Key Points
DOCK2-deficienct NK cells fail to effectively kill leukemia cells in vitro and major histocompatibility complex class I–deficient bone marrow cells in vivo.
Activating NK receptor–mediated Rac activation and the lytic synapse formation are severely impaired in DOCK2-deficient NK cells.
Abstract
Natural killer (NK) cells play an important role in protective immunity against viral infection and tumor progression, but they also contribute to rejection of bone marrow grafts via contact-dependent cytotoxicity. Ligation of activating NK receptors with their ligands expressed on target cells induces receptor clustering and actin reorganization at the interface and triggers polarized movement of lytic granules to the contact site. Although activation of the small GTPase Rac has been implicated in NK cell–mediated cytotoxicity, its precise role and the upstream regulator remain elusive. Here, we show that DOCK2, an atypical guanine nucleotide exchange factor for Rac, plays a key role in NK cell–mediated cytotoxicity. We found that although DOCK2 deficiency in NK cells did not affect conjugate formation with target cells, DOCK2-deficienct NK cells failed to effectively kill leukemia cells in vitro and major histocompatibility complex class I–deficient bone marrow cells in vivo, regardless of the sorts of activating receptors. In DOCK2-deficient NK cells, NKG2D-mediated Rac activation was almost completely lost, resulting in a severe defect in the lytic synapse formation. Similar results were obtained when the Rac guanine nucleotide exchange factor activity of DOCK2 was selectively abrogated. These results indicate that DOCK2-Rac axis controls NK cell–mediated cytotoxicity through the lytic synapse formation.
Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system that play an important role in protective immunity against viral infection and tumor progression but also contribute to rejection of bone marrow (BM) grafts via contact-dependent cytotoxicity.1,2 Activation of NK cells is regulated by a balance of activating and inhibitory signals delivered through the germ-line-encoded receptors that recognize ligands expressed on the surface of target cells.1,2 NK cells express multiple activating receptors including NKG2D, Ly49D, and the low-affinity receptor for immunoglobulin G (IgG), FcγRIIIa.3 NKG2D recognizes endogenous major histocompatibility complex (MHC) class I–related molecules such as Rae1α-Rae1ε, which were originally identified as retinoic acid–inducible proteins but were expressed at high levels primarily in virally infected cells and tumor cells.4 On the other hand, Ly49D triggers the killing of Chinese hamster ovary (CHO) cells by recognizing hamster MHC class I molecules,5 and FcγRIIIa is involved in antibody-dependent cell-mediated cytotoxicity (ADCC).1,2 Because these receptors lack cytoplasmic signaling elements, the delivery of activating signal is mediated through the associating transmembrane protein, such as DAP12, FcRγ, CD3ζ, and DAP10, depending on the receptor engaged.1,3,4
NK cell–mediated cytotoxicity involves the secretion of cytolytic effector molecules such as perforin and granzyme from specialized organelles that are known as lytic granules. Ligation of activating receptors with their ligands expressed on target cells induces receptor clustering at the interface and triggers polarized movement of lytic granules to the contact site.6 Because this lytic synapse formation requires remodeling of the actin cytoskeleton,6 activation of Rac may be involved in this process. Similar to other small GTPases, conversion of the GDP-bound Rac to the active state is catalyzed by guanine nucleotide exchange factors (GEFs).7 To date, Vav proteins (Vav1, Vav2, and Vav3) have been considered to be the major Rac GEFs acting downstream of activating NK receptors.8-12 Indeed, Vav1 deficiency in NK cells is sufficient to disrupt DAP10-mediated cytotoxicity, and lack of Vav2 and Vav3 profoundly impairs FcRγ- and DAP12-mediated cytotoxicity.11 However, in DAP10-mediated cytotoxicity, the importance of the Vav1–Grb2 interaction through the Src homology 3 (SH3) domains also has been demonstrated.13 Therefore, it remains unclear whether Vav proteins regulate NK cell–mediated cytotoxicity through the Rac GEF activity or the adaptor functions.
DOCK2 is a member of the CDM family of proteins (Caenorhabditis elegans CED-5, mammals DOCK180, and Drosophila melanogaster Myoblast City) and is predominantly expressed in hematopoietic cells.14 Although DOCK2 does not contain the Dbl homology (DH) domain typically found in GEFs,7 DOCK2 mediates the GTP–GDP exchange reaction for Rac via its DOCK homology region (DHR)-2 (also known as Docker) domain.15-17 DOCK2 is essential for chemokine receptor– and antigen receptor–mediated Rac activation and plays a key role in migration and activation of T cells and neutrophils.14,18-22 However, the role of DOCK2 in NK cell–mediated cytotoxicity remains unknown. In this study, we show that DOCK2-deficient (DOCK2−/−) NK cells fail to kill effectively leukemia cells in vitro and MHC class I–deficient BM cells in vivo, regardless of the sorts of activating receptors. Unlike Vav deficiency,11-13 lack of DOCK2 did not affect phosphorylations of Erk, Akt, SLP-76, and PLCγ2. However, NKG2D-mediated Rac activation was almost completely lost in DOCK2−/− NK cells, resulting in a severe defect in the lytic synapse formation. Our results thus indicate that DOCK2 is a Rac GEF critical for the lytic synapse formation during NK cell–mediated cytotoxic responses.
Methods
Mice
DOCK2−/− mice were backcrossed onto a C57BL/6 background for more than 8 generations before use,23 and age-matched and sex-matched C57BL/6 mice were used as wild-type (WT) controls. The knock-in mice expressing DOCK2-GFP fusion protein (DOCK2-GFP mice), transgenic mice expressing coxsackie-adenovirus receptor (CAR), and β2-microglobulin deficient (β2m−/−) mice have been described previously.20,24,25 Mice were kept under specific pathogen-free conditions in the animal facility of Kyushu University. The protocol of animal experiments was approved by the committee of Ethics of Animal Experiments, Kyushu University.
Flow cytometric analysis
To compare NK cell development, spleen or BM cells were stained with anti-NK1.1 (PK136; BD Pharmingen), anti-CD3ε (145-2C11; eBioscience), and anti-CD27 (LG.3A10; BD Pharmingen) antibodies in combination with anti-CD49b (DX5) (HM alpha2; BD Pharmingen) or anti-CD11b (M1/70; eBioscience) antibody. The expression of activating receptors in NK cells was analyzed by staining with anti-NKG2D (A10; eBioscience), anti-Ly49D (4E5; BioLegend), or anti-NKp46 (29A1.4; BD Pharmingen) antibody. Flow cytometric analysis was performed with FACS Calibur (BD Bioscience).
NK cell preparation and51Cr release assay
Splenic NK cells were purified with microbeads coated with anti-CD49b antibody (Miltenyi Biotec). Unless stated otherwise, isolated NK cells were cultured for 1 to 6 days in RPMI 1640 medium supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol (Nacalai tesque), 2 mM l-glutamine (Gibco), 100 U/mL penicillin (Gibco), 100 μg/mL streptomycin (Gibco), 1 mM sodium pyruvate (Gibco), MEM nonessential amino acids (Gibco), and 1000 U/mL interleukin 2 (IL-2; PeproTech) (complete RPMI medium). The purity of NK1.1+CD3ε– cells was above 83%, irrespective of DOCK2 expression and their activation status.
To measure NK cell–mediated cytotoxicity, target cells (5 × 106) were labeled with 3.7 MBq 51Cr for 1 hour. The labeled cells were washed 3 times with serum-free medium, resuspended in complete RPMI medium, and plated in 96-well V-bottom plates (1 × 104 cells/well). NK cells were added at a specified ratio and incubated for 4 hours. Maximum release was determined by incubating labeled target cells with 10% Triton X-100. The percentage of cytotoxicity was calculated with the following equation: 100 × (experimental release − spontaneous release) / (maximum release − spontaneous release). For CHO killing assays, NK cell activation was blocked by treating cells with anti-Ly49D antibody (4E5) before the assay. For ADCC, 51Cr labeled EL-4 cells were preincubated with anti-Thy1.2 antibody (30-H12; BD Bioscience) and washed before adding NK cells.
Assays for NKp46-mediated degranulation
IL-2-activated NK cells (1 × 105) were incubated in the presence of fluorescein isothiocyanate–conjugated anti-CD107a antibody (1D4B; BD Bioscience) for 4 hours in a 96-well flat bottom plate coated with anti-NKp46 antibody (10 μg/mL; R&D Systems). Cells were then stained for NK1.1 and CD3ε. Degranulation response was evaluated by analyzing the cell surface expression of CD107a.26
Cytokine production
IL-2 activated NK cells were cultured in 96-well U-bottom plates (1 × 105 cells/well) in the presence of IL-12 (10 ng/mL) or with YAC-1 cells at the ratio of 1:1. After 24 hours of culture, the supernatants were collected and the level of IFN-γ was measured, using the ELISA kit (Thermo Scientific).
Plasmids and transfection
The gene encoding Rae1ε (residues 1-702) lacking a putative glycosylphosphatidylinositol (GPI) attachment site (SVGLIFISLLFAFAFAM) was amplified by polymerase chain reaction, using the cDNA obtained from mouse dendritic cells as a template. enhanced green fluorescent protein (EGFP) was derived from pEGFP vector (Clontech). The GPI anchor domain coding DNA has been described previously27 and was prepared by annealing the following oligonucleotides: 5′-GATCCTATCAGCTGGTACTACCACTACGACCACTACAACGCTGTTGCTATTACTCCTATTGCTATTATTGCTCCTGTAGC-3′ and 5′-GGCCGCTACAGGAGCAATAATAGCAATAGGAGTAATAGCAACAGCGTTGTAGTGGTCGTAGTGGTAGTACCAGCTGATAG-3′. The C-terminally truncated Rae1ε, EGFP, and GPI anchor fragments were cloned into a pBJ1 expression vector, which was used for transfection into EL-4 cells by electroporation. The adenoviral vector pAd-DOCK2-GFP with or without mutation was transfected into human embryonic kidney–293A cells (Invitrogen) to amplify the recombinant adenovirus. The resultant virus was purified with cesium chloride centrifugation and was used to infect CAR-expressing DOCK2−/− NK cells. After infection, cells were washed 3 times and cultured for 2 days with complete RPMI medium.
BM transplantation, colony-forming unit (CFU) assay, and histology
A day after lethal irradiation (9.5 Gy), DOCK2−/− or WT mice received 3 × 106 BM cells from β2m−/− or WT mice intravenously. Four days later, recipients were killed, and spleen cells (1 × 105) were cultured in α-modification of Eagle medium with nucleosides (α-MEM; Gibco), supplemented with 0.86% methyl cellulose (Wako), 30% fetal calf serum, 250 μM 2-mercaptoethanol, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 U/mL GM-CSF or IL-3 (both from PeproTech). Triplicate cultures were maintained for another 4 days. Cell aggregates containing 25 cells or more were scored as individual colonies. For histology, splenic sections were stained with hematoxylin and eosin.
Cell conjugation assay
Target cells and NK cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) and PKH26 (Sigma-Aldrich), respectively. After washing, labeled NK cells (3.5 × 104) were mixed with CFSE-loaded target cells at a ratio of 1:1, centrifuged, and incubated for 10, 20, or 40 minutes at 37°C. After suspending gently, cells were analyzed on a FACS Calibur.
Immunofluorescence staining and confocal imaging
NK cells (5 × 105) and target cells were mixed at a ratio of 1:1 and centrifuged at 500 rpm for 5 minutes. Then, cells were gently resuspended and transferred to a poly-d-lysine-coated dish and incubated for specified lengths of time. Cells were then fixed with 4% paraformaldehyde for 10 minutes and permeabilized with 0.1% Triton X-100 in PBS for 5 minutes. After blocking with 1% bovine serum albumin in PBS for 1 hour, cells were stained with Alexa Fluor 546-phalloidin (Invitrogen), anti-perforin (CB5.4; Enzo Life Science), and/or anti-GFP antibody (Invitrogen), followed by appropriately labeled secondary antibodies. Cells were washed with PBS and mounted with fluorescent mounting medium (Dako). Samples also were analyzed with a laser scanning confocal microscope (LSM510 META; Carl Zeiss).
Pull-down assay, immunoprecipitation, and immunoblotting
NK cells were incubated for 30 minutes on ice with anti-NKG2D (10 μg/mL; A10) or anti-NKp46 antibody (20 μg/mL; R&D Systems). Cells were then stimulated by cross-linking these antibodies with anti-hamster IgG1 antibody (20 μg/mL; G94-56; BD Pharmingen; for anti-NKG2D antibody) or anti-goat IgG antibody (40 μg/mL; Jackson ImmunoResearch Laboratories; for anti-NKp46 antibody) at 37°C for the specified times. To assess Rac activation, cell extracts (350-560 μg) were incubated with glutathione S-transferase (GST)-fusion Rac-binding domain (RBD) of PAK1 at 4°C for 1 hour. The GST-PAK1-RBD-bound proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and blots were probed with anti-Rac1 antibody (23A8; Millipore). To analyze tyrosine phosphorylation of Vav or PLCγ2, cell extracts (250-660 μg) were immunoprecipitated with anti-Vav (C-14; Santa Cruz) or anti-PLCγ2 (Q-20; Santa Cruz) antibody, and the precipitates were analyzed with antiphosphotyrosine antibody (pY99; Santa Cruz). Activation of Erk, JNK, SLP76, and Akt were assessed with the phosphorylation-specific antibodies against Thr202/Tyr204 of Erk (D13.14.4E; Cell Signaling), Thr183/Tyr185 of JNK (G9; Cell Signaling), Tyr128 of SLP76 (BD Pharmingen), and Ser473 of Akt (D9E; Cell Signaling), respectively, using 60-140 μg of NK cell extracts.
Statistical analysis
Statistical analysis was performed using analysis of 2-tailed student’s t test.
Results
DOCK2 deficiency does not affect NK cell development
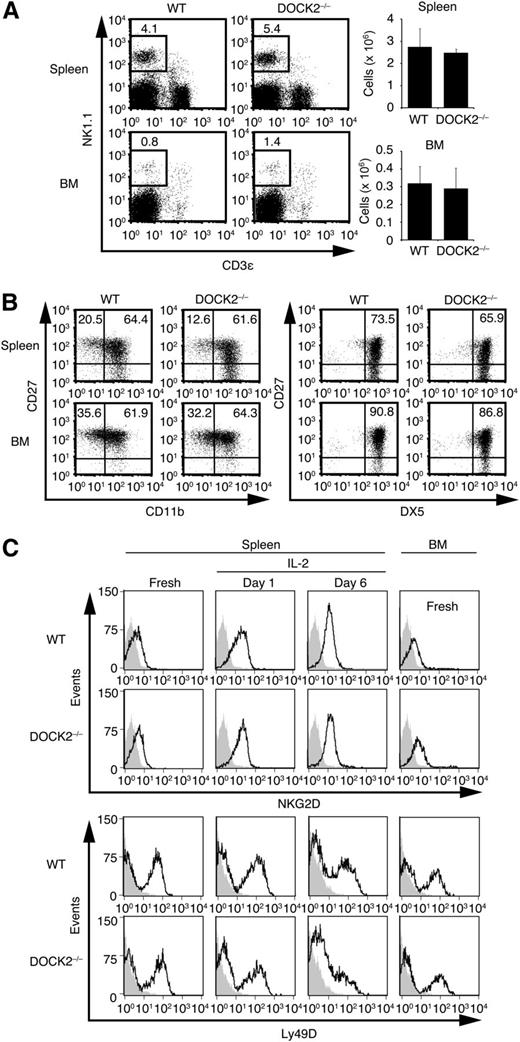
DOCK2 is expressed in all hematopoietic cells tested, including NK cells (supplemental Figure 1). To examine the role of DOCK2 in NK cell functions, we first compared the NK cell numbers between WT and DOCK2−/− mice that had been backcrossed onto a C57BL/6 background. The numbers of NK cells in the spleen and the BM were comparable between these mouse lines (Figure 1A). Although murine NK cells can be divided into functionally distinct subsets on the basis of their surface expression of CD27, CD11b, and DX5,28 the proportion of CD27highCD11bhighDX5high mature NK cells was unchanged between WT and DOCK2−/− mice (Figure 1B). In addition, WT and DOCK2−/− NK cells expressed similar amounts of NKG2D, Ly49D, and another activating receptor NKp46 on their surface, in terms of the intensity or the percentage (Figure 1C and supplemental Figure 2A). These results indicate that DOCK2 deficiency does not affect NK cell development and the surface expression of activating receptors.
DOCK2 deficiency does not affect NK cell development and surface expression of activating receptors. (A) Spleen cells and BM cells were stained for NK1.1 and CD3ε, and the number of NK cells (NK1.1+CD3ε– cells) was compared between WT and DOCK2−/− mice. Data are expressed as the mean ± standard deviation (SD) of 3 mice. (B) The expression of CD27, CD11b, and/or DX5 on NK1.1+CD3ε– NK cells in the spleen and BM was compared between WT and DOCK2−/− mice. Data are representative of 2 independent experiments. (C) Fresh or activated NK cells with IL-2 (1000 U/mL) for the indicated times were prepared from WT and DOCK2−/− mice, and the expression levels of NKG2D and Ly49D were compared by staining the cells with relevant antibody followed by Alexa Fluor 488–labeled secondary antibody. As a control, the flow cytometric profile for cells stained with the secondary antibody only is shown. Data are representative of 2 independent experiments.
DOCK2 deficiency does not affect NK cell development and surface expression of activating receptors. (A) Spleen cells and BM cells were stained for NK1.1 and CD3ε, and the number of NK cells (NK1.1+CD3ε– cells) was compared between WT and DOCK2−/− mice. Data are expressed as the mean ± standard deviation (SD) of 3 mice. (B) The expression of CD27, CD11b, and/or DX5 on NK1.1+CD3ε– NK cells in the spleen and BM was compared between WT and DOCK2−/− mice. Data are representative of 2 independent experiments. (C) Fresh or activated NK cells with IL-2 (1000 U/mL) for the indicated times were prepared from WT and DOCK2−/− mice, and the expression levels of NKG2D and Ly49D were compared by staining the cells with relevant antibody followed by Alexa Fluor 488–labeled secondary antibody. As a control, the flow cytometric profile for cells stained with the secondary antibody only is shown. Data are representative of 2 independent experiments.
DOCK2 is required for NK cell–mediated cytotoxicity and cytokine production
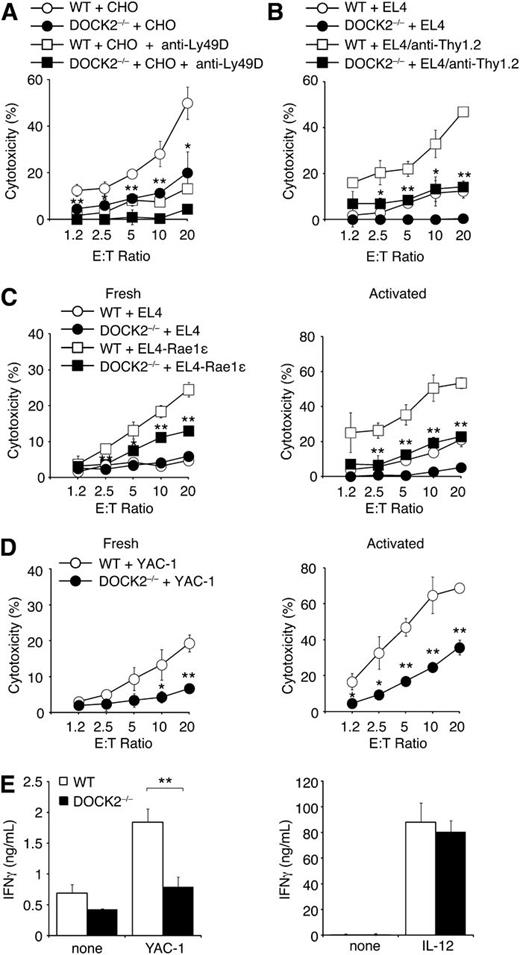
Next we examined the role of DOCK2 in NK cell–mediated cytotoxicity, using various target cells. Ly49D is known to signal mainly through DAP12.1 When WT NK cells were incubated with CHO cells, they effectively killed CHO cells in a manner dependent on Ly49D (Figure 2A). Similarly, WT NK cells killed EL-4 cells coated with anti-Thy1.2 antibody (Figure 2B). However, both Ly49D/DAP12-mediated cytotoxicity and FcγRIIIa-mediated ADCC were severely impaired in the absence of DOCK2 (Figure 2A,B). Similar to FcγRIIIa, NKp46 transmits the signal via FcRγ and CD3ζ,1 yet its endogenous ligands remain unclear. To examine the role of DOCK2 in NKp46-mediated cytotoxicity, we monitored surface exposure of the lytic granule marker CD107a. Although cell surface expression of CD107a in WT NK cells markedly increased in response to anti-NKp46 antibody stimulation, only a modest increase was observed in the case of DOCK2−/− NK cells (supplemental Figure 2B). These results suggest that DOCK2 deficiency also impairs NKp46-mediated cytotoxicity.
NK cell–mediated cytotoxicity is impaired in the absence of DOCK2. (A) WT and DOCK2−/− NK cells were incubated with CHO cells at the indicated ratio in the presence or absence of anti-Ly49D blocking antibody (10 μg/mL). (B) WT and DOCK2−/− NK cells were incubated with EL4 cells with or without pretreatment with anti-Thy1.2 antibody (10 μg/mL) at the indicated ratio. (C) Fresh (left) or in vitro-activated (right) WT and DOCK2−/− NK cells were incubated with EL-4 or EL4-Rae1ε cells at the indicated ratio. (D) Fresh (left) or in vitro-activated (right) WT and DOCK2−/− NK cells were incubated with YAC-1 cells at the indicated ratio. (E) In vitro–activated WT and DOCK2−/− NK cells were incubated with YAC-1 cells at the ratio of 1:1 or cultured alone in the presence of IL-12 to measure IFN-γ production. In (A–E), data are expressed as the mean ± SD of triplicate wells and are representative of at least 2 independent experiments. *P < .05; **P < .01.
NK cell–mediated cytotoxicity is impaired in the absence of DOCK2. (A) WT and DOCK2−/− NK cells were incubated with CHO cells at the indicated ratio in the presence or absence of anti-Ly49D blocking antibody (10 μg/mL). (B) WT and DOCK2−/− NK cells were incubated with EL4 cells with or without pretreatment with anti-Thy1.2 antibody (10 μg/mL) at the indicated ratio. (C) Fresh (left) or in vitro-activated (right) WT and DOCK2−/− NK cells were incubated with EL-4 or EL4-Rae1ε cells at the indicated ratio. (D) Fresh (left) or in vitro-activated (right) WT and DOCK2−/− NK cells were incubated with YAC-1 cells at the indicated ratio. (E) In vitro–activated WT and DOCK2−/− NK cells were incubated with YAC-1 cells at the ratio of 1:1 or cultured alone in the presence of IL-12 to measure IFN-γ production. In (A–E), data are expressed as the mean ± SD of triplicate wells and are representative of at least 2 independent experiments. *P < .05; **P < .01.
Murine NK cells express 2 isoforms of NKG2D: NKG2D-L, and NKG2D-S.3,4 NKG2D-L signals through DAP10, whereas NKG2D-S signals through both DAP10 and DAP12.29,30 The relative proportion of NKG2D-L and NKG2D-S in NK cells varies on in vitro activation with IL-2. Indeed, it is known that freshly isolated NK cells predominantly express NKG2D-L, whereas in vitro activation with IL-2 leads to an increase in NKG2D-S expression.29 We prepared fresh and in vitro–activated NK cells from WT and DOCK2−/− mice and examined their cytotoxicity against EL-4 or EL-4 transfectants expressing the NKG2D ligand Rae1ε as a GFP-fused form (EL4-Rae1ε). Both types of WT NK cells killed EL4-Rae1ε, but not EL4, indicating that this cytotoxicity is mainly mediated by NKG2D (Figure 2C). However, DOCK2−/− NK cells failed to kill effectively EL4-Rae1ε under the both culture conditions (Figure 2C). Similar results were obtained when NK cell–mediated cytotoxicity was tested for YAC-1 cells, which also express NKG2D ligands (Figure 2D).11 Collectively, these results indicate that DOCK2 regulates NK cell-mediated cytotoxicity, regardless of the sorts of activating receptors and adaptor molecules.
In addition to cytotoxicity, an important NK effector function is cytokine production.2 When IL-2 activated WT NK cells were incubated with YAC-1 cells, they produced considerable amounts of IFN-γ (Figure 2E). However, IFN-γ production by DOCK2−/− NK cells was significantly reduced under the same condition (Figure 2E). In contrast, WT and DOCK2−/− NK cells comparably produced IFN-γ in response to IL-12 stimulation (Figure 2E). These results suggest that DOCK2 is also required for activating receptor-mediated cytokine production.
DOCK2 deficiency enables engraftment of MHC class I–deficient BM cells
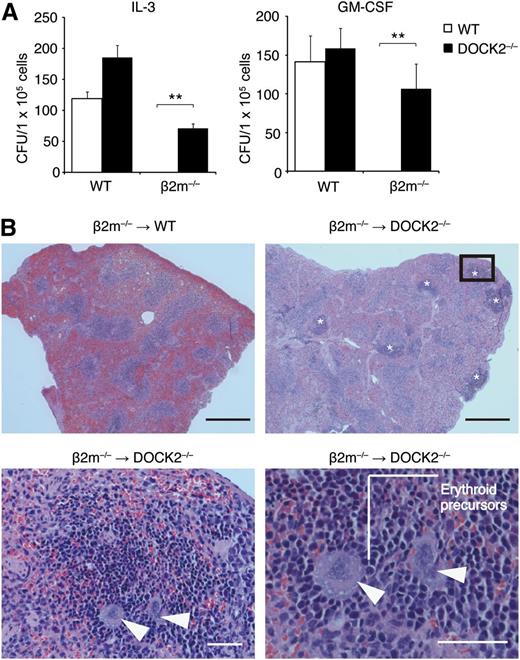
Although NK cells are beneficial in host protection against viral infection and tumor progression, irradiation-resistant NK cells are also involved in rejection of BM cell grafts.31-33 To examine the effect of DOCK2 deficiency on BM transplantation, we used β2m−/− C57BL/6 mice as donors. As β2m−/− BM cells lack the expression of MHC class I molecules that serve as ligands for several inhibitory NK receptors,1,2 they are highly susceptible to NK cell-mediated cytotoxicity. When β2m−/− BM cells were transferred into lethally irradiated C57BL/6 mice, they were rejected within 4 days (Figure 3A). However, β2m−/− BM cells transplanted into DOCK2−/− C57BL/6 mice successfully reconstituted hematopoiesis in splenic CFU assays (Figure 3A). Consistent with this finding, histologic examination revealed that extramedullary hematopoiesis with erythroid islands and large megakaryocytes occurred in splenic red pulp of DOCK2−/−, but not WT, mice transplanted with β2m−/− BM cells (Figure 3B). In contrast, no rejection was found when syngeneic control BM cells were transferred into WT and DOCK2−/− mice (Figure 3A). These results indicate that DOCK2−/− NK cells fail to kill effectively MHC class I–deficient BM cells in vivo.
DOCK2 deficiency enables engraftment of MHC class I–deficient BM cells. (A) Four days after intravenous injection of WT or β2m−/− BM cells into lethally irradiated WT (n = 3) or DOCK2−/− (n = 3) mice, splenic CFU assays were performed in the presence of IL-3 (50 U/mL) or GM-CSF (50 U/mL). Data are expressed as the mean ± SD of 3 mice, and are representative of 2 independent experiments. **P < .01. (B) Four days after intravenous injection of β2m−/− BM cells into lethally irradiated WT or DOCK2−/− mice, splenic sections were stained with hematoxylin and eosin. Data are representative of 6 mice per category. Asterisks or arrowheads indicate extramedullary hematopoiesis or megakaryocytes, respectively. The lower panels indicate high-magnification images of the boxed area. Black bar, 500 μm; White bar, 50 μm.
DOCK2 deficiency enables engraftment of MHC class I–deficient BM cells. (A) Four days after intravenous injection of WT or β2m−/− BM cells into lethally irradiated WT (n = 3) or DOCK2−/− (n = 3) mice, splenic CFU assays were performed in the presence of IL-3 (50 U/mL) or GM-CSF (50 U/mL). Data are expressed as the mean ± SD of 3 mice, and are representative of 2 independent experiments. **P < .01. (B) Four days after intravenous injection of β2m−/− BM cells into lethally irradiated WT or DOCK2−/− mice, splenic sections were stained with hematoxylin and eosin. Data are representative of 6 mice per category. Asterisks or arrowheads indicate extramedullary hematopoiesis or megakaryocytes, respectively. The lower panels indicate high-magnification images of the boxed area. Black bar, 500 μm; White bar, 50 μm.
DOCK2 is critical for the lytic synapse formation
To explore the mechanism by which DOCK2 controls NK cell–mediated cytotoxicity, we first compared conjugate formation with target cells between WT and DOCK2−/− NK cells. Although it has been reported that NK cells lacking all Vav proteins exhibit nearly abolished conjugate formation,34 DOCK2−/− NK cells formed conjugates with YAC-1 and EL4-Rae1ε cells at comparable efficiency to that of WT NK cells at any times tested (Figure 4A and supplemental Figure 3). Thus, unlike Vav deficiency, DOCK2 deficiency in NK cells did not affect conjugate formation with target cells.
DOCK2 deficiency impairs the lytic synapse formation. (A) PKH26-labeled WT and DOCK2−/− NK cells were mixed with CFSE-loaded target cells, YAC-1 and EL4-Rae1ε, for 10 minutes at a ratio of 1:1, and conjugate formation was analyzed without fixation. Data are representative of 2 independent experiments. (B) WT and DOCK2−/− NK cells were mixed with EL4-Rae1ε cells for the indicated times to analyze localization of GFP-tagged Rae1ε in the conjugates. Scale bar, 5 μm. Data are expressed as the percentages of the conjugates with polarized accumulation of Rae1ε (the mean ± SD) of 3 independent experiments. In each experiment, more than 30 cells were analyzed per group. **P < .01. (C) WT and DOCK2−/− NK cells were mixed with YAC-1 cells for the indicated times to analyze localization of perforin and F-actin in the conjugates. Scale bar, 5 μm. (D) After pretreatment with latrunculin B (8 μg/mL) for 2 hours, WT NK cells were mixed with YAC-1 cells for the indicated times, and the effect of blockade of actin polymerization on perforin localization was analyzed. Scale bar, 5 μm. Mock, DMSO. In (C,D), data are expressed as the percentages of the conjugates with polarized accumulation of perforin or F-actin (the mean ± SD) of 3 independent experiments. In each experiment, more than 30 cells were analyzed per group. *P < .05; **P < .01.
DOCK2 deficiency impairs the lytic synapse formation. (A) PKH26-labeled WT and DOCK2−/− NK cells were mixed with CFSE-loaded target cells, YAC-1 and EL4-Rae1ε, for 10 minutes at a ratio of 1:1, and conjugate formation was analyzed without fixation. Data are representative of 2 independent experiments. (B) WT and DOCK2−/− NK cells were mixed with EL4-Rae1ε cells for the indicated times to analyze localization of GFP-tagged Rae1ε in the conjugates. Scale bar, 5 μm. Data are expressed as the percentages of the conjugates with polarized accumulation of Rae1ε (the mean ± SD) of 3 independent experiments. In each experiment, more than 30 cells were analyzed per group. **P < .01. (C) WT and DOCK2−/− NK cells were mixed with YAC-1 cells for the indicated times to analyze localization of perforin and F-actin in the conjugates. Scale bar, 5 μm. (D) After pretreatment with latrunculin B (8 μg/mL) for 2 hours, WT NK cells were mixed with YAC-1 cells for the indicated times, and the effect of blockade of actin polymerization on perforin localization was analyzed. Scale bar, 5 μm. Mock, DMSO. In (C,D), data are expressed as the percentages of the conjugates with polarized accumulation of perforin or F-actin (the mean ± SD) of 3 independent experiments. In each experiment, more than 30 cells were analyzed per group. *P < .05; **P < .01.
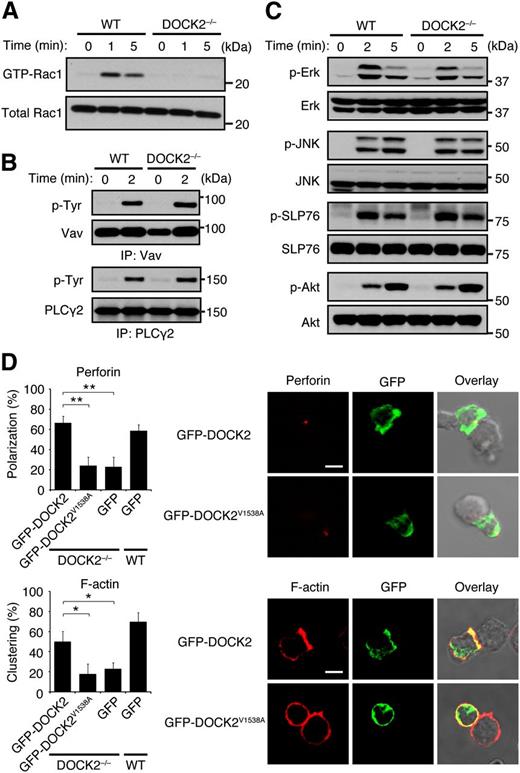
This finding led us to examine whether DOCK2 is involved in the lytic synapse formation. When WT NK cells were incubated with EL4-Rae1ε cells, GFP-fused Rae1ε accumulated at the interface in the majority of the conjugates (Figure 4B). However, such localization was scarcely found in the absence of DOCK2 (Figure 4B), indicating that DOCK2 is required for NKG2D clustering at the interface with target cells. In addition, we found that DOCK2 deficiency in NK cells severely impaired actin polymerization and perforin accumulation at the interface (Figure 4C). Actin polymerization seemed to precede perforin redistribution, because pretreatment of WT NK cells with latrunculin B, an inhibitor of actin assembly, impaired polarized movement of perforin to the contact site (Figure 4D).
DOCK2 regulates the lytic synapse formation through Rac activation
To obtain the evidence that DOCK2 acts as a Rac GEF downstream of activating NK receptors, we compared NKG2D-mediated Rac activation between WT and DOCK2−/− NK cells. When WT NK cells were stimulated with anti-NKG2D antibody, activated Rac1 was readily detected (Figure 5A). However, such Rac activation was almost completely lost in DOCK2−/− NK cells (Figure 5A), indicating that DOCK2 is a major Rac GEF acting downstream of NKG2D. Although it has been reported that tyrosine phosphorylation of Vav augments its Rac GEF activity,35 NKG2D-mediated Vav phosphorylation was unchanged between WT and DOCK2−/− NK cells (Figure 5B). In addition, we found that NKG2D-mediated phosphorylations of Erk, Akt, SLP-76, and PLCγ2, all of which are known to be defective in Vav-deficient NK cells,11-13 occurred normally in DOCK2−/− NK cells (Figure 5B,C). Similar results were obtained when NKp46-mediated signals were compared between WT and DOCK2−/− NK cells (supplemental Figure 4A,B).
DOCK2 regulates the lytic synapse formation through Rac activation. (A) NKG2D-mediated Rac activation was compared between WT and DOCK2−/− NK cells. (B) NKG2D-mediated phosphorylations of Vav and PLCγ2 were compared between WT and DOCK2−/− NK cells. (C) NKG2D-mediated phosphorylations of Erk, JNK, SLP76, and Akt were compared between WT and DOCK2−/− NK cells. (D) After adenoviral transfer of GFP-tagged WT DOCK2, DOCK2V1538A, or GFP alone, WT and DOCK2−/− NK cells were mixed with YAC-1 cells for 5 minutes to analyze localization of perforin and F-actin in the conjugates. Scale bar, 5 μm. Data are expressed as the percentages of the conjugates with polarized accumulation of perforin or F-actin (the mean ± SD) of 3 independent experiments. In each experiment, more than 30 cells were analyzed per group. *P < .05; **P < .01.
DOCK2 regulates the lytic synapse formation through Rac activation. (A) NKG2D-mediated Rac activation was compared between WT and DOCK2−/− NK cells. (B) NKG2D-mediated phosphorylations of Vav and PLCγ2 were compared between WT and DOCK2−/− NK cells. (C) NKG2D-mediated phosphorylations of Erk, JNK, SLP76, and Akt were compared between WT and DOCK2−/− NK cells. (D) After adenoviral transfer of GFP-tagged WT DOCK2, DOCK2V1538A, or GFP alone, WT and DOCK2−/− NK cells were mixed with YAC-1 cells for 5 minutes to analyze localization of perforin and F-actin in the conjugates. Scale bar, 5 μm. Data are expressed as the percentages of the conjugates with polarized accumulation of perforin or F-actin (the mean ± SD) of 3 independent experiments. In each experiment, more than 30 cells were analyzed per group. *P < .05; **P < .01.
The valine residue at position 1538 of DOCK2 DHR-2 is known to function as a nucleotide sensor,17 and the DOCK2 mutant encoding alanine instead of valine at this position (DOCK2V1538A) almost completely lacks the Rac GEF activity.36 To examine whether DOCK2 regulates the lytic synapse formation by acting as the Rac GEF, we wanted to express GFP-tagged WT DOCK2 and DOCK2V1538A in DOCK2−/− NK cells. For this purpose, we crossed DOCK2−/− mice with transgenic mice expressing the gene encoding CAR under the Lck promoter24 and developed the experimental system with which full-length DOCK2 can be expressed in DOCK2−/− NK cells by adenoviral transfer.36 When WT DOCK2 was expressed in DOCK2−/− NK cells, accumulation of F-actin and perforin to the synapse markedly improved compared with the control expressing GFP alone (Figure 5D). However, the expression of DOCK2V1538A in DOCK2−/− NK cells failed to rescue the defect in the lytic synapse formation (Figure 5D) in spite of the fact that WT DOCK2 and DOCK2V1538A were comparably expressed in these cells (supplemental Figure 5). In addition, we found that the lytic synapse formation was impaired in WT NK cells by treating them with CPYPP, a small-molecule inhibitor of DOCK2 that binds to the DOCK2 DHR-2 domain and inhibits its Rac GEF activity (supplemental Figure 6).37 These results indicate that DOCK2 regulates lytic synapse formation through Rac activation.
DOCK2 is recruited to the synapse in a manner dependent on the activity of phosphatidylinositol 3-kinases (PI3Ks)
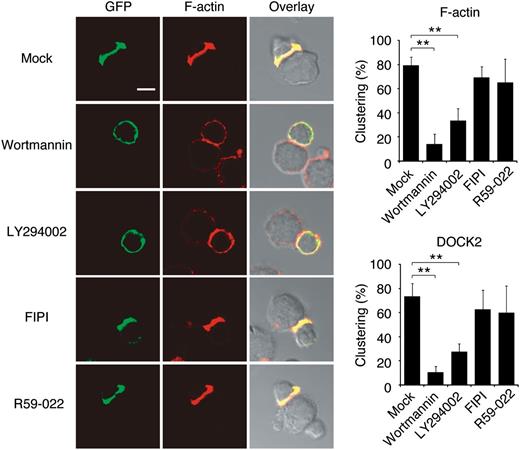
Having found that DOCK2 regulates lytic synapse formation through Rac activation, we next examined how intracellular DOCK2 dynamics are regulated during NK cell-mediated cytotoxicity. When NK cells from DOCK2-GFP mice were incubated with YAC-1 cells, DOCK2 rapidly accumulated at the interface in the majority of the conjugates (Figure 6). However, this polarized localization of DOCK2 was inhibited by treating NK cells with PI3K inhibitors, wortmannin and LY294002 (Figure 6). Because wortmannin treatment did not affect NKG2D-mediated Rac activation in a biochemical assay (supplemental Figure 7), it seems likely that PI3K activity is required to recruit DOCK2 to the interface and activate Rac locally for the lytic synapse formation. In contrast, although DOCK2 binds to phosphatidic acid through the C-terminal polybasic amino acid cluster,22 the inhibitor specific for phospholipase D or diacylglycerol kinase, 2 major enzymes that produce signaling pools of phosphatidic acid,38 did not affect DOCK2 localization during the lytic synapse formation.
DOCK2 is recruited to the synapse in a manner dependent on the PI3K activity. NK cells from DOCK2-GFP mice were treated with wortmannin (100 nM), LY294002 (10 μM), FIPI (750 nM), R59-022 (10 μM), or Mock (DMSO) for 30 minutes at 37°C. Cells were then mixed with YAC-1 cells for 5 minutes to analyze localization of DOCK2 (GFP) and F-actin in the conjugates. Scale bar, 5 μm. Data are expressed as the percentages of the conjugates with polarized accumulation of F-actin or DOCK2 (the mean ± SD) of 3 independent experiments. In each experiment, more than 30 cells were analyzed per group. **P < .01.
DOCK2 is recruited to the synapse in a manner dependent on the PI3K activity. NK cells from DOCK2-GFP mice were treated with wortmannin (100 nM), LY294002 (10 μM), FIPI (750 nM), R59-022 (10 μM), or Mock (DMSO) for 30 minutes at 37°C. Cells were then mixed with YAC-1 cells for 5 minutes to analyze localization of DOCK2 (GFP) and F-actin in the conjugates. Scale bar, 5 μm. Data are expressed as the percentages of the conjugates with polarized accumulation of F-actin or DOCK2 (the mean ± SD) of 3 independent experiments. In each experiment, more than 30 cells were analyzed per group. **P < .01.
Discussion
In this study, we investigated the physiological functions of DOCK2 in NK cells. We found that although DOCK2 deficiency in NK cells did not affect conjugate formation with target cells, DOCK2−/− NK cells failed to effectively kill leukemia cells in vitro and MHC class I–deficient BM cells in vivo, regardless of the sorts of activating receptors. Our results thus indicate that DOCK2 plays a key role in NK cell–mediated cytotoxicity.
So far, Vav proteins have been considered to be the Rac GEFs acting downstream of activating NK receptors.8-12 However, as Vav functions as an adaptor molecule and can assemble signaling complexes independent of its action on Rac activation,39 it was still unclear whether Vav proteins regulate NK cell-mediated cytotoxicity by acting as the Rac GEFs. Here we have shown that DOCK2 deficiency in NK cells almost totally abolishes NKG2D-mediated Rac activation without affecting Vav phosphorylation. This finding clearly indicates that DOCK2 is a major Rac GEF acting downstream of NKG2D and regulates Rac activation independently of the Vav activity. We also found that NKG2D-mediated phosphorylations of Erk, Akt, SLP-76, and PLCγ2, all of which are known to be defective in Vav-deficient NK cells,11-13 occur normally in DOCK2−/− NK cells. Therefore, these signaling pathways are likely to be regulated by Vav proteins, independent of Rac activation and probably through the adaptor functions.
Although it has been shown that the expression of dominant-negative Rac mutant impairs NK cell–mediated cytotoxicity,8,40,41 the precise role of Rac activation in this process was unknown. Ligation of activating NK receptors with their ligands expressed on target cells induces receptor clustering and actin reorganization at the interface and triggers polarized movement of perforin to the contact site.6 We found that this lytic synapse formation was severely impaired in DOCK2−/− NK cells. This defect was rescued by expressing WT DOCK2, but not DOCK2V1538A, which lacks the Rac GEF activity, indicating that DOCK2 regulates the lytic synapse formation through Rac activation. Because blockade of actin polymerization prevents accumulation of perforin at the interface, it is clear that the integrity and reorganization of the actin cytoskeleton is required for polarization of lytic granules to the synapse. It is thus suggested that the primary function of Rac activation in NK cell–mediated cytotoxicity is to induce F-actin assembly at the interface and to trigger the lytic granule polarization.
Several lines of evidence indicate that the PI3K activity is required for NK cell–mediated cytotoxicity and the lytic synapse formation.13,40,42 NKG2D stimulation induces accumulation of phosphatidylinositol-3,4,5-triphosphate (PIP3), a lipid product of PI3Ks, at the interface with target cells.42 However, the pathway linking PIP3 production to actin polymerization remained to be determined. In this study, we have shown that DOCK2 is recruited to the synapse in a manner dependent on the PI3K activity. Although DOCK2 does not contain the pleckstrin homology domain typically found in GEFs,7 DOCK2 binds to PIP3 through the DHR-1 domain.20 Therefore, it seems likely that DOCK2 translocates to the synapse through the interaction of DHR-1 domain with PIP3. Our results thus suggest that PI3Ks regulate the lytic synapse formation, at least in part, by controlling DOCK2 localization.
In summary, we have shown that DOCK2 is a Rac GEF acting downstream of activating NK receptors and critically regulates NK cell–mediated cytotoxicity through the lytic synapse formation. Although NK cells play an important role in protective immunity, NK cell–mediated cytotoxicity is involved in transplant rejection and autoimmune diseases. Because DOCK2 expression is limited to hematopoietic cells, DOCK2 may be a novel therapeutic target for controlling these immunological disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Akira Shibuya for helpful comments.
This work was supported by the Core Research for Evolutional Science and Technology program (Y.F.) and the Strategic Japanese-Swiss Cooperative Program (Y.F.) of Japan Science and Technology Agency; Grants-in-Aid for Scientific Research (Y.F.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and Grants-in-Aid for Scientific Research (Y.F.) from the Japan Society for the Promotion of Science.
Authorship
Contribution: Y.F. designed research; Y.S., Y.T., T.Y., M.W., X.D., M.T., A.N., and F.S. performed research; Y.S. and Y.T. analyzed data; and Y.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshinori Fukui, Division of Immunogenetics, Department of Immunobiology and Neuroscience, Medical Institute of Bioregulation, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; email: fukui@bioreg.kyushu-u.ac.jp.