Key Points
This breakthrough involves the role of the aryl hydrocarbon receptor in the expansion and specification of hematopoietic progenitor cells.
This work sets a precedent for the use of an in vitro platform for the clinically relevant production of blood products.
Abstract
The evolutionarily conserved aryl hydrocarbon receptor (AhR) has been studied for its role in environmental chemical-induced toxicity. However, recent studies have demonstrated that the AhR may regulate the hematopoietic and immune systems during development in a cell-specific manner. These results, together with the absence of an in vitro model system enabling production of large numbers of primary human hematopoietic progenitor cells (HPs) capable of differentiating into megakaryocyte- and erythroid-lineage cells, motivated us to determine if AhR modulation could facilitate both progenitor cell expansion and megakaryocyte and erythroid cell differentiation. Using a novel, pluripotent stem cell–based, chemically-defined, serum and feeder cell–free culture system, we show that the AhR is expressed in HPs and that, remarkably, AhR activation drives an unprecedented expansion of HPs, megakaryocyte-lineage cells, and erythroid-lineage cells. Further AhR modulation within rapidly expanding progenitor cell populations directs cell fate, with chronic AhR agonism permissive to erythroid differentiation and acute antagonism favoring megakaryocyte specification. These results highlight the development of a new Good Manufacturing Practice–compliant platform for generating virtually unlimited numbers of human HPs with which to scrutinize red blood cell and platelet development, including the assessment of the role of the AhR critical cell fate decisions during hematopoiesis.
Introduction
The aryl hydrocarbon receptor (AhR) is a member of the evolutionarily conserved Per/ARNT/SIM (PAS) family of transcription factors.1 It is the only PAS family member known to be activated by endogenous or exogenous ligands. PAS proteins contribute to several important physiological processes.2 Historically, the evolutionarily conserved AhR was studied in the context of its activation by a variety of ubiquitous environmental pollutants including dioxins, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons, and subsequent transactivation of cytochrome P450–encoding genes.3,4 However, the AhR field has recently undergone a major paradigm shift following the demonstration that the AhR plays important physiological roles in the absence of environmental ligands.3,4 For example, several studies have demonstrated that the AhR contributes to regulation of autoimmune responses,5-11 inflammation,9,12 cell growth,13 cell migration,14,15 apoptosis,16,17 and cancer progression.18-20 Specifically with regard to hematopoietic cells, the AhR regulates development of Th17 cells, regulatory T-cell subsets, and gut-associated T cells.5-10,21 Importantly, recent breakthrough studies suggest that the AhR plays a critical role in nominal hematopoietic stem cell (HSC) growth and differentiation.22,23 For example, AhR−/− mice are characterized by an increased number of bone marrow HSCs22 and a commensurate increased propensity to develop lymphomas.24 These insights led to the hypothesis that the AhR, activated by endogenous ligands, regulates stem cell growth and/or differentiation.25 Despite these early results, little is known about the effects of AhR modulation on the development of megakaryocyte- or erythroid-lineage cells from bipotential progenitors. Involvement of the AhR in this process is suggested by decreased numbers of erythrocytes and platelets in young AhR−/− mice and the skewing of the blood cell repertoire toward myeloid and B lineage cells as AhR−/− mice age.22,26,27
The differentiation of HSCs into all 8 blood cell lineages is a critical and tightly regulated physiological process.28 Disruption of this regulation can have a profound downstream effect on multiple hematopoietic cell types, potentially leading to a myriad of blood cell disorders ranging from leukemia to stem cell exhaustion.22,29 However, definition of the molecular mechanisms that control specification of primary human blood cells has been hampered by the lack of a model system in which sufficient numbers of stem or progenitor cells can be grown and the absence of practical and efficient techniques for directing differentiation of hematologic progenitors into end-stage cells. For example, several teams have published proof-of-principle examples of the derivation of megakaryocyte-30-32 and erythroid-lineage cells30,32,33 from embryonic stem cells and induced pluripotent stem cells (iPSCs). However, development of a model system that results in robust expansion of these cell populations or their immediate precursors and with which molecular signals driving cell differentiation can readily be studied has been problematic.
Our conceptual approach to addressing this need was to mimic in vitro the natural sequence of blood cell development in vivo to derive the number and range of cell types needed for the creation of a genetically tractable iPSC-based platform. Key components of this new platform, as shown here, include the definition of feeder cell–free, chemically defined conditions under which iPSCs can be differentiated into modest numbers of hematopoietic progenitor cells (HPs) and a stimulus (AhR hyperactivation) that results in a dramatic expansion of this megakaryocyte-erythroid progenitor (MEP)–like population and the production of virtually unlimited numbers of megakaryocyte- and erythroid-lineage cells. Thus, this culture system allows for the efficient and consistent capture in culture and expansion of populations of HPs that exist only transiently during in vivo development of platelets and red blood cells (RBCs). Furthermore, continued AhR manipulation, presumably in rapidly expanding MEP populations, dictates cell fate choices including the decision to differentiate into megakaryocyte- vs erythroid-lineage cells. In addition to demonstrating a critical and previously unappreciated role for the AhR in the HP, megakaryocyte, and erythroid lineages, the platform detailed here provides a flexible, consistent, and genetically tractable system for studying blood cell differentiation at multiple defined stages of development. Because this system can be initiated from both normal and disease-specific iPSCs, it is also amenable to the generation and study of megakaryocyte- and erythroid-lineage cells from patients with blood diseases. Finally, this work represents a significant step toward the in vitro production of virtually unlimited numbers of therapeutic, patient-specific RBCs and platelets.
Materials and methods
iPSC derivation and culture
iPSC derivation was achieved using the hSTEMCCA lentiviral vector as described previously.49
Directed differentiation of iPSCs into hematopoietic cells
iPSCs were plated onto matrigel-coated 6-well plates in iPSC media conditioned on inactivated murine embryonic fibroblasts for 24 hours and were supplemented with 2 ng/mL of Rho kinase inhibitor and 20 ng/mL of basic fibroblast growth factor (bFGF). After 2 days, iPSC media were replaced with media cocktails designed to initiate hematopoietic specification: D0 1 media: RPMI (Invitrogen; A1049101) supplemented with 5 ng/mL of human bone morphogenetic protein 4 (hBMP-4) (R&D; 314-BP-010), 50 ng/mL of human vascular endothelial growth factor (hVEGF) (R&D; 293-VE-010), 25 ng/mL of hWnt3a (R&D; 287-TC-500), and 5% KOSR; D2 media: RPMI supplemented with 5 ng/mL of hBMP-4, 50 ng/mL of hVEGF, 20 ng/mL of bFGF, and 5% KOSR; D3 media: StemPro 34 (Invitrogen; 10639011), 5 ng/mL of hBMP-4, 50 ng/mL of hVEGF, and 20 ng/mL of bFGF; D4-5 media: StemPro 34, 15 ng/mL of hVEGF, and 5 ng/mL of bFGF; D6 media: 74% Iscove modified Dulbecco medium (IMDM) (Invitrogen; 12330061), 24% Hams F12 (Mediatech; 10-080-CV), 1% B27 supplement (Invitrogen; 12587-010), 0.5% N2-supplement (Invitrogen; 17502-048), 0.5% bovine serum albumin (BSA) (Sigma; A3059), 50 ng/mL of hVEGF, 100 ng/mL of bFGF, 100 ng/mL of human stem cell factor (R&D; 255-SC-010), 25 ng/mL of hFlt3 ligand (R&D; 308-FKN-005); D7 media: 74% IMDM, 24% Hams F12, 1% B27 supplement, 0.5% N2-supplement, 0.5% BSA, 50 ng/mL of hVEGF, 100 ng/mL of bFGF, 100 ng/mL of human stem cell factor, 25 ng/mL of hFlt3 ligand, 50 ng/mL of human thrombopoietin (TPO) (Genentech; G140BT), 10 ng/mL of IL-6 (R&D; 206-IL-010), 0.5 U/mL of hEPOgen, and 0.2 μM of 6-formylindolo[3,2-b]carbazole (FICZ) (a gift of Michael Pollastri, PhD, Northeastern University) After day 7, 0.5 mL of day 7 media was added to the culture daily without aspirating the media from the previous day. All base media mixes included 2 mM of l-glutamine (Invitrogen; 25030081), 4 × 10-4 M of monothioglycerol (Sigma; M1753), 100 μg/mL of Primocin, and 50 ug/mL of ascorbic acid. Cells in suspension were collected and assayed on days 10 to 15 or were split for long-term culture.
Megakaryocyte- and erythroid-lineage specification of HPs
Megakaryocyte-lineage cells were generated from day 15 HPs by first washing the cells and placing them in megakaryocyte specification media containing IMDM, 0.5% BSA, 10 μg/mL of nucleoside triphosphates and deoxynucleoside triphosphates (Invitrogen), 40 μg/mL of low-density lipoprotein (Sigma; L8292), 200 μg/mL of human holotransferrin (Sigma; T0665), 10 μg/mL of human insulin (Sigma; I3536), 50 μM of 2 ME, and 100 ng/mL of human TPO. Mature megakaryocytes were grown on an OP9 stromal layer for 3 to 5 days to allow for terminal megakaryocyte differentiation and platelet production. Erythroid-lineage cells were generated from day 15 HPs by first washing the cells and placing them in erythroid specification media containing IMDM, 10% Plasmanate (human plasma mimetic; Talecris Biotherapeutics, Research Triangle Park, NC), and 3U/mL of human erythropoietin (EPO).
Methylcellulose assays
Nonadherent day 15 HPs were collected and plated in MethoCult H4034 Optimum (Stem Cell Technologies; 4034) at a density of 56 000 cells per 3 mL of MethoCult. The MethoCult and cell mixture was plated in 35-mm culture dishes per the manufacturer's instructions. Resultant colonies were scored on day 12 and were classified as mixed, myeloid, or erythroid in origin.
Mass spectrometry analyses
Whole blood was diluted 1:500 in an electrospray ionization buffer and was analyzed directly. iPSC-derived erythroid-lineage cells were lysed with the addition of formic acid (0.8%), and the resulting solution was passed through a C18 spin column. Proteins were eluted with 60% acetonitrile/0.3% formic acid/water and were assayed.
Quantitative reverse-transcription polymerase chain reaction (RT-PCR)
RNA was extracted using the RNeasy kit (Qiagen) according to the manufacturer’s instructions and DNase treated using the DNA-free kit (Ambion; AM1906). Reverse transcription into cDNA was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; 4368814).
Flow cytometry
Roughly 105 cells were collected, spun, and resuspended in 0.5% BSA in phosphate-buffered solution. Samples were incubated for 30 minutes at an ambient temperature with human antibodies including CD41a-fluorescein isothiocyanate (BD 555466), CD235-PE (BD 555570), and CD71-fluorescein isothiocyanate (BD 555536); washed and spun at 3300 rpm for 7 minutes, and resuspended in 0.5% BSA in phosphate-buffered solution with 1 μg/mL of propidium iodide.
Image capture and analysis
All images were captured on a Nikon Eclipse TS100 microscope equipped with a Diagnostic Instruments, Inc., model 18.2 Color Masonic Camera. Images were processed using Adobe Photoshop software.
Statistical analysis
Results are presented as the mean ± the standard deviation (SD) of experiments performed in triplicate. Statistical significance was confirmed using the Student t test.
Results
Analysis of human hematopoietic cell differentiation genomic mapping (dMap) data suggests a role for AhR in normal hematopoietic specification
As a guide for assessing the possible role of the AhR in hematopoietic cells, we analyzed the “dMap” data set (www.broadinstitute.org/dmap),34 a publicly available compendium of expression profiles from 71 distinct purified populations of human hematopoietic cells. For our purposes, we focused on the HSC-to-megakaryocyte/erythroid differentiation path, and we analyzed the expression of a manually curated list of putative AhR target genes. Hierarchical clustering was carried out to evaluate the coexpression patterns of Ahr and these genes. This analysis revealed upregulated Ahr mRNA expression in HSCs and MEPs (supplemental Figure 1). Erythroid cells were clustered into 2 groups. Earlier-phase erythroid cells maintained elevated expression of the Ahr and genes upregulated in MEPs. Later-phase erythroid cells exhibited a largely reciprocal pattern. Ahr levels were consistently upregulated in megakaryocytes. The levels of 21 genes, including several of considerable import to stem cells (eg, c-myc, EGR1, ALDHA1) correlated significantly with Ahr levels (false discovery rate ≤0.004). Other important hematopoietic-specific genes such as NFE2, a critical regulator of both the erythroid and megakaryocyte lineages, correlated negatively with Ahr expression. These results indicate that Ahr expression is evident in HPs and suggest that the AhR may play a role in the development of human bipotential MEPs and megakaryocyte- and erythroid-lineage cells.
The AhR agonist FICZ allows for the exponential expansion of iPSC-derived HPs
The clinical translation of iPSC-based technologies will require many modifications to improve the efficiency of generation and the safety profile of iPSC-derived cells. Using a novel, feeder cell–free, chemically defined system for the production of HPs from human iPSCs that is not beholden to the use of stromal cell lines or xenogeneic agents, our primary goal was the production of large numbers of clinically relevant, high-purity hematopoietic cells. Our approach follows the roadmap provided by the developing embryo. Because embryonic stem cells and iPSC resemble pluripotent, undifferentiated cells of the early blastocyst embryo, the signals active in the early embryo were harnessed to direct the differentiation of iPSC in vitro. Because of the known variability in the formation of human embryoid bodies,35 this protocol uses a 2D culture system optimized to produce HPs within 10 to 15 days (Figure 1A). Because of the expression of Ahr mRNA early in the MEP differentiation process (supplemental Figure 1), a strong AhR agonist, FICZ, was added on day 7.
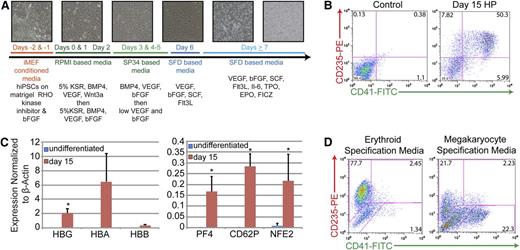
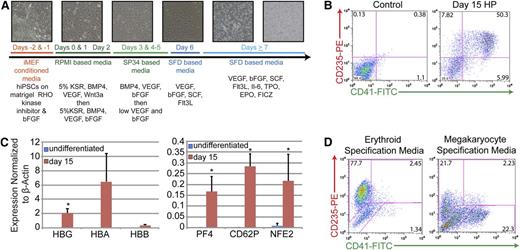
The feeder-free, chemically defined production of HPs from iPSCs produces populations of cells that express markers of both the megakaryocyte and erythroid lineages. (A) Differentiation strategy from iPSC to HP stage. Phase-contrast images (10×) of cultures depicting morphologic changes and the production of both an initial adherent layer followed by nonadherent HPs. (B) Representative fluorescence-activated cell sorter (FACS) analysis of day 15 HPs that coexpress CD235 (red cells) and CD41 (megakaryocytes). (C) Quantitative PCR analysis of undifferentiated iPSCs vs day 15 HPs. Relative gene expression was normalized to β-actin. Data are the average of 3 independent experiments ± SD: *P < .05. (D) Representative FACS analysis of day 15 HPs that have been exposed to either erythroid- or megakaryocyte-specific specification media for 5 days.
The feeder-free, chemically defined production of HPs from iPSCs produces populations of cells that express markers of both the megakaryocyte and erythroid lineages. (A) Differentiation strategy from iPSC to HP stage. Phase-contrast images (10×) of cultures depicting morphologic changes and the production of both an initial adherent layer followed by nonadherent HPs. (B) Representative fluorescence-activated cell sorter (FACS) analysis of day 15 HPs that coexpress CD235 (red cells) and CD41 (megakaryocytes). (C) Quantitative PCR analysis of undifferentiated iPSCs vs day 15 HPs. Relative gene expression was normalized to β-actin. Data are the average of 3 independent experiments ± SD: *P < .05. (D) Representative FACS analysis of day 15 HPs that have been exposed to either erythroid- or megakaryocyte-specific specification media for 5 days.
In this system, differentiating iPSCs produce an endothelial-like adherent layer characterized by the expression of Tie2 and kinase insert domain-containing receptor from which nonadherent CD43+ hematopoietic cells emerge beginning at day 7 (Figure 1A and supplemental Figure 2A). Days 7 to 15 are characterized by the rapid outgrowth of nonadherent cells (Figure 1A and supplemental Figure 2A). As judged by immunophenotyping on day 15, greater than 50% of these nonadherent cells coexpress CD235-glycophorin A (erythroid lineage) and CD41-integrin αIIb (megakaryocyte lineage), suggesting that at least a portion of the HPs generated were bipotential MEPs (Figure 1B). When placed in colony-forming assays, this total, unsorted population of cells produced roughly equal numbers of mixed, myeloid-, and erythroid-lineage colonies suggesting the presence of bipotential MEPs, as well as committed megakaryocyte- and erythroid-lineage cells (supplemental Figure 2B). In comparison with undifferentiated iPSCs, these cells also significantly upregulated globin genes characteristic of erythroid-lineage cells and PF4, CD62P, and NFE2 genes characteristic of megakaryocyte-lineage cells (Figure 1C). Note that the timeframe to generate what phenotypically appear to be MEPs is significantly shorter than that noted in previously described protocols31,36 and that no fractionation or further manipulation of the cells is required. Importantly, maintenance of cultures in erythroid specification media containing EPO, or megakaryocyte specification media containing TPO, resulted in a final fate choice leading to the appearance of distinct populations of CD235+/CD41− erythroid-lineage cells or CD41+/CD235− megakaryocyte-lineage cells, respectively (Figure 1D). These results are consistent with the presence of functionally bipotential MEPs.
A crucial roadblock in the translation of iPSC technology is the ability to produce sufficient, clinically relevant quantities of cells. Even for basic research studies, the numbers and quality of hematopoietic cells that can be produced through the directed differentiation of iPSCs can be limiting.37 With the outgrowth of HPs by day 15 of culture, we were presented with the possibility that ongoing AhR activation could continue to drive progenitor expansion. Here, we demonstrate that the AhR agonist FICZ has the ability to allow for the exponential expansion of iPSC-derived HPs. In comparison with untreated control samples, FICZ-treated HPs demonstrate significantly less cell death as judged by propidium iodide and Hoechst dye exclusion, as well as significantly more proliferation as judged by 5-ethynyl-2′-deoxyuridine (EdU) incorporation (Figure 2A-D). Furthermore, in contrast to untreated cells in which 500 000 HPs yielded 4 million cells (eightfold increase), FICZ-treated day 15 HPs demonstrated logarithmic expansion such that FICZ treatment of 500 000 HPs yielded 300 million cells (600-fold increase) within 10 days (Figure 2E).
The AhR agonist FICZ inhibits apoptosis and allows for the exponential expansion of iPSC-derived HPs. (A) Representative FACS analysis of live vs dead or dying cells (propidium iodide [PI] vs Hoechst) from day 15 HPs ± FICZ. Plots were gated first in forward light scatter (FSC) vs side light scatter (SSC) and then from that population for PI+ and PI- Hoechst+. (B) FICZ increases the population of live cells, as delineated by FSC and SSC, and decreases the number of compromised or apoptotic cells. For the live-cell gate, data are the average of 3 independent experiments ± SD: *P < .01. For the apoptotic cell gate, data are the average of 3 independent experiments ± SD: *P < .02. (C) EdU proliferation assay comparing day 15 HPs ± FICZ. After FICZ stimulation on day 7, EdU incorporation into treated HPs was significantly increased compared with untreated controls, indicative of increased proliferation. Data are the average of 3 independent experiments ± SD: *P < .01. (D) Representative phase-contrast images of HP population ± FICZ. (E) Growth curve of day 15 HPs ± 0.2 μm of FICZ. Cells were counted manually using trypan blue exclusion. Graphical data and the associated statistics are the result of 3 independent experiments per group.
The AhR agonist FICZ inhibits apoptosis and allows for the exponential expansion of iPSC-derived HPs. (A) Representative FACS analysis of live vs dead or dying cells (propidium iodide [PI] vs Hoechst) from day 15 HPs ± FICZ. Plots were gated first in forward light scatter (FSC) vs side light scatter (SSC) and then from that population for PI+ and PI- Hoechst+. (B) FICZ increases the population of live cells, as delineated by FSC and SSC, and decreases the number of compromised or apoptotic cells. For the live-cell gate, data are the average of 3 independent experiments ± SD: *P < .01. For the apoptotic cell gate, data are the average of 3 independent experiments ± SD: *P < .02. (C) EdU proliferation assay comparing day 15 HPs ± FICZ. After FICZ stimulation on day 7, EdU incorporation into treated HPs was significantly increased compared with untreated controls, indicative of increased proliferation. Data are the average of 3 independent experiments ± SD: *P < .01. (D) Representative phase-contrast images of HP population ± FICZ. (E) Growth curve of day 15 HPs ± 0.2 μm of FICZ. Cells were counted manually using trypan blue exclusion. Graphical data and the associated statistics are the result of 3 independent experiments per group.
AhR mediates the expansion and specification of HPs
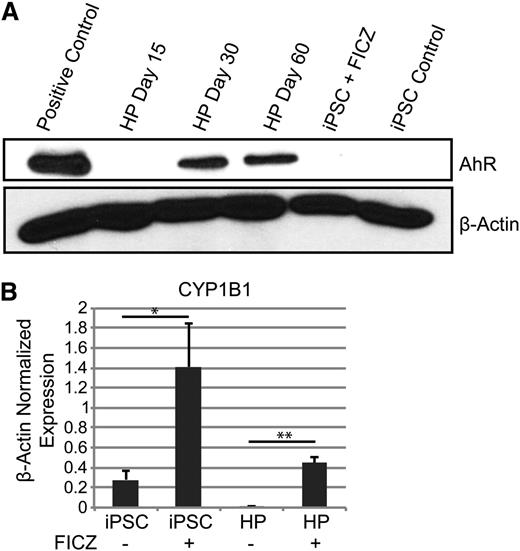
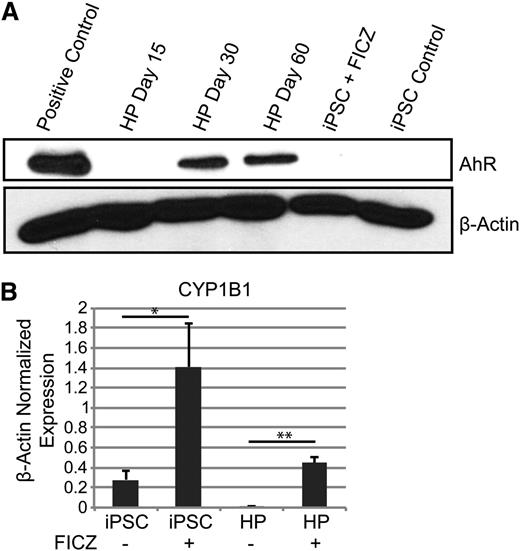
The results presented above strongly suggest that AhR hyperactivation drives human HP expansion. To further test this hypothesis, AhR expression and functionality in both undifferentiated iPSCs and differentiated HPs were investigated. Little or no AhR receptor protein was detected in human iPSCs by western blotting (Figure 3A). However, a significant increase in expression of the prototypic AhR target gene, CYP1B1, was seen by quantitative PCR at 72 hours after treatment with 0.2 μM of FICZ, suggesting that iPSCs express AhR but at levels below those detectable on western blots (Figure 3B). Similarly, AhR protein was not detected in day 15 HPs. However, treatment of day 15 HPs with FICZ significantly induced CYP1B1 expression (Figure 3B). Similar data were obtained with 2 other AhR agonists, β-naphthoflavone and the prototypic environmental AhR ligand, 2,3,7,8-tetrachlorodibenzo[p]dioxin (TCDD) (supplemental Figure 2). In contrast to iPSCs or day 15 HPs, AhR was robustly expressed in day 30 and day 60 HPs (Figure 3A).
AhR agonists induce CYP1B1 target gene expression in human iPSCs and HPs. (A) Western blot analysis for AhR and β-actin protein expression in iPSCs and HPs. (B) Quantitative PCR data of iPSCs and day 15 HPs with and without 0.2 μm of FICZ for 3 days. Expression is normalized to β-actin levels. Data are the average of 3 independent experiments ± SD: *P < .05, **P < .005 (supplemental Figure 2).
AhR agonists induce CYP1B1 target gene expression in human iPSCs and HPs. (A) Western blot analysis for AhR and β-actin protein expression in iPSCs and HPs. (B) Quantitative PCR data of iPSCs and day 15 HPs with and without 0.2 μm of FICZ for 3 days. Expression is normalized to β-actin levels. Data are the average of 3 independent experiments ± SD: *P < .05, **P < .005 (supplemental Figure 2).
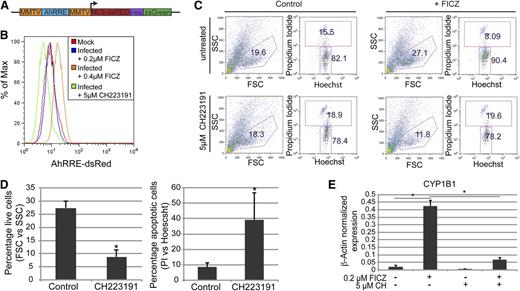
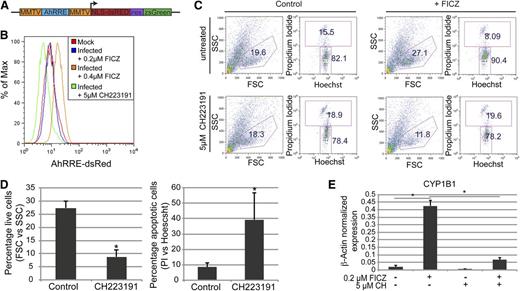
To quantify baseline AhR transcriptional activity, presumably enforced by an endogenous AhR ligand, we cloned a human AhR-driven promoter38,39 into a lentivirus reporter vector that encodes for dsRed and ZsGreen (Figure 4A). This dual gene AhR-driven reporter construct allowed for the normalization of transduction efficiency, correction for autoflorescence, and the quantification of baseline or FICZ-induced AhR transcriptional activity without bias for specific gene targets. Day 30 HPs were transduced with an AhR-driven reporter lentivirus or were mock infected at a multiplicity of infection of 10 and grown in basal medium containing 0.2 μM of FICZ for 72 hours. HPs were then subjected to 3 different growth conditions to assess the activity of AhR in this cell population: (1) the steady-state condition consisting of cells maintained in 0.2 μM of FICZ; (2) exposure to an increased FICZ concentration to 0.4 μM; or (3) growth in 0.2 μM of FICZ but also in the presence of 5 μM of a known AhR inhibitor, CH223191.40 In contrast to the mock-infected HPs, the AhR-driven reporter-infected HPs displayed a modest increase in dsRed expression, suggesting that the AhR-responsive elements in the reporter were being transactivated in the HPs (Figure 4B). An increase in the FICZ concentration to 0.4 μM significantly increased DsRed expression (Figure 4B). These results are consistent with the induction of CYP1B1 expression in primary HPs after FICZ exposure (Figure 3B). Importantly, a significant decrease in DsRed expression (below the level of expression in the mock-infected populations) was noted when the reporter virus-infected cells, maintained in a basal level of 0.2 μM of FICZ, were treated with 5 μM of CH223191 (Figure 4B). Similar results were obtained using a lentivirus encoding an AhR-driven luciferase or green-fluorescent reporter (data not shown). These studies confirm the presence of a functional, FICZ-responsive AhR in human HPs.
AhR mediates the expansion and specification of HPs. (A) Schematic representation of pHAGE2 lentiviral reporter constructs that contain the mouse mammary tumor virus flanking the dioxin response element (MMTV-AhRRE-MMTV) driving the expression of NLS-dsRed (pHAGE2-MMTV-AhRRE-MMTV-NLS-dsRed-IRES-zsGreen) (B) Representative FACS analysis for AhRRE-dsRED in HPs infected with pHAGE2-MMTV-AhRRE-MMTV-NLS-dsRed-IRES-ZsGreen. Infected cells were untreated or treated with 5 μM of CH223191, or 0.4 μM of FICZ. (C) Representative flow cytometry dot plots of live vs dead cells (PI vs Hoechst) from day 15 HPs ± FICZ and/or CH223191. For these experiments, HPs were pretreated with the known AhR inhibitor CH223191 on day 6 before the addition of FICZ on day 7. (D) Graphical representation of experiments performed in panel C. For the live-cell gate, data are the average of 3 independent experiments ± SD: *P < .005. For the apoptotic cell gate, data are the average of 3 independent experiments ± SD: *P < .04. (E) Expression of CYP1B1 as detected by quantitative PCR of MEPs from panel C, normalized to β-actin. Data are the average of 3 independent experiments ± SD: *P < .005.
AhR mediates the expansion and specification of HPs. (A) Schematic representation of pHAGE2 lentiviral reporter constructs that contain the mouse mammary tumor virus flanking the dioxin response element (MMTV-AhRRE-MMTV) driving the expression of NLS-dsRed (pHAGE2-MMTV-AhRRE-MMTV-NLS-dsRed-IRES-zsGreen) (B) Representative FACS analysis for AhRRE-dsRED in HPs infected with pHAGE2-MMTV-AhRRE-MMTV-NLS-dsRed-IRES-ZsGreen. Infected cells were untreated or treated with 5 μM of CH223191, or 0.4 μM of FICZ. (C) Representative flow cytometry dot plots of live vs dead cells (PI vs Hoechst) from day 15 HPs ± FICZ and/or CH223191. For these experiments, HPs were pretreated with the known AhR inhibitor CH223191 on day 6 before the addition of FICZ on day 7. (D) Graphical representation of experiments performed in panel C. For the live-cell gate, data are the average of 3 independent experiments ± SD: *P < .005. For the apoptotic cell gate, data are the average of 3 independent experiments ± SD: *P < .04. (E) Expression of CYP1B1 as detected by quantitative PCR of MEPs from panel C, normalized to β-actin. Data are the average of 3 independent experiments ± SD: *P < .005.
To determine if FICZ-mediated transactivation of the AhR receptor was responsible for the exponential expansion of iPSC-derived HPs, the effect of AhR inhibition with CH223191 on cell viability and expansion of the HP population was tested. As previously shown in Figure 2, addition of 0.2 μM of FICZ on day 7 of iPSC culture significantly increased the percentage of cells captured in the viable cell gate, as defined by forward and side scatter parameters (eg, 15.5% vs 8.09%) (Figure 4C). However, treatment with 5 μM of CH223191 at 1 day before the addition of FICZ completely blocked the increase in viable cells, as measured either by forward and side scatter parameters (FSC and SSC, respectively) or propidium iodide uptake (Figure 4C-D). Furthermore, no significant expansion of HPs was seen in cultures treated with CH223191 plus FICZ (data not shown). These data indicate that AhR activation with FICZ mediates an increase in HP viability and drives expansion of HP populations. The efficacy of the CH223191 was confirmed by its ability to block CYP1B1 induction as assayed by quantitative RT-PCR (Figure 4E).
Chronic AhR activation is permissive to erythroid cell maturation
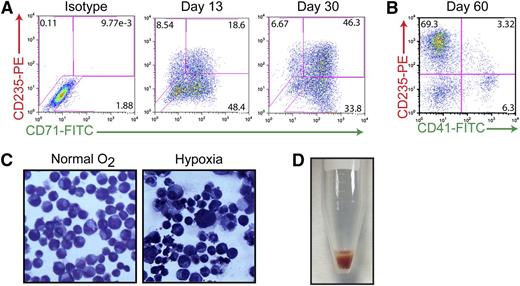
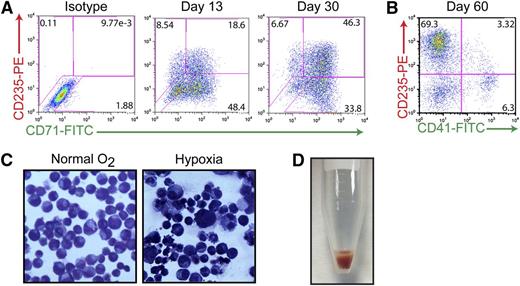
Previous studies have suggested that the AhR may play a critical role in hematopoietic cell development and function, possibly including the growth and differentiation of HSCs.7-9,41 Having shown that AhR activation results in exponential expansion of HP populations (Figure 2), and that the presence of EPO or TPO and FICZ generates either erythroid-lineage or megakaryocyte-lineage cells in the short term (Figure 1D), we then were in a position to determine if the AhR also contributes to HP differentiation into megakaryocyte- or erythroid-lineage cells during longer periods. To this end, HPs were cultured for extended periods (>120 days) in the presence of basal medium containing 0.2 μM of FICZ. Immunophenotyping of cell cultures maintained in these feeder-free conditions during a 60-day period revealed a progressive erythroid specification and maturation. As demonstrated in Figure 5A, the majority of early-passage (day 13) iPSC-derived HPs expressed CD71 (transferrin receptor) with a portion of the cells also expressing CD235 (glycophorin A), suggesting an immature erythroid cell phenotype. Under prolonged exposure to FICZ (30 days), these cells begin to downregulate CD71 and a larger percentage of cells express CD235, suggesting a more mature phenotype (Figure 5A). By day 60, most (∼70%) of the cells are CD235+/CD41−, indicating specification to the erythroid lineage under our basal growth conditions (Figure 5B). These populations of iPSC-derived erythroid-lineage cells demonstrate functional maturity, as assessed by their ability to respond to hypoxia (Figure 5C). For example, when cultured under low oxygen concentrations (5% O2) to simulate stress erythropoiesis, cells display hallmark characteristics of maturing erythroblasts, including a reduction in cell size and the condensation of chromatin within the nuclei of the cells (Figure 5C). More strikingly, when cells are centrifuged, bright red pellets can be seen indicating the production of hemoglobin (Figure 5D). In contrast to peripheral blood cells, which only express α-globin and β-globin (forming the most common form of hemoglobin in adult humans), iPSC-derived erythroid-lineage cells express α-globin, γ-globin (fetal), as well as 2 embryonic globins (ζ- and ε-globin) as judged by mass spectrometry analysis (supplemental Figure 4A). These results, as well as the lack of adult globin (β-globin) in this population, suggest that iPSC-derived erythroid-lineage cells are at an embryonic/fetal developmental stage.
Continuous AhR activation is permissive to erythroid cell maturation. (A) Representative FACS analysis of cells coexpressing 2 hallmark markers of the erythroid lineage: CD71 (transferrin receptor, early) and CD235 (glycophorin a, late) on day 15 and day 30 of specification. (B) Representative FACS analysis of cells coexpressing CD235E and CD41-demonstrating that by day 60, virtually all of the cells are committed to the erythroid lineage. (C) Wright-Giemsa staining of day 30 erythroid-lineage cells pre- and postexposure to hypoxic conditions demonstrating decreased size and condensed chromatin under low oxygen conditions. (D) Hemoglobin-expressing cell pellet of iPSC-derived erythroid-lineage cells.
Continuous AhR activation is permissive to erythroid cell maturation. (A) Representative FACS analysis of cells coexpressing 2 hallmark markers of the erythroid lineage: CD71 (transferrin receptor, early) and CD235 (glycophorin a, late) on day 15 and day 30 of specification. (B) Representative FACS analysis of cells coexpressing CD235E and CD41-demonstrating that by day 60, virtually all of the cells are committed to the erythroid lineage. (C) Wright-Giemsa staining of day 30 erythroid-lineage cells pre- and postexposure to hypoxic conditions demonstrating decreased size and condensed chromatin under low oxygen conditions. (D) Hemoglobin-expressing cell pellet of iPSC-derived erythroid-lineage cells.
AhR repression promotes megakaryocyte specification
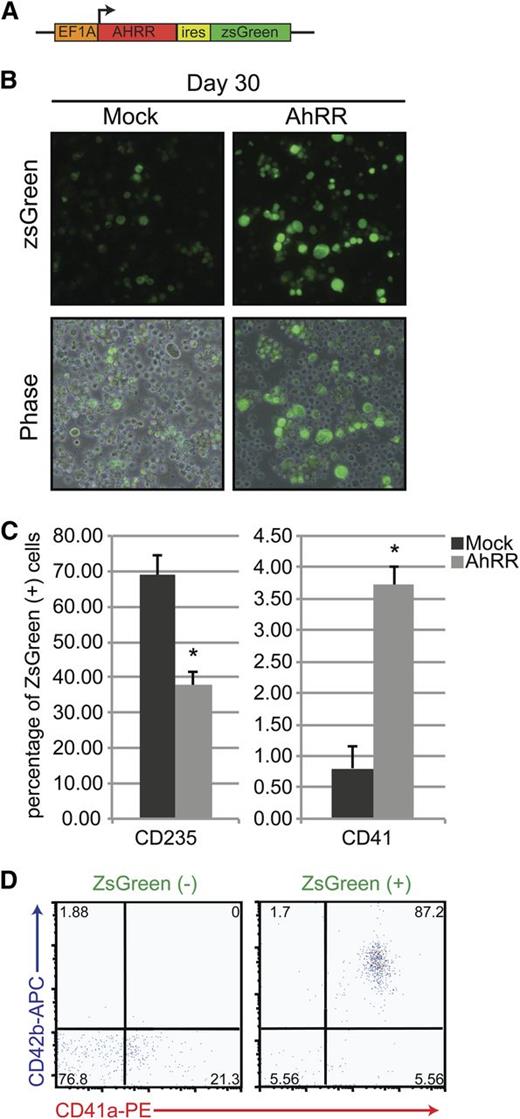
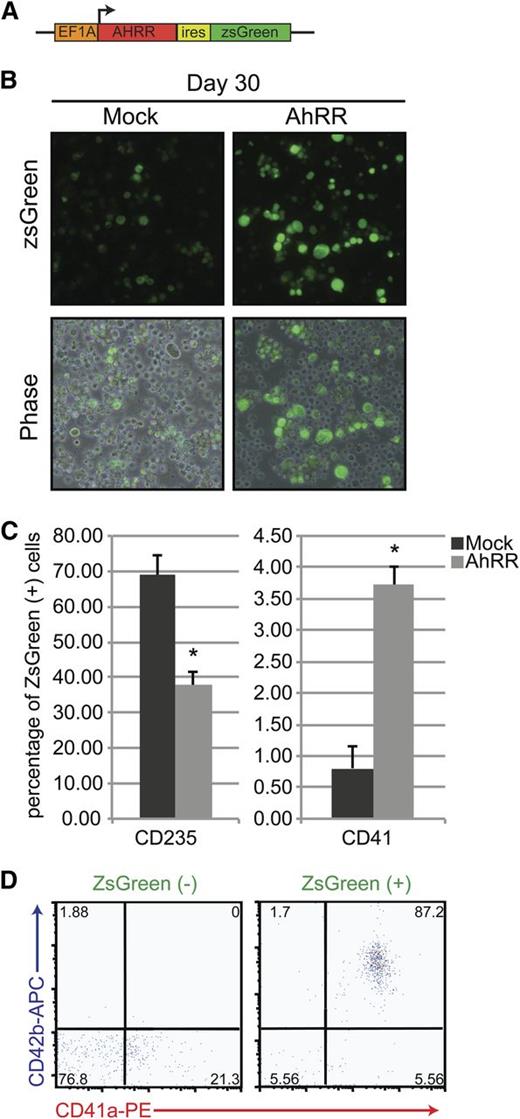
As the default pathway in our system seemed to allow for the specification and maturation of iPSC-derived HPs into the red cell lineage under chronic AhR agonism, we hypothesized that AhR downregulation in these long-term cultures might allow for the emergence of an alternative lineage, megakaryocytes. To test this hypothesis, we evaluated the effects of AhR inhibition using ectopic expression of an AhR reporter plasmid previously shown to block human AhR transcriptional activity.42,43 We constructed and used a lentiviral vector that encoded an AhR repressor (AhRR) element, along with a ZsGreen reporter (Figure 6A). This AhRR element potently and specifically inhibits either baseline or AhR agonist–induced AhR transcriptional activity.42,43 In contrast to cells transduced with a control ZsGreen reporter, cells infected with the AhRR-ZsGreen lentivirus and cultured for 5 days produce a significantly higher number of large, CD41+ megakaryocyte-lineage cells (Figure 6B-C). Interestingly, although the AhRR-transduced populations were capable of producing megakaryocyte-lineage cells, they also contained fewer CD235+ cells, suggesting that AhR antagonism initiates a transcriptional switch from erythroid- to megakaryocyte-lineage specification (Figure 6C).
AhR repression promotes megakaryocyte specification. (A) Schematic representation of a pHAGE2 lentiviral reporter construct containing the AhRR and ZsGreen under the control of the constitutive promoter Ef1α (pHAGE2-Ef1α-AhRR-IRES-zsGreen). (B) Phase-contrast and fluorescent images of D30 HPs after infection with a constitutively active ZsGreen control virus or pHAGE2-Ef1α-AhRR-IRES-zsGreen. Large cells (megakaryocytes) are noted in the populations infected with the AhRR. (C) Graphical representation of the percentage of ZsGreen+ cells that express CD235 (erythroid lineage) or CD41 (megakaryocyte lineage) after mock or AhRR infection. Data are presented as means of 3 independent experiments ± SD: *P < .005. (D) FACS analysis of AhRR-ZsGreen negative vs positive fractions for hallmark megakaryocyte markers CD41a/CD42b.
AhR repression promotes megakaryocyte specification. (A) Schematic representation of a pHAGE2 lentiviral reporter construct containing the AhRR and ZsGreen under the control of the constitutive promoter Ef1α (pHAGE2-Ef1α-AhRR-IRES-zsGreen). (B) Phase-contrast and fluorescent images of D30 HPs after infection with a constitutively active ZsGreen control virus or pHAGE2-Ef1α-AhRR-IRES-zsGreen. Large cells (megakaryocytes) are noted in the populations infected with the AhRR. (C) Graphical representation of the percentage of ZsGreen+ cells that express CD235 (erythroid lineage) or CD41 (megakaryocyte lineage) after mock or AhRR infection. Data are presented as means of 3 independent experiments ± SD: *P < .005. (D) FACS analysis of AhRR-ZsGreen negative vs positive fractions for hallmark megakaryocyte markers CD41a/CD42b.
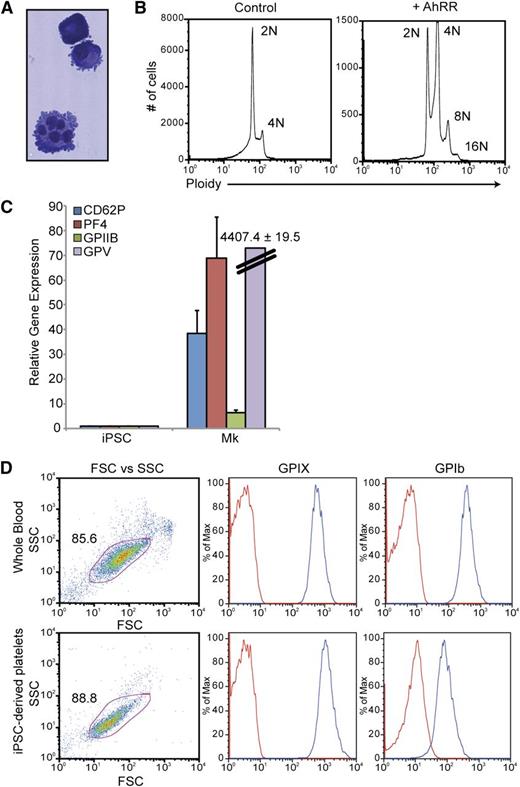
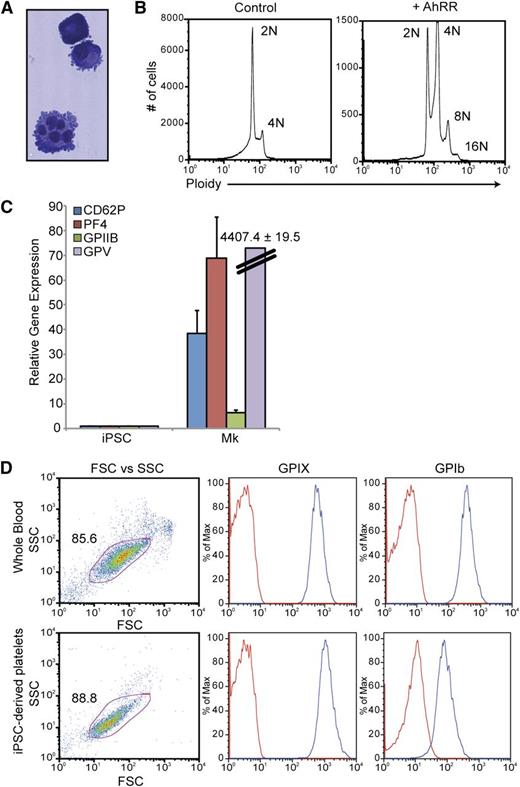
To further study the megakaryocyte-lineage cells produced via AhR antagonism, a discontinuous BSA gradient (0%, 1.5%, 3%) was used to isolate mature megakaryocytes from AhRR-ZsGreen–transduced populations. In contrast to the ZsGreen-AhRR− population, the majority of the ZsGreen-AhRR+ population was positive for a combination of 2 hallmark megakaryocyte-lineage markers: CD41a and CD42b (Figure 6D). These cells also demonstrated hallmark characteristics of mature megakaryocytes, including lobular nuclei and membrane blebbing at the surface of the cells (Figure 7A) and the ability to endoreplicate to 8N and 16N (Figure 7B). Quantitative PCR analysis of these cells also revealed robust upregulation of a spectrum of megakaryocyte-related genes including glycoprotein (GP) V, GPIIb, PF4, and CD62P (Figure 7C). Functionality of iPSC-derived megakaryocytes was assessed by their ability to produce platelets. Mature megakaryocytes were grown on an OP9 stromal layer for 3 to 5 days to allow for terminal megakaryocyte differentiation and platelet production. Cultures were initially gated using GPIIb (CD41a) expression and demonstrated that iPSC megakaryocyte–derived platelets were similar to platelet populations from whole blood with respect to size (FSC), granularity (SSC), as well as the expression of GPIX and GPIb, 2 subunits of the GPIb/V/IX complex, that are characteristic of platelets (Figure 7D).
Characterization and functional analyses of iPSC-derived megakaryocyte-lineage cells. (A) Wright-Giemsa stain of megakaryocytes produced via AhR antagonism. (B) Ploidy analysis of iPSC-derived megakaryocytes demonstrating endoreplication. (C) Quantitative PCR analysis comparing undifferentiated iPSCs vs iPSC-derived megakaryocytes for a spectrum of megakaryocyte-specific markers. Expression is normalized to β-actin levels. Data are the average of 3 independent experiments ± SD. (D) FACS analysis comparing platelets in whole blood vs iPSC megakaryocyte–derived platelets. Mature megakaryocytes were grown on an OP9 stromal layer for 3 to 5 days to allow for terminal megakaryocyte differentiation and platelet production. Cultures were initially gated using GPIIb (CD41a) expression and demonstrated that iPSC megakaryocyte–derived platelets were similar to platelet populations from whole blood with respect to size (FSC), granularity (SSC), as well as the expression of GPIX and GPIb, 2 subunits of the GPIb/V/IX complex, that are characteristic of platelets.
Characterization and functional analyses of iPSC-derived megakaryocyte-lineage cells. (A) Wright-Giemsa stain of megakaryocytes produced via AhR antagonism. (B) Ploidy analysis of iPSC-derived megakaryocytes demonstrating endoreplication. (C) Quantitative PCR analysis comparing undifferentiated iPSCs vs iPSC-derived megakaryocytes for a spectrum of megakaryocyte-specific markers. Expression is normalized to β-actin levels. Data are the average of 3 independent experiments ± SD. (D) FACS analysis comparing platelets in whole blood vs iPSC megakaryocyte–derived platelets. Mature megakaryocytes were grown on an OP9 stromal layer for 3 to 5 days to allow for terminal megakaryocyte differentiation and platelet production. Cultures were initially gated using GPIIb (CD41a) expression and demonstrated that iPSC megakaryocyte–derived platelets were similar to platelet populations from whole blood with respect to size (FSC), granularity (SSC), as well as the expression of GPIX and GPIb, 2 subunits of the GPIb/V/IX complex, that are characteristic of platelets.
Collectively, these data suggest that production of erythroid-lineage cells is the default pathway of HPs chronically stimulated with an AhR ligand. Inhibition of that default pathway results in an increase in the percentage of megakaryocyte-lineage cells, an outcome that could reflect either a switch in cell fate decision to favor megakaryocyte production or the maintenance of a small population of megakaryocytes during a decrease in the production of AhR-dependent erythroid-lineage cells.
Discussion
Our results indicate that AhR has a physiological and functional role in normal hematopoietic development and that modulation of the receptor in HPs can direct cell fate. We demonstrate a novel methodology for the directed differentiation of pluripotent stem cells in serum- and feeder cell–free, chemically defined culture conditions into HPs capable of final specification into megakaryocyte- and/or erythroid-lineage cells.
As a starting point for these studies, we used human hematopoietic cell differentiation genomic (dMap) array data as a roadmap for assessing the possible role of AhR in hematopoietic cells. These analyses suggested that the AhR plays an important role in blood cell development and were consistent with previous studies.22,23,26,44
In our studies, we have found that the use of a nontoxic AhR agonist in a directed differentiation scheme dramatically increases the number of HPs and resultant cells. This finding is important in that, traditionally, the evolutionarily conserved AhR has been studied for its role in environmental chemical-induced toxicity, and in our system it is shown to be involved in the growth and the differentiation of at least 2 crucial blood cell types. After the addition of the potent AhR ligand FICZ to our cultures, we observed exponential expansion of HPs from 500 000 to 300 million cells in 10 days. Importantly, the role of AhR in the HP population was confirmed using a highly specific AhR inhibitor. This logarithmic expansion of cells appears to be a function of decreased cell death and is consistent with previous studies, which suggest that the AhR can control apoptosis.16,17
It is important to note that these results can be contrasted with work performed by Gasiewicz et al48 in which AhR activation leads to exhaustion of the HSC pool, as opposed to hematopoietic cell expansion. This apparent contradiction could reflect different stages in hematopoiesis (HSCs vs HPs) or the nature of the ligand. Considerable evidence also indicates that functional outcomes may differ when different AhR ligands are used (eg, TCDD vs FICZ).45
Interestingly, FICZ, the AhR ligand used throughout our work, is a photometabolite of tryptophan originally described by Rannug et al.46 On the basis of previous studies demonstrating the ubiquity of FICZ47 and taken together with our data demonstrating the activity of this ligand, it is possible that FICZ plays a role in regulating hematopoiesis in vivo, possibly with other endogenous AhR ligands also playing a role. The ability to expand HPs with an AhR ligand also suggests that blood cell development may be affected by a variety of environmental ligands.26,48
In addition to allowing for the exponential expansion of HPs, our results indicate that AhR modulation is also involved in the further specification of both the erythroid and megakaryocyte lineages, with AhR agonism permissive to the differentiation of erythroblasts and antagonism or downregulation of AhR leading to megakaryocyte specification. Although EPO and TPO are the major drivers in RBC and platelet development, AhR may play a cytokine-independent role in the development and specification of these lineages and warrants further study in this capacity.
During the course of our studies, we derived putative progenitors known to express markers of both the megakaryocyte and erythroid lineages. A particularly striking outcome of our experiments is the development of a simple protocol for the rapid and highly efficient derivation of putative MEPs, which expand exponentially under AhR agonism. In addition to the ability to answer basic biological questions concerning hematopoietic development, a useful outcome for this work will be the use of this in vitro platform for the production of blood products, as the combination of AhR modulation with a completely chemically defined and xenobiotic agent–free differentiation scheme makes Good Manufacturing Practice production and clinical translation feasible. Blood transfusion is an indispensable cell therapy, and the safety and adequacy of the blood supply are of national and international concern. An iPSC-based system, such as the one described in our work in which large numbers of cells can be produced, could allow for RBC and platelet transfusion without problems related to immunogenicity, contamination, or supply. Furthermore, the ability to produce both populations of cells from a single source, and that both platelets and mature RBCs contain no nuclear genetic material, may mitigate some safety concerns usually associated with suggestions to transfuse or transplant iPSC-derived cells in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) U01 HL107443-01, an American Society of Hematology Scholar Award, and an Affinity Research Collaborative award from the Evans Center for Interdisciplinary Research at Boston University (G.J.M.); an NIH training grant 5T32HL007501-30 (S.S.R.); NIH P01-ES11624, P42ES007381, and the Art beCAUSE Breast Cancer Foundation (J.U., A.P., and D.H.S.); and by NIH Mass Spectrometer Resource (P41 GM104603), an NIH National Heart, Lung, and Blood Institute Proteomics contract (HHSN268201000031C), and a Shared Instrument grant: MALDI-TOF/TOF (matrix-assisted laser desorption/ionization-time-of-flight/time-of-flight) Mass Spectrometer (S10 OD010724) (C.E.C., M.E.M., and R.T.).
National Institutes of Health
Authorship
Contribution: B.W.S. and S.S.R. designed and performed research; J.U., A.P., and A.L. performed and analyzed research; S.K.N. performed research; D.F., P.G., S.M., D.H.K.C., and M.H.S. analyzed data; A.L.F. designed experiments and analyzed data; A.D.M. analyzed data; R.T. performed research; M.E.M., C.E.C., D.N.K., and G.M. analyzed data; D.H.S. designed experiments and analyzed data; and G.J.M. designed and performed experiments, analyzed data, and wrote the paper.
Conflict-of interest disclosure: The authors declare no competing financial interests.
Correspondence: George J. Murphy, Center for Regenerative Medicine, Boston University School of Medicine, 650 Albany St, Suite 444, Boston, MA 02118; e-mail: gjmurphy@bu.edu.
References
Author notes
B.W.S. and S.S.R. contributed equally to this study.

![Figure 2. The AhR agonist FICZ inhibits apoptosis and allows for the exponential expansion of iPSC-derived HPs. (A) Representative FACS analysis of live vs dead or dying cells (propidium iodide [PI] vs Hoechst) from day 15 HPs ± FICZ. Plots were gated first in forward light scatter (FSC) vs side light scatter (SSC) and then from that population for PI+ and PI- Hoechst+. (B) FICZ increases the population of live cells, as delineated by FSC and SSC, and decreases the number of compromised or apoptotic cells. For the live-cell gate, data are the average of 3 independent experiments ± SD: *P < .01. For the apoptotic cell gate, data are the average of 3 independent experiments ± SD: *P < .02. (C) EdU proliferation assay comparing day 15 HPs ± FICZ. After FICZ stimulation on day 7, EdU incorporation into treated HPs was significantly increased compared with untreated controls, indicative of increased proliferation. Data are the average of 3 independent experiments ± SD: *P < .01. (D) Representative phase-contrast images of HP population ± FICZ. (E) Growth curve of day 15 HPs ± 0.2 μm of FICZ. Cells were counted manually using trypan blue exclusion. Graphical data and the associated statistics are the result of 3 independent experiments per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/3/10.1182_blood-2012-11-466722/4/m_376f2.jpeg?Expires=1769341305&Signature=R6GSiBmXdNya-b5UEm8EP-VME1spN~kk-7jUHeG3z1tM-pMNFiWe8JqbYtiqdKRiUsE9SDcQEI6LB3Cx8UT4gO886931SNjpc~FxqZPTnC9Q84DWc6xjo1KamdxbEJmZk90-eJanh5An3Zc--jLlWvoy7Qwe~W86yRpvlUzyJYfKIP2hhkRzgLAtp09e435dc-g2a2GbpU-WdvuNWyIdJorxsOQHB3Bt06n9x3vOy8ApylRS9gGwK1Y1F7gqcXWuMZQXQ2DqFaHHgswmEZS-b7tMRMpD~WG0msCUBXvZDGegGkwGWmTzcNhrsyAR3BC3B7GRRk4lH6e72q-tDsL82w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. The AhR agonist FICZ inhibits apoptosis and allows for the exponential expansion of iPSC-derived HPs. (A) Representative FACS analysis of live vs dead or dying cells (propidium iodide [PI] vs Hoechst) from day 15 HPs ± FICZ. Plots were gated first in forward light scatter (FSC) vs side light scatter (SSC) and then from that population for PI+ and PI- Hoechst+. (B) FICZ increases the population of live cells, as delineated by FSC and SSC, and decreases the number of compromised or apoptotic cells. For the live-cell gate, data are the average of 3 independent experiments ± SD: *P < .01. For the apoptotic cell gate, data are the average of 3 independent experiments ± SD: *P < .02. (C) EdU proliferation assay comparing day 15 HPs ± FICZ. After FICZ stimulation on day 7, EdU incorporation into treated HPs was significantly increased compared with untreated controls, indicative of increased proliferation. Data are the average of 3 independent experiments ± SD: *P < .01. (D) Representative phase-contrast images of HP population ± FICZ. (E) Growth curve of day 15 HPs ± 0.2 μm of FICZ. Cells were counted manually using trypan blue exclusion. Graphical data and the associated statistics are the result of 3 independent experiments per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/3/10.1182_blood-2012-11-466722/4/m_376f2.jpeg?Expires=1769341306&Signature=JeGoLE7sWevtVtCdzPNH662327JR~XNaDDTtNMYgAhHPrd9qZY3CTjuGa5M1ipoY6tZ66M7XC1Di3f0GHdOSZzvpG8Y0aCMmIbJ0DMrRCRFmyDaLgkiZBDPKrhlMn4NeidxMLBqVr5aVbKvvKP1SXMzQW6CLcpRYWhA7fyLHCrE31iAXwlwns3gDqE61eS0yGUoQPv1bytFh5Yy6yeB0NtNbjP15hVR5qxKecEF-NlVnxd6yJ0ACdrQsD5afiDDMs5buq5tpHi8SUwiGEdYcCK2uo2-DE0RsTXJbF7hpf4mQjkrVmkSTsQyG6VtGq7lCROyR3Ib8AVDeEASFZicIRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)