplemental Methods (see the Blood Web site).18 The T-cell culture process involved stimulation of T cells with the anti-CD3 monoclonal antibody OKT3 and culture in IL-2–containing media. The cells were in culture for a total of 8 days. The vector encoding the CAR (Figure 1A) and generation of the replication incompetent gammaretroviruses used in transductions have been described.32 Release criteria for clinical T-cell products were at least 200 pg/mL of IFN-γ release against CD19+ targets in a standard enzyme-linked immunosorbent assay and at least 30% CAR expression on T cells as measured by anti-Fab flow cytometry. Cells were tested for sterility, endotoxin, and replication competent retroviruses as detailed in the supplemental Methods. Flow cytometry, immunohistochemistry, and quantitative polymerase chain reaction (qPCR) are described in the supplemental Methods.18,32 A CAR-specific antibody was used.33
An anti-CD19 CAR gives T cells the ability to specifically recognize CD19. (A) The CAR incorporated an anti-CD19 single chain Fv (scFv), part of the CD28 costimulatory molecule, and the cytoplasmic part of the CD3ζ molecule. (B) PBMCs from healthy allogeneic donors were activated with an anti-CD3 monoclonal antibody and transduced with gammaretroviruses encoding an anti-CD19 CAR. The cells were infused after 8 days of culture. (C) Expression of the CAR was detected on the surface of the infusion cells of patient 1 on day 7 of culture by staining with anti–antigen-binding fragment (Fab) antibodies. Staining with isotype-matched control antibodies is also shown. The plots are gated on CD3+ lymphocytes. (D) The infusion cells of patient 1 were stained for CD45RA and CC chemokine receptor 7 (CCR7). Staining with isotype-matched control antibodies is also shown. The staining was performed on day 7 of culture, and the plots are gated on CAR-expressing CD3+ lymphocytes. (E) On day 8 of culture, infusion cells of patient 1 were cultured for 4 hours with either the CD19+ cell line CD19-K562 or the negative-control cell line NGFR-K562 that does not express CD19. CD19-specific upregulation of CD107a occurred. Plots are gated on CD3+ lymphocytes. Similar results were obtained with the infusion cells of all patients treated. (F-H) A representative example of CD19-specific cytokine production by anti-CD19-CAR T cells is shown. Infusion cells of patient 4 were stimulated with either CD19-K562 cells or NGFR-K562 cells for 6 hours on day 8 of culture. Intracellular cytokine staining followed by flow cytometry revealed that large fractions of the T cells produced interferon γ (IFN-γ), tumor necrosis factor (TNF), and interleukin (IL) 2 in a CD19-specific manner. Plots are gated on CD3+ lymphocytes.
An anti-CD19 CAR gives T cells the ability to specifically recognize CD19. (A) The CAR incorporated an anti-CD19 single chain Fv (scFv), part of the CD28 costimulatory molecule, and the cytoplasmic part of the CD3ζ molecule. (B) PBMCs from healthy allogeneic donors were activated with an anti-CD3 monoclonal antibody and transduced with gammaretroviruses encoding an anti-CD19 CAR. The cells were infused after 8 days of culture. (C) Expression of the CAR was detected on the surface of the infusion cells of patient 1 on day 7 of culture by staining with anti–antigen-binding fragment (Fab) antibodies. Staining with isotype-matched control antibodies is also shown. The plots are gated on CD3+ lymphocytes. (D) The infusion cells of patient 1 were stained for CD45RA and CC chemokine receptor 7 (CCR7). Staining with isotype-matched control antibodies is also shown. The staining was performed on day 7 of culture, and the plots are gated on CAR-expressing CD3+ lymphocytes. (E) On day 8 of culture, infusion cells of patient 1 were cultured for 4 hours with either the CD19+ cell line CD19-K562 or the negative-control cell line NGFR-K562 that does not express CD19. CD19-specific upregulation of CD107a occurred. Plots are gated on CD3+ lymphocytes. Similar results were obtained with the infusion cells of all patients treated. (F-H) A representative example of CD19-specific cytokine production by anti-CD19-CAR T cells is shown. Infusion cells of patient 4 were stimulated with either CD19-K562 cells or NGFR-K562 cells for 6 hours on day 8 of culture. Intracellular cytokine staining followed by flow cytometry revealed that large fractions of the T cells produced interferon γ (IFN-γ), tumor necrosis factor (TNF), and interleukin (IL) 2 in a CD19-specific manner. Plots are gated on CD3+ lymphocytes.
Results
Characteristics of patients treated with donor-derived allogeneic anti-CD19-CAR T cells
Patients with B-cell malignancies persisting despite prior alloHSCT and subsequent treatment with at least 1 standard DLI received a single infusion of allogeneic anti-CD19-CAR T cells. No antimalignancy therapy except CAR T cells was administered to patients on this clinical trial, and for all patients, at least 4 weeks had elapsed from the time of the most recent prior treatment before anti-CD19-CAR T cells were infused. The patients had all received extensive prior treatment, and none of the 10 patients had obtained a CR with their most recent standard DLI (Table 1). Nine of the 10 patients had either progressive malignancy or stable malignancy at the time of anti-CD19-CAR T-cell infusion; 1 patient was in PR at the time of CAR T-cell infusion (Table 2).
Because the patients did not receive chemotherapy or radiotherapy prior to the CAR T-cell infusions, the patients all had large numbers of endogenous blood T cells at the time of their anti-CD19-CAR T-cell infusion (Table 2). Although depletion of endogenous lymphocytes has been shown to enhance the antimalignancy activity of adoptively transferred T cells in mouse models,13,23,34 we did not include lymphocyte-depleting chemotherapy or radiotherapy in this clinical trial. At the time the trial was initiated, no patient had been treated with allogeneic anti-CD19-CAR T cells, and we were concerned that the allogeneic anti-CD19-CAR T cells in a lymphocyte-depleted recipient could have caused severe GVHD or cytokine-mediated toxicities.
T cells capable of specifically recognizing CD19 were generated in 8 days
The anti-CD19 CAR used in this work includes part of the CD28 costimulatory molecule and the cytoplasmic portion of the CD3ζ molecule (Figure 1A).32 To produce anti-CD19-CAR T cells, we obtained PBMCs from the healthy allogeneic transplant donors of each patient by apheresis. Potential advantages to obtaining cells from healthy donors include lack of leukemia contamination and lack of T-cell impairment by prior immunosuppressive drugs or chemotherapy. T cells were transduced with a gammaretroviral vector encoding the anti-CD19 CAR (Figure 1B). The cells were infused on day 8 of culture. A mean of 98% of the infused cells were CD3+ T cells, and a mean of 58% (range 34% to 78%) of the infused T cells expressed the anti-CD19 CAR (Table 2; Figure 1C). The reasons for the lower percentage of CAR+ T cells among the infused cells of patients 1 and 2 are not known. The most common phenotype of the infused anti-CD19-CAR–expressing T cells was CD45RA– and CCR7+, which is associated with central memory T cells (supplemental Table 1).35 The CAR-transduced T cells of all patients degranulated in a CD19-specific manner and produced IFN-γ, TNF, and IL-2 in a CD19-specific manner (Figure 1E-H).
Anti-CD19-CAR T cells caused regressions of malignancies in some patients with B-cell malignancies that were resistant to standard DLIs
All patients treated with allogeneic anti-CD19-CAR T cells had highly treatment-resistant B-cell malignances that persisted despite allogeneic transplantation and standard DLIs. Patients 1, 5, and 9 had >50% decreases in malignant lymph node masses as measured by computed tomography (CT) scans within 1 month after receiving anti-CD19-CAR T cells. Patient 5 obtained an ongoing CR, and patient 9 obtained an ongoing PR (Table 3). The malignancies of patients 1, 2, 4, 6, 8, and 10 were staged as SD after anti-CD19-CAR T-cell infusion. Patients 3 and 7 had progressive malignancy after their infusions.
Regression of CLL and tumor lysis syndrome in the first patient treated with allogeneic anti-CD19-CAR T cells
Patient 1’s CLL relapsed after a URD transplant, and the relapsed CLL was treated with 4 standard DLIs and an infusion of experimental tumor-derived lymphocytes.36 None of these cell infusions caused a substantial regression of his CLL. Next, patient 1 received a multiagent chemotherapy regimen followed immediately by a fifth DLI that contained 5.9 × 107 CD3+ cells per kg of bodyweight. This treatment caused only a modest decrease in the CLL that was staged as SD; <3 months after the fifth DLI, the CLL started to progress. He received a course of rituximab that ended 6 weeks before his anti-CD19-CAR T-cell infusion. The rituximab did not cause any regression of the CLL and was the last CLL therapy that the patient received prior to anti-CD19-CAR T cells.
At the time of anti-CD19-CAR T-cell infusion, patient 1 had progressive CD19+ CLL with bulky adenopathy. PBMCs were obtained from the patient’s transplant donor and used to produce anti-CD19-CAR T cells. Patient 1 received a single infusion of 1 × 106 total cells per kg of bodyweight (6.2 × 107 total cells). No chemotherapy was administered with the CAR-transduced T cells.
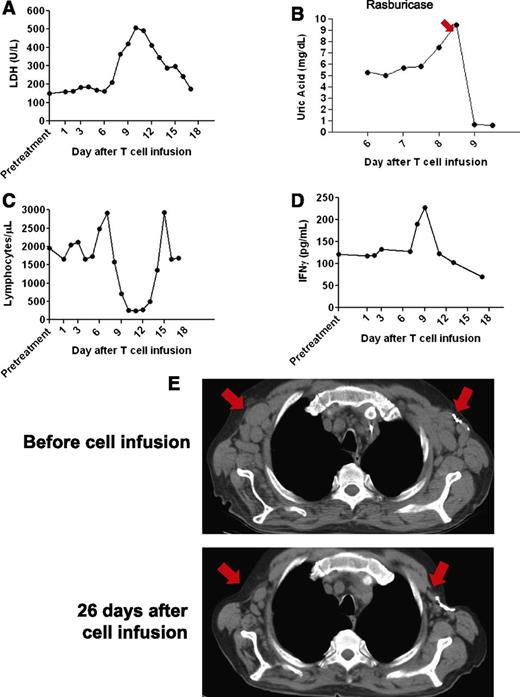
After the anti-CD19-CAR T-cell infusion, patient 1 was unchanged for 6 days. From day 6 until day 12 after the T-cell infusion, he experienced fatigue and fevers of up to 40.3°C. The patient’s serum lactate dehydrogenase increased before returning to normal levels by 17 days after the T-cell infusion (Figure 2A). On days 7 through 9 after the anti-CD19-CAR T-cell infusion, he had mild, intermittent hypotension that was successfully treated with intravenous fluid boluses. He developed laboratory evidence of tumor lysis syndrome with elevations in serum magnesium and phosphorous. Despite prophylactic allopurinol that was started 1 day before the anti-CD19-CAR T-cell infusion, the serum uric acid increased rapidly to 9.5 mg/dL 8 days after the T-cell infusion (Figure 2B). Administration of the recombinant urate oxidase drug rasburicase reduced the uric acid level. A transient decrease in the total lymphocyte count occurred (Figure 2C).
Tumor lysis syndrome and regression of adenopathy occurred after infusion of allogeneic anti-CD19-CAR T cells into patient 1. (A) Serum lactate dehydrogenase (LDH) levels increased after the CAR-transduced T-cell infusion and then decreased as toxicity resolved. (B) The serum uric acid was 5.4 mg/dL (normal range 3.7-8.6 mg/dL) before the CAR T-cell infusion. Eight days after the infusion, the serum uric acid increased sharply before the recombinant urate oxidase drug rasburicase was given to reduce the uric acid. (C) Shortly after infusion of anti-CD19-CAR T cells, the total blood lymphocyte count abruptly decreased and then recovered to normal levels. (D) Serum IFN-γ levels at the indicated days after anti-CD19-CAR T-cell infusion were determined by enzyme-linked immunosorbent assay. A transient increase in serum IFN-γ occurred during the period of clinical toxicity. (E) CT scans were performed before the anti-CD19-CAR T-cell infusion and 26 days after the anti-CD19-CAR T-cell infusion. A decrease in adenopathy occurred at the locations indicated by the arrows.
Tumor lysis syndrome and regression of adenopathy occurred after infusion of allogeneic anti-CD19-CAR T cells into patient 1. (A) Serum lactate dehydrogenase (LDH) levels increased after the CAR-transduced T-cell infusion and then decreased as toxicity resolved. (B) The serum uric acid was 5.4 mg/dL (normal range 3.7-8.6 mg/dL) before the CAR T-cell infusion. Eight days after the infusion, the serum uric acid increased sharply before the recombinant urate oxidase drug rasburicase was given to reduce the uric acid. (C) Shortly after infusion of anti-CD19-CAR T cells, the total blood lymphocyte count abruptly decreased and then recovered to normal levels. (D) Serum IFN-γ levels at the indicated days after anti-CD19-CAR T-cell infusion were determined by enzyme-linked immunosorbent assay. A transient increase in serum IFN-γ occurred during the period of clinical toxicity. (E) CT scans were performed before the anti-CD19-CAR T-cell infusion and 26 days after the anti-CD19-CAR T-cell infusion. A decrease in adenopathy occurred at the locations indicated by the arrows.
Treatment-refractory CLL regressed after infusion of anti-CD19-CAR T cells
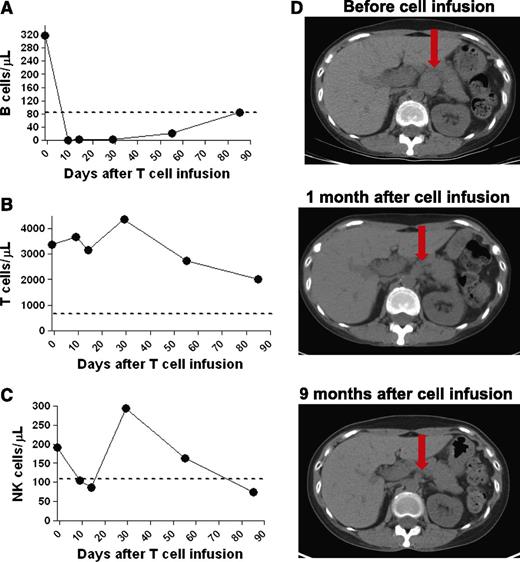
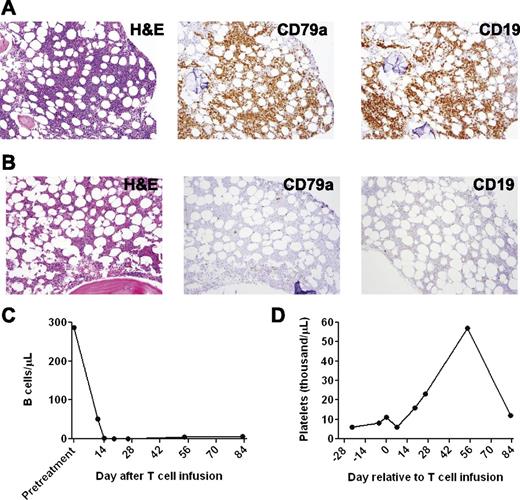
CT scans revealed a dramatic general regression of patient 1’s adenopathy after the anti-CD19-CAR T-cell infusion (Figure 2E). A bone marrow biopsy performed 2 days before the anti-CD19-CAR T-cell infusion showed extensive CLL involvement (Figure 3A). A bone marrow biopsy performed 26 days after the anti-CD19-CAR T-cell infusion showed no morphologic evidence of CLL and only rare B-lineage cells (Figure 3B). Blood CD19+ CLL cells and normal B cells were eliminated after the anti-CD19-CAR T-cell infusion (Figure 3C). After the anti-CD19-CAR T-cell infusion, patient 1 became independent of platelet transfusions because his platelet count increased (Figure 3D). This improving platelet count was consistent with recovery of platelet production as the bone marrow was cleared of CLL (Figure 3B). Of particular interest, the number of T cells in the blood of patient 1 was slightly above normal at the time of the anti-CD19-CAR T-cell infusion, and the blood natural killer (NK) cells were at normal levels before the CAR T-cell infusion (Table 2); therefore, this case demonstrated, for the first time, malignancy regression after transfer of anti-CD19-CAR T cells to a recipient who was not depleted of endogenous lymphocytes. Two months after the anti-CD19-CAR T-cell infusion, patient 1’s CLL progressed in multiple lymph nodes, and the platelet count started to decrease, which indicated CLL progression in the bone marrow. The patient died of progressive CLL 6 months after the anti-CD19-CAR T-cell infusion.
A profound eradication of CLL cells from the bone marrow and blood occurred after infusion of anti-CD19-CAR T cells into patient 1. (A) A bone marrow biopsy was performed 2 days before anti-CD19-CAR T cells were infused. Bone marrow immunohistochemistry staining showed that 80% to 90% of the bone marrow cells consisted of atypical lymphoid cells that had a morphology consistent with CLL. The atypical lymphoid cells were CD79a+ and CD19+. Flow cytometry staining for immunoglobulin light chain restriction showed that the cells were clonal (not shown). (B) Twenty-six days after the anti-CD19-CAR T-cell infusion, immunohistochemistry staining of a bone marrow specimen showed no morphologic evidence of CLL. Only rare CD79a+ cells were present. (C) Blood B cells, of which 99% were CLL cells, dropped dramatically after infusion of anti-CD19-CAR T cells. (D) Patient 1 was dependent on platelet transfusions before the infusion of anti-CD19-CAR T cells. After the CAR T-cell infusion, the patient no longer required platelet transfusions. His platelet count increased for ∼2 months after the anti-CD19-CAR T-cell infusion; subsequently, his platelet count decreased when his CLL progressed.
A profound eradication of CLL cells from the bone marrow and blood occurred after infusion of anti-CD19-CAR T cells into patient 1. (A) A bone marrow biopsy was performed 2 days before anti-CD19-CAR T cells were infused. Bone marrow immunohistochemistry staining showed that 80% to 90% of the bone marrow cells consisted of atypical lymphoid cells that had a morphology consistent with CLL. The atypical lymphoid cells were CD79a+ and CD19+. Flow cytometry staining for immunoglobulin light chain restriction showed that the cells were clonal (not shown). (B) Twenty-six days after the anti-CD19-CAR T-cell infusion, immunohistochemistry staining of a bone marrow specimen showed no morphologic evidence of CLL. Only rare CD79a+ cells were present. (C) Blood B cells, of which 99% were CLL cells, dropped dramatically after infusion of anti-CD19-CAR T cells. (D) Patient 1 was dependent on platelet transfusions before the infusion of anti-CD19-CAR T cells. After the CAR T-cell infusion, the patient no longer required platelet transfusions. His platelet count increased for ∼2 months after the anti-CD19-CAR T-cell infusion; subsequently, his platelet count decreased when his CLL progressed.
Sustained CR of CLL after infusion of anti-CD19-CAR T cells
Patient 5 had undergone extensive treatment of CLL, including 2 MSD alloHSCTs, before undergoing a URD transplant. She obtained a CR after this third alloHSCT, but her CLL relapsed ∼1 year after the transplant. Her relapsed CLL was treated with chemotherapy and a series of 5 DLIs. The maximum DLI cell dose was 1 × 108 CD3+ cells per kg of bodyweight. The patient developed GVHD of the gastrointestinal tract that resolved. None of the DLIs that patient 5 received resulted in a remission. The last standard DLI was administered 13 months before her anti-CD19-CAR T-cell infusion and was her last CLL therapy before her anti-CD19-CAR T-cell infusion. At the time of enrollment on the anti-CD19-CAR trial, her CLL was progressing. PBMCs were obtained from her URD transplant donor, and patient 5 received a single infusion of 1.5 × 106 total cells per kg of bodyweight (6.4 × 107 total cells). No chemotherapy or other CLL therapy was administered around the time of the CAR T-cell infusion. After the anti-CD19-CAR T-cell infusion, patient 5 was asymptomatic for 7 days. Fevers of up to 39.8°C started 8 days after the T-cell infusion and persisted for 3 days. The patient developed mild intermittent hypotension that required treatment with intravenous fluid boluses for 4 days.
A specific eradication of blood B cells occurred during the 9 days after the anti-CD19-CAR T-cell infusion (Figure 4A). At the time of treatment on our protocol, patient 5’s persisting CLL was found in lymph nodes, and the blood B cells were polyclonal (data not shown). Patient 5’s blood T cells and NK cells were at normal levels at the time of the anti-CD19-CAR T-cell infusion, and the blood T cells and NK cells were retained as the B cells were eradicated (Figure 4B-C). During the time that the blood B cells were being eradicated, physical examination revealed a decrease in size of an enlarged supraclavicular lymph node. CT scans revealed resolution of abdominal adenopathy, and the CLL entered a CR (Figure 4D).
Patient 5 obtained a CR after infusion of anti-CD19-CAR T cells. (A) Within 9 days after infusion of anti-CD19-CAR T cells, B cells were eradicated from the blood of patient 5. T cells (B) and NK cells (C) were at normal levels in the blood of patient 5 at the time of anti-CD19-CAR T-cell infusion. T-cell levels remained in the normal range, whereas NK cell levels fluctuated. The dashed line on the plots in panels A-C is the lower limit of the normal range of each cell type. (D) CT scans show that abdominal adenopathy regressed in patient 5 after infusion of anti-CD19-CAR T cells. The red arrows indicate a lymph node mass that regressed to a normal-sized lymph node.
Patient 5 obtained a CR after infusion of anti-CD19-CAR T cells. (A) Within 9 days after infusion of anti-CD19-CAR T cells, B cells were eradicated from the blood of patient 5. T cells (B) and NK cells (C) were at normal levels in the blood of patient 5 at the time of anti-CD19-CAR T-cell infusion. T-cell levels remained in the normal range, whereas NK cell levels fluctuated. The dashed line on the plots in panels A-C is the lower limit of the normal range of each cell type. (D) CT scans show that abdominal adenopathy regressed in patient 5 after infusion of anti-CD19-CAR T cells. The red arrows indicate a lymph node mass that regressed to a normal-sized lymph node.
Because patient 5 did not receive any other antimalignancy therapy around the time of the anti-CD19-CAR T-cell infusion, the eradication of blood B cells and resolution of adenopathy in this patient was attributable to the anti-CD19-CAR T cells. No CLL therapy has been administered to patient 5 since her anti-CD19-CAR T-cell infusion, and her CLL currently remains in CR more than 9 months after the infusion.
MCL regressed in patient 9 after infusion of anti-CD19-CAR T cells
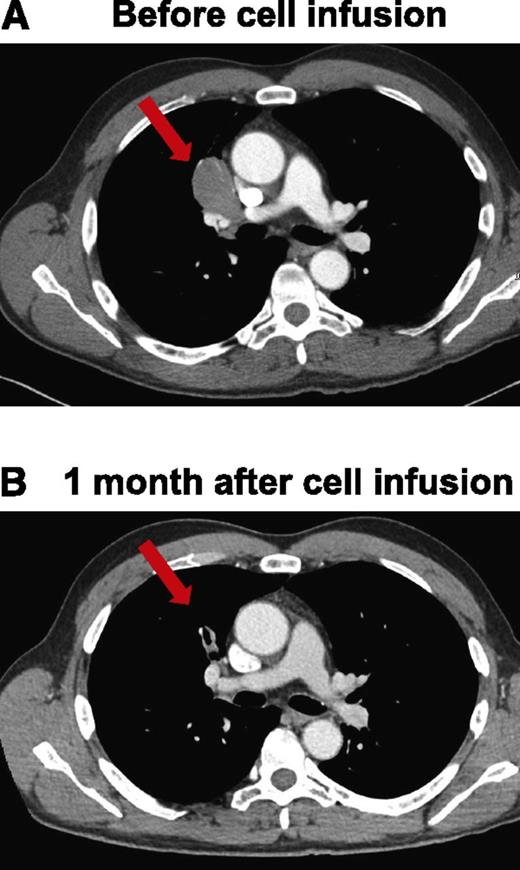
Patient 9 had MCL that was treated with a URD transplant. Following this transplant, the patient obtained a CR, but his lymphoma relapsed 18 months after transplant. In addition to multiple courses of chemotherapy, monoclonal antibodies, and radiation therapy, he underwent 2 DLIs to treat his relapsed lymphoma. Neither of the DLIs caused a significant regression of his lymphoma. His last DLI was 9 months before he received anti-CD19-CAR–transduced T cells. His last lymphoma treatment prior to receiving anti-CD19-CAR T cells was a course of ofatumumab that ended 1 month before the CAR T-cell infusion and resulted in SD. Patient 9 received an infusion of 4 × 106 anti-CD19-CAR–transduced T cells per kg of bodyweight. CT scans obtained 1 month after the CAR T-cell infusion revealed a dramatic regression of a mediastinal lymphoma mass, which was the patient’s only area of lymphoma (Figure 5). The patient continues in an ongoing PR 3 months after the anti-CD19-CAR T-cell infusion.
Regression of adenopathy in patient 9 within 1 month after anti-CD19-CAR T-cell infusion. CT scans before the cell infusion (A) and after infusion of anti-CD19-CAR T cells showing that mediastinal adenopathy had regressed in patient 9 (B). The red arrows indicate a lymph node mass that regressed.
Regression of adenopathy in patient 9 within 1 month after anti-CD19-CAR T-cell infusion. CT scans before the cell infusion (A) and after infusion of anti-CD19-CAR T cells showing that mediastinal adenopathy had regressed in patient 9 (B). The red arrows indicate a lymph node mass that regressed.
Toxicities associated with infusions of allogeneic anti-CD19-CAR T cells
Overall, the most common toxicities attributable to anti-CD19-CAR T cells were fatigue, fever, and hypotension (Table 3). Despite the fact that 6 of the 10 treated patients had GVHD at some point after their most recent alloHSCT, none of the patients treated on this study developed signs of GVHD after their anti-CD19-CAR T-cell infusions. Patient 1’s tumor lysis syndrome has already been described (Figure 1). Patient 1 had a history of temporarily depressed cardiac function with previous illnesses before receiving anti-CD19-CAR T cells. His cardiac left ventricular ejection fraction decreased from 66% before the anti-CD19-CAR T-cell infusion to 25% 12 days after the CAR T-cell infusion. The cardiac ejection fraction recovered to normal by 4 months after the anti-CD19-CAR T-cell infusion.
Patient 3 had pneumonitis and fever among other toxicities listed in Table 3. The etiology of patient 3’s toxicities is unclear because he had pulmonary infiltrates and mild dyspnea before his CAR T-cell infusion, and respiratory syncytial virus was detected in his bronchoalveolar washings at the time of the pneumonitis by direct fluorescent antibody staining. Importantly, with the exceptions of the depressed cardiac function in patient 1 and B-cell depletion in patients 1, 5, and 10, all toxicities experienced by patients receiving anti-CD19-CAR T cells resolved completely in <2 weeks after CAR T-cell infusion.
The only patients to have elevations in serum IFN-γ were patient 1 (peak level 227 pg/mL), patient 9 (peak level 161 pg/mL), and patient 10 (peak level 64 pg/mL). Patient 1’s serum IL-6 level increased 3.5-fold from baseline to its peak 11 days after CAR T-cell infusion. Elevations in serum IFN-γ have been previously reported with CLL.37 In accordance with these previous reports, patient 1’s serum IFN-γ was elevated before the anti-CD19-CAR T-cell infusion and increased to higher levels during the time of his acute toxicities (Figure 2D). No patient had a significant serum TNF elevation.
Persistence of allogeneic anti-CD19-CAR T cells in the blood
We measured persistence of the infused CAR T cells by qPCR (Figure 6A-B). The most common pattern of persistence was observed in patients 1, 2, 5, 9, and 10. In these patients, cells containing the CAR gene were not detectable in the blood for at least the first 5 days after infusion. Consistent with in vivo proliferation, the percentage of PBMCs that were CAR+ then increased rapidly to a peak 7 to 14 days after the infusion. The CAR+ T cells then decreased rapidly so that minimal numbers of CAR-expressing T cells were detectable beyond 1 month after infusion (Figure 6A). CAR+ T cells were detected in the blood of all patients except patients 6 and 7. We detected CAR-expressing T cells ex vivo by staining with a CAR-specific monoclonal antibody (Figure 6C-D).33
Anti-CD19-CAR–expressing T cells were detected in the blood of patients. (A) qPCR was used to determine the percentage of total PBMCs that contained the anti-CD19-CAR gene. The highest percentages of PBMCs containing the CAR gene were generally found between 6 and 14 days after infusion. (B) The absolute number of PBMCs containing the CAR gene per microliter of blood was determined by qPCR. (C) PBMCs from patient 8 were stained with a CAR-specific monoclonal antibody. Pretreatment PBMCs and PBMCs from 9 days after infusion were stained. Most CAR+ cells detected 9 days after infusion were CD4+. (D) PBMCs from patient 9 were stained with a CAR-specific monoclonal antibody. Pretreatment PBMCs and PBMCs from 7 days after infusion were stained. CAR+ cells included both CD4+ and CD8+ T cells. The plots in panels C and D are gated on live CD3+ lymphocytes.
Anti-CD19-CAR–expressing T cells were detected in the blood of patients. (A) qPCR was used to determine the percentage of total PBMCs that contained the anti-CD19-CAR gene. The highest percentages of PBMCs containing the CAR gene were generally found between 6 and 14 days after infusion. (B) The absolute number of PBMCs containing the CAR gene per microliter of blood was determined by qPCR. (C) PBMCs from patient 8 were stained with a CAR-specific monoclonal antibody. Pretreatment PBMCs and PBMCs from 9 days after infusion were stained. Most CAR+ cells detected 9 days after infusion were CD4+. (D) PBMCs from patient 9 were stained with a CAR-specific monoclonal antibody. Pretreatment PBMCs and PBMCs from 7 days after infusion were stained. CAR+ cells included both CD4+ and CD8+ T cells. The plots in panels C and D are gated on live CD3+ lymphocytes.
Phenotype of CAR-expressing T cells during eradication of CLL
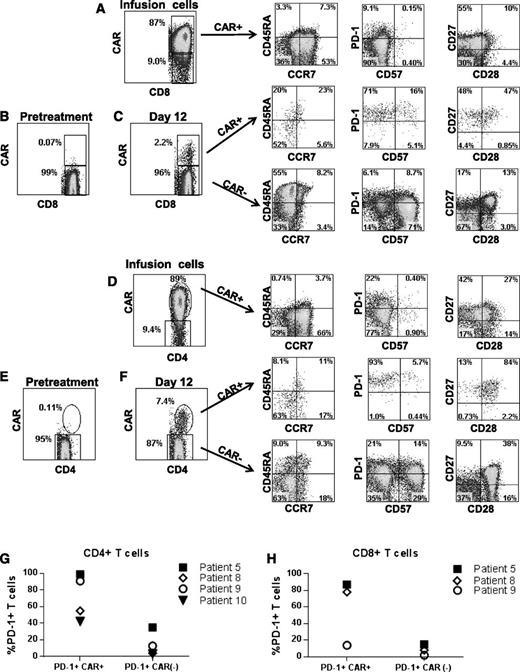
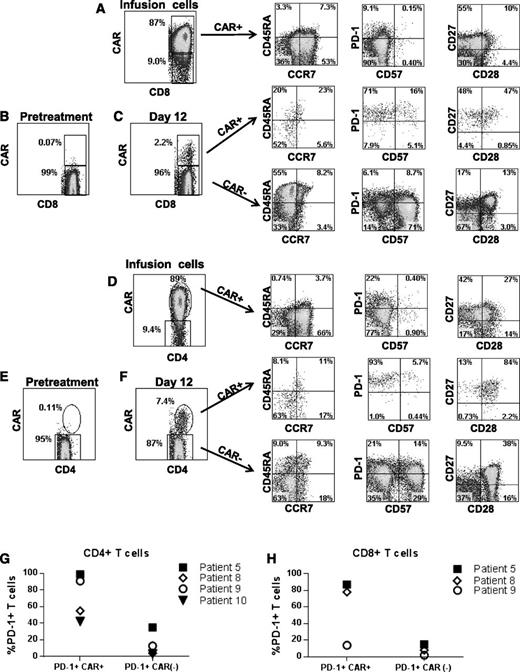
We assessed several T-cell phenotype markers on cells from the time of infusion and at the time that the CAR+ T cells were at their peak levels in the blood. Results from patient 5 are shown as a representative example of the phenotypes of the CAR+ T cells (Figure 7). Compared with the CAR+ T cells at the time of infusion, CAR+ T cells at the time of peak blood levels had higher frequencies of cells with a CD45RA–, CCR7– effector memory phenotype in 3 of 4 assessed patients; in addition, there was a lower frequency of T cells with a CD45RA–, CCR7+ central memory phenotype among both the CD8+ and the CD4+ T cells at the time of peak blood levels after infusion compared with at the time of infusion.35 Expression of the critical T-cell inhibitory receptor PD-138 was increased on CAR+CD8+ and CAR+CD4+ T cells at the time of peak blood CAR+ T-cell levels compared with CAR+ T cells at the time of infusion. The low PD-1 and CD57 expression levels of the CAR+ T cells infused into patient 5 are representative of other patients on the trial. Another example of the PD-1 and CD57 phenotype of infused cells (patient 1) is in supplemental Figure 1. At the time of peak blood CAR+ T-cell levels, PD-1 expression was increased on CAR+ blood T cells compared with CAR– blood T cells from the same patients (Figure 7C,F-H). In 3 of 4 assessed patients, CAR+ T cells at the time of peak blood levels expressed substantially higher levels of CD27 relative to CAR– T cells from the same time point (Figure 7C,F).
Phenotype of anti-CD19-CAR–expressing T cells. (A) Infusion cells of patient 5 are shown after staining with a CAR-specific antibody. The first plot is gated on CD3+CD8+ cells. The phenotype of the CD3+CD8+CAR+ cells is shown on the subsequent plots of CD45RA vs CCR7, programmed cell death protein 1 (PD-1) vs CD57, and CD27 vs CD28. (B) CAR expression was assessed on pretreatment lymphocytes of patient 5 by flow cytometry. The plot shows minimal background staining when the cells were stained with a CAR-specific monoclonal antibody. The plot is gated on CD3+CD8+ lymphocytes. (C) The first plot is gated on CD3+CD8+ lymphocytes that were obtained from patient 5 12 days after CAR T-cell infusion. CAR+ cells made up 2.2% of the CD3+CD8+ lymphocytes. The subsequent CD45RA vs CCR7, PD-1 vs CD57, and CD27 vs CD28 plots are gated on CD3+CD8+ lymphocytes that are either CAR+ or CAR–. (D) Infusion cells of patient 5 are shown. The first plot is gated on CD3+CD4+ cells. Subsequent plots show the phenotype of CD3+CD4+CAR+ cells. (E) The plot shows minimal background staining when pretreatment lymphocytes from patient 5 were stained with a CAR-specific antibody. The plot is gated on CD3+CD4+ lymphocytes. (F) The first plot is gated on CD3+CD4+ lymphocytes that were obtained from patient 5 12 days after CAR T-cell infusion. CAR+ cells made up 7.4% of the CD3+CD4+ lymphocytes. Subsequent plots show the phenotype of CD3+CD4+ cells that were either CAR+ or CAR–. Increased expression of the T-cell inhibitory protein PD-1 was present on CD4+CAR+ T cells (G) and CD8+CAR+ T cells (H) relative to CAR– T cells from the same patient at the time of the peak in the level of CAR+ blood T cells in each patient. The percentage of PD-1+ T cells is shown for the CAR+ and CAR– T cells of the indicated patients. Note that an insufficient number of CAR+CD8+ T cells were present in the blood of patient 10 to allow accurate determination of PD-1–expressing CD8+ cells in this patient. PD-1 expression was determined as shown in panels C and F. For CD4+ T cells, the difference in PD-1 expression between CAR+ cells and CAR– cells was statistically significant (P = .03, Mann-Whitney U test). PD-1 levels are only shown for 4 patients because some patients had very low or undetectable levels of CAR+ T cells in their blood and because we lacked sufficient PBMCs from relevant time points to conduct the experiment in some other patients.
Phenotype of anti-CD19-CAR–expressing T cells. (A) Infusion cells of patient 5 are shown after staining with a CAR-specific antibody. The first plot is gated on CD3+CD8+ cells. The phenotype of the CD3+CD8+CAR+ cells is shown on the subsequent plots of CD45RA vs CCR7, programmed cell death protein 1 (PD-1) vs CD57, and CD27 vs CD28. (B) CAR expression was assessed on pretreatment lymphocytes of patient 5 by flow cytometry. The plot shows minimal background staining when the cells were stained with a CAR-specific monoclonal antibody. The plot is gated on CD3+CD8+ lymphocytes. (C) The first plot is gated on CD3+CD8+ lymphocytes that were obtained from patient 5 12 days after CAR T-cell infusion. CAR+ cells made up 2.2% of the CD3+CD8+ lymphocytes. The subsequent CD45RA vs CCR7, PD-1 vs CD57, and CD27 vs CD28 plots are gated on CD3+CD8+ lymphocytes that are either CAR+ or CAR–. (D) Infusion cells of patient 5 are shown. The first plot is gated on CD3+CD4+ cells. Subsequent plots show the phenotype of CD3+CD4+CAR+ cells. (E) The plot shows minimal background staining when pretreatment lymphocytes from patient 5 were stained with a CAR-specific antibody. The plot is gated on CD3+CD4+ lymphocytes. (F) The first plot is gated on CD3+CD4+ lymphocytes that were obtained from patient 5 12 days after CAR T-cell infusion. CAR+ cells made up 7.4% of the CD3+CD4+ lymphocytes. Subsequent plots show the phenotype of CD3+CD4+ cells that were either CAR+ or CAR–. Increased expression of the T-cell inhibitory protein PD-1 was present on CD4+CAR+ T cells (G) and CD8+CAR+ T cells (H) relative to CAR– T cells from the same patient at the time of the peak in the level of CAR+ blood T cells in each patient. The percentage of PD-1+ T cells is shown for the CAR+ and CAR– T cells of the indicated patients. Note that an insufficient number of CAR+CD8+ T cells were present in the blood of patient 10 to allow accurate determination of PD-1–expressing CD8+ cells in this patient. PD-1 expression was determined as shown in panels C and F. For CD4+ T cells, the difference in PD-1 expression between CAR+ cells and CAR– cells was statistically significant (P = .03, Mann-Whitney U test). PD-1 levels are only shown for 4 patients because some patients had very low or undetectable levels of CAR+ T cells in their blood and because we lacked sufficient PBMCs from relevant time points to conduct the experiment in some other patients.
Discussion
Standard DLIs can induce remissions in patients with B-cell malignancies persisting after alloHSCT; however, these unmanipulated allogeneic lymphocytes can also cause the sometimes fatal complication of GVHD.4,7,9 Many approaches have been tried to separate the cell-mediated allogeneic graft-versus-malignancy effect from GVHD.39 Nonantigen-specific approaches include culturing donor lymphocytes in sirolimus in an effort to change the functional properties of the donor lymphocytes.40 Antigen-specific approaches to T-cell therapy have been developed to treat virus-associated malignancies after alloHSCT41,42 and to target minor histocompatibility antigens.43 Lymphocytes were collected from a single patient with acute lymphoblastic leukemia persisting after allogeneic cord blood transplantation, genetically modified to express an anti-CD19 CAR, and reinfused to treat the acute lymphoblastic leukemia after the patient had received chemotherapy.27 Because clinical trials of autologous anti-CD19-CAR T cells have generated encouraging early results, we decided to study allogeneic anti-CD19-CAR T cells as a way of enhancing the graft-versus-malignancy activity of allogeneic donor-derived lymphocytes.13,15,18,19,21,27,44
The most impressive antimalignancy responses among the patients on this study were observed in patients 1, 5, and 9. These patients had some characteristics that might be related to why they had objective regressions of malignancy while the other patients did not. Patients 1 and 5 both had CLL, and both of these patients had substantial numbers of blood B cells at the time of CAR T-cell infusion. It is conceivable that blood B cells promoted proliferation or survival of the CAR T cells in vivo. Patients 1, 5, and 9 were all recipients of URD transplants. Patients 1, 5, and 9 had 3 of the 4 highest peak levels of blood CAR+ T cells when expressed as a percentage of PBMCs (Figure 6A). Finally, patients 1, 5, and 9 experienced cytokine release–associated toxicities including hypotension and fever.
We conclude that the regressions of CLL in patients 1, 5, and 9 were caused by CD19-specific immune responses rather than general donor-versus-host responses against allogeneic antigens. Four characteristics of the clinical courses of these patients support this conclusion. First, the regressions of CLL in patients 1 and 5 were evident <2 weeks after the CAR T-cell infusions. Such rapid regressions of malignancy are not consistent with the slower regressions of malignancy that are typically observed after standard DLIs.9,45 Second, regression of malignancy after anti-CD19-CAR T-cell infusions was associated with rapid depletion of all B cells (Figures 3 and 4). Third, in patients 1 and 5, the regressions of CLL after anti-CD19-CAR T-cell infusions were more extensive than the regressions of CLL obtained when these patients received standard DLIs containing much higher numbers of T cells (Tables 1 and 3). Finally, none of the 3 patients with regression of malignancy after infusion of anti-CD19-CAR T cells had any evidence of GVHD after receiving anti-CD19-CAR T cells. GVHD, which is caused by immune responses against allogeneic antigens, is often associated with antimalignancy responses after alloHSCT and standard DLIs.3,7,9
A unique aspect of this study is that regressions of malignancy and eradication of normal B cells occurred in patients who were not lymphocyte depleted. All of the patients on the study had substantial numbers of endogenous blood T cells at the time of their anti-CD19-CAR T-cell infusions (Table 2). There is extensive evidence that the antimalignancy activity of adoptively transferred T cells can be enhanced by depleting endogenous lymphocytes before adoptive T-cell transfer.13,23,34 Despite this evidence, we did not include lymphocyte-depleting chemotherapy in this clinical trial because of concern over excessive T-cell activation, which could have led to GVHD or cytokine-release toxicities. Because we did not include any chemotherapy in our protocol and because at least 4 weeks had elapsed between any prior treatment and infusions of anti-CD19-CAR T cells, our results provide clear evidence of the antimalignancy activity of these T cells.
CAR T cells persisted in the blood of patients for <1 month (Figure 6A-B), and PD-1, a critical marker of T-cell exhaustion,38 was increased on CAR+ T cells relative to CAR– T cells (Figure 7G-H). We hypothesize that higher peak blood levels of CAR T cells and increased persistence of CAR T cells will be necessary to improve response rates. One approach that we are following to increase the efficacy of anti-CD19-CAR T-cell infusions is to escalate the dose of cells. In another effort to improve the antimalignancy activity and persistence of allogeneic CAR T cells, we plan to give repeat doses of CAR T cells preceded by lymphocyte-depleting chemotherapy to patients with residual malignancy and mild toxicity after an initial CAR T-cell infusion. Finally, persistence of CAR-expressing T cells might be improved by changing costimulatory domains or other components of CARs.
For the first time, we administered anti-CD19-CAR T cells that were derived from healthy allogeneic donors to patients with persisting B-cell malignancies after alloHSCT. GVHD did not develop in patients receiving allogeneic anti-CD19-CAR T cells, and regressions of malignancy occurred in patients with malignancies refractory to standard DLIs. Administration of T cells genetically modified to target malignancy-associated antigens is a promising approach to improve outcomes of patients undergoing alloHSCT.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the National Marrow Donor Program, Dr David Stroncek, and the staff of the National Institutes of Health Department of Transfusion Medicine for obtaining URD cells. The National Gene Vector Biorepository performed replication-competent-retrovirus monitoring.
This work was supported by intramural funding of the National Institutes of Health National Cancer Institute, Center for Cancer Research. This project has been funded in part with federal funds from the National Institutes of Health National Cancer Institute (contract no. HHSN261200800001E).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authorship
Contribution: J.N.K. designed research, conducted experiments, provided patient care, and wrote the manuscript; J.J.R., R.O.C., S.H.K., I.M., S.A.F., and W.G.T. conducted experiments and edited the manuscript; M.E.D., F.T.H., M.R.B., R.E.G., and S.A.R. designed research and edited the manuscript; D.C.H., D.H.F., N.M.H., A.R.M., D.D.H., J.C.G.-B., S.Z.P., C.S., B.G.H., B.B.-S., and J.S.W. provided patient care and edited the manuscript; and B.J. provided critical reagents and advice and edited the manuscript.
Conflict-of-interest disclosure: M.E.D., S.H.K., S.A.F., and S.A.R. are affiliated with the Surgery Branch, National Cancer Institute, which receives research funding from Kite Pharma for development of T-cell therapies; however, Kite Pharma did not support this study. The remaining authors declare no competing financial interests.
Correspondence: James N. Kochenderfer, National Institutes of Health, 10 Center Dr, CRC Room 3-3330, Bethesda, MD 20892; e-mail: kochendj@mail.nih.gov.
References
Author notes
R.E.G. and S.A.R. contributed equally to this study.