Key Points
Replacing rituximab with ofatumumab in second-line therapy for intermediate grade lymphoma does not increase toxicity; ORR/CR are encouraging.
An ongoing randomized phase III trial will compare rituximab with ofatumumab, combined with chemotherapy, in relapsed or refractory DLBCL.
Abstract
Standard treatment of transplant-eligible patients with relapsed diffuse large B-cell lymphoma (DLBCL) consists of rituximab and platinum-based chemotherapy, either ifosfamide, carboplatin, and etoposide (ICE) or dexamethasone, cytarabine, and cisplatin (DHAP), with autologous transplant consolidation for those with chemosensitive disease. Nonetheless, outcomes are suboptimal for patients failing rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). We performed a multi-center phase II trial investigating the safety and efficacy of ofatumumab, a monoclonal antibody against CD20, combined with ICE or DHAP second-line therapy in patients with relapsed or refractory DLBCL, grade 3b follicular lymphoma, or transformed follicular lymphoma. Sixty-one patients were treated with either ofatumumab-ICE (35) or ofatumumab-DHAP (26). The overall response rate (ORR) was 61%, and the complete response (CR) rate was 37%. In patients with 2 or 3 adverse risk factors according to the second-line, age-adjusted, international prognostic index, the ORR was 59% and CR 31%, and in patients with early-relapsing or primary refractory disease, the ORR was 55% and CR 30%. Toxicity was largely hematologic, and stem cell mobilization was successful in 43 of 45 patients. Substitution of ofatumumab for rituximab in standard second-line regimens following failure of R-CHOP is a promising approach. This trial was registered at www.clinicaltrials.gov as NCT00823719.
Introduction
Outcomes in the treatment of diffuse large B-cell lymphoma (DLBCL) and other intermediate grade B-cell non-Hodgkin’s lymphomas (NHLs) have significantly improved in the rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) era. Population-based data from Vancouver showed an improvement in 2-year progression-free survival (PFS) of 51% to 69% and in 2-year overall survival (OS) from 52% to 78% following the routine inclusion of rituximab in first-line therapy.1 Data from the Surveillance, Epidemiology, and End Results database in the United States showed an improvement in median OS from 20 to 47 months during the last 2 decades.2 Despite this marked improvement, relapsed or refractory disease continues to carry a poor prognosis, even among transplant-eligible patients. Indeed, the effectiveness of standard second-line therapy and consolidative high-dose therapy with autologous stem cell rescue (HDT/ASCR) in patients who failed R-CHOP or R-CHOP–like therapy was elucidated by the Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) trial, a randomized comparison of rituximab and ifosfamide, carboplatin, and etoposide to rituximab and dexamethasone, cytarabine, and cisplatin (R-DHAP) followed by consolidative HDT/ASCR, with a second randomization to posttransplant maintenance rituximab or observation.3 Patients with an initial remission duration of <1 year or those primarily refractory to R-CHOP had a 51% overall response rate (ORR) and a 3-year PFS of 23%. However, among responding patients who were able to proceed to autologous transplant, the 3-year PFS was 39% compared with 14% among those who did not undergo autologous transplantation.
Possible reasons underlying the suboptimal effectiveness of second-line rituximab-based therapy include resistance to rituximab, which may be mediated by changes in apoptotic thresholds, sensitivity to complement-mediated cytotoxicity, antibody-dependent cellular cytotoxicity, as well as mutation in or down-regulation of CD20.4 Ofatumumab is a human monoclonal IgG1κ antibody against CD20 that targets a different epitope than rituximab; in vitro data suggest that ofatumumab induces more potent complement-mediated cytotoxicity than rituximab, and clinical data demonstrate activity of ofatumumab in rituximab-refractory indolent lymphomas.5-11 Substitution of ofatumumab for rituximab in second-line therapy for intermediate grade NHL following failure of first-line rituximab-containing therapy can potentially circumvent or overcome rituximab resistance, thereby improving response rates, the ability to proceed to consolidative HDT/ASCR, and ultimately, OS.
Methods
Patients
Patients eligible for participation in this study had a diagnosis of relapsed or primary refractory DLBCL, grade 3b follicular lymphoma, or transformed follicular lymphoma. CD20 positivity must have been confirmed at initial diagnosis and also when a diagnostic biopsy or aspiration was performed prior to enrollment to determine eligibility. Primary therapy for NHL must have included rituximab and either an anthracycline or anthracenedione, with or without consolidative radiation therapy. Patients with responsive disease must have received at least 6 cycles of therapy or, for patients with early-stage disease, must have received at least 3 cycles of therapy and consolidative radiation therapy. Patients with stable disease (SD) as best response to first-line therapy must have received at least 3 cycles of chemoimmunotherapy. Patients were required to have 2 or more clearly demarcated lesions with a long axis >1.5 cm and a short axis ≥1.0 cm or 1 clearly demarcated lesion with a long axis >2.0 cm and a short axis ≥1.0 cm as assessed by computed tomography (CT) scan. All lesions defined as anatomical tumor sites by CT were required to demonstrate (18F) fluorodeoxyglucose avidity on a positron emission tomography (PET) scan. Patients enrolled were at least 18 years of age, had an Eastern Cooperative Oncology Group performance status ≤2, and were deemed potentially eligible for high-dose chemotherapy and autologous stem cell transplantation by the treating physician; no upper-age cutoff was dictated by the protocol. Written informed consent was provided by all patients. Treatment with corticosteroid was allowed up to doses of <1 mg/kg per day of prednisolone or equivalent dose of other glucocorticoid. Exclusion criteria included any previous therapy for NHL with the exception of first-line therapy and maintenance rituximab, central nervous system involvement, and chronic infection, including hepatitis B (core antibody-positive but DNA-negative patients by polymerase chain reaction testing were permitted to participate with prophylaxis against reactivation encouraged), hepatitis C, or HIV. Patients were also excluded if they had major comorbid illness, including significant cardiac or cerebrovascular disease or either prior or concurrent malignancy within 5 years. Additional exclusion criteria included prior treatment with anti-CD20 monoclonal antibody therapy other than rituximab, known or suspected hypersensitivity to any component of planned treatment, or impairment in baseline hematologic, hepatic, or renal function. Protocol, amendments, and consent forms were approved by the institutional review board at each center. The study was conducted in accordance with the Guidelines for Good Clinical Practice and the Declaration of Helsinki. All authors had access to primary clinical data, which were analyzed and interpreted by Matthew J. Matasar, Michael Fennessy, Qiming Liao, Klaus Edvardsen, Roxanne C. Jewell, Doug Fecteau, Rajendra P. Singh, Steen Lisby, and Craig H. Moskowitz.
Study design and treatment
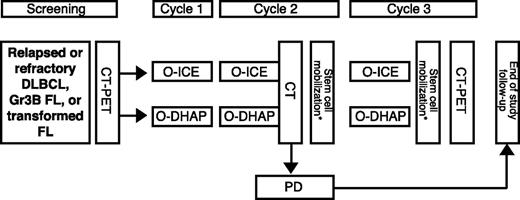
The study was registered with clinicaltrials.gov (NCT00823719). The study design was a multi-center phase II clinical trial of ofatumumab and standard salvage chemotherapy, either DHAP or ifosfamide, carboplatin, and etoposide (ICE), as depicted in Figure 1. Each participating center prospectively selected the salvage regimen of choice, and all patients at the center received the chosen regimen. Crossover between ICE and DHAP was permitted only in the event of significant toxicity from cytotoxic agents; crossover due to inadequate response was not permitted. Data from crossover patients were analyzed according to initial regimen.
Treatment protocol. Routine cycle length was 21 days. *Stem cell mobilization could be performed in cycle 2 and/or cycle 3. FL, follicular lymphoma; Gr3B, grade 3B; O, ofatumumab; PD, progressive disease.
Treatment protocol. Routine cycle length was 21 days. *Stem cell mobilization could be performed in cycle 2 and/or cycle 3. FL, follicular lymphoma; Gr3B, grade 3B; O, ofatumumab; PD, progressive disease.
Three cycles, each consisting of ofatumumab followed by cytotoxic therapy, were planned. Initially, cycle 1 consisted of ofatumumab 300 mg intravenously on day 1 (or up to 3 days prior to day 1) and 1000 mg intravenously on day 8, with the initial lower dose intended to abrogate infusion reactions; this approach was amended during the study, increasing the day 1 ofatumumab dose to 1000 mg based upon emerging safety data. Ofatumumab 1000 mg was administered on day 1 of cycles 2 and 3 for a total of 4 doses. DHAP consisted of dexamethasone 40 mg orally or intravenously on days 1 to 4, cisplatin 100 mg/m2 by 24-hour continuous intravenous infusion on day 1, and 2 doses of cytarabine 2000 mg/m2 intravenously, given 12 hours apart, on day 2. ICE consisted of etoposide 100 mg/m2 intravenously on days 1 to 3, ifosfamide 5 g/m2 by 24-hour continuous intravenous infusion with an equal dose of mesna on day 2, and carboplatin intravenously at an area under the curve (AUC) of 5 (maximum dose, 800 mg) based on 12-hour measured creatinine clearance on day 1 or 2. Growth factor support with filgrastim or pegfilgrastim was recommended, and the planned cycle length was 21 days. Peripheral blood stem cell (PBSC) mobilization was performed during cycle 2 and/or 3 according to local policy using filgrastim with or without plerixafor, with a target minimum of 2 × 106 CD34+ cells/kg to be collected and cryopreserved. If unsuccessful, additional attempts at PBSC mobilization or operative bone marrow harvest were permissible according to the standards of the treating center. Study treatment concluded at completion of second-line therapy; thus, subsequent consolidative therapy was not specified by the protocol.
Safety and response assessments
Patients were closely monitored for adverse reactions during ofatumumab and chemotherapy infusions, and standard laboratory parameters, including hepatic, renal, and hematologic testing, were obtained up to 3 days prior to each cycle. Grading of adverse events (AEs) was according to the Common Terminology Criteria for Adverse Events version 3.0. Ofatumumab infusion reactions included all AEs that could potentially be associated with infusion reactions and occurred during or within 24 hours of the end of the ofatumumab infusion. Samples were collected for the detection of anti-ofatumumab antibodies prior to the first dose of ofatumumab and at the follow-up or early withdrawal visit.
Treatment responses were determined by local investigators according to the Revised Response Criteria for Malignant Lymphoma.12 Contrast-enhanced CT scans of the chest, abdomen, and pelvis (and other anatomical regions when clinically indicated) were obtained within 4 weeks prior to the start of therapy and in the last week of cycles 2 and 3. A fluorodeoxyglucose-PET scan was performed within 4 weeks prior to the start of therapy and in the last week of cycle 3. For those patients with bone marrow infiltration at baseline and who were determined to have a complete response (CR) to treatment, a repeat bone marrow biopsy was performed for confirmation of response. Patients were followed for disease status and survival status posttreatment, although the schedule of restaging studies was not specified by the protocol.
Statistical analysis
The primary end point for the study was ORR at the completion of therapy, with secondary end points including CR rate, PFS, OS, safety, and the rate of successful PBSC mobilization (with a minimum yield of 2 × 106 CD34+ cells/kg). The null hypothesis was an ORR ≤40%, and the specified alternative hypothesis was an ORR ≥60%. A Simon 2-stage minimax design was employed,13 with α = 0.05 and a power of 90% to test H0: P ≤ .4 against H1: P ≥ .6. Twenty-nine evaluable patients were required for the first-stage analysis and if 12 or fewer responses were observed in the first stage, the study would be stopped. The first stage analysis confirmed continuation of the study and an additional 25 patients were enrolled for the second stage. An enrollment target of 60 patients was planned to ensure the required 54 patients evaluable for efficacy. Patients with major protocol deviations affecting efficacy (eg, CD20 negative lymphoma) were excluded from the per-protocol (PP) analysis of efficacy. All patients commencing study therapy were included in the safety analysis. For efficacy parameters of ORR and CR rate, number, percentage, and associated 95% confidence interval were calculated. For safety parameters and success rates of PBSC mobilization, number and percentage were calculated. For analysis of PFS and OS, the Kaplan-Meier survival method and Cox proportional hazards model were used. All efficacy analyses were in the PP population, according to study therapy (O-ICE or O-DHAP) and other clinical characteristics. P values provided were solely descriptive.
Pharmacokinetic assessments
Limited blood samples for the quantification of ofatumumab levels in plasma were collected prior to each dose, at the end of infusion for the first and third cycles, and at follow-up. Sample bioanalysis was performed using a validated antibody capture sandwich enzyme-linked immunosorbent assay with an Fc-specific anti-human IgG1 coupled to horseradish peroxidase for detection, and included the addition of a heterophilic blocking reagent to reduce interference from heterophilic antibodies (GlaxoSmithKline, data on file). The validated assay range was 0.1 to 1606.5 μg/mL.
Maximum plasma concentration, minimum plasma concentration prior to next dose, and time of maximum concentration were directly determined from the concentration-time data. Nonlinear mixed-effects modeling using a linear 2-compartment structural model was performed using NONMEM, v. 7.1.2 (ICON). Covariate exploration identified body surface area as a significant covariate for clearance (CL), volume of the central compartment (V1), and volume of the peripheral compartment; gender was a significant covariate for V1, with the V1 in females ∼20% smaller than that in males. Individual post hoc estimates of CL, V1, and volume of the peripheral compartment were generated based on the model, and half-life, volume of distribution at steady state, and AUC values (AUC[0-∞] at cycle 1, day 1 and cycle 3, AUC[0-504 h] at cycle 3) were calculated. Pharmacokinetic parameter values were summarized as geometric mean and between-patient coefficient of variation.
Results
Response
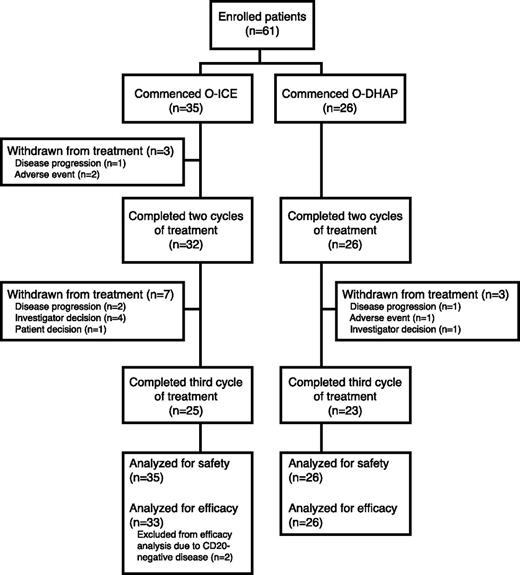
A total of 61 patients were enrolled in the study beginning May 2009, and all had completed the study therapy by July 2011. Thirty-five patients were registered at centers administering ICE and 26 at centers administering DHAP; patient distribution is depicted in Figure 2, and baseline patient characteristics are detailed in Table 1. Accrued patients represented a population with poor prognosis, with 48% having a second-line age-adjusted international prognostic index (saaIPI) score of 2 or 3, 48% having primary refractory disease, and an additional 33% having either a duration of remission or unknown best response to first-line therapy of <12 months. All 61 patients were included in safety analyses, but only 59 were evaluable in the per-protocol analysis of efficacy, as 2 patients were enrolled in the study despite the absence of expression of CD20 on biopsy confirming relapse or refractory disease. For 54 (92%) of 59 patients, the ORR evaluation was determined with both CT and PET.
CONSORT diagram of distribution of patients according to treatment assignation. O, ofatumumab.
CONSORT diagram of distribution of patients according to treatment assignation. O, ofatumumab.
Seven patients were withdrawn from the study for reasons other than progression or an AE: 4 patients had SD after cycle 2 and were withdrawn at the preference of the treating physician, and 3 patients with responsive disease after cycle 2 did not have a response assessment at cycle 3 (one with a CR was withdrawn after cycle 2 to proceed directly to autologous transplant, one patient chose to discontinue treatment, and one failed to undergo restaging following cycle 3). All 7 were retained in the per-protocol analysis, with the response following cycle 2 used for the ORR analysis.
The ORR for the PP patients was 61%, with rates of CR of 37%, partial response 24%, SD 22%, and progressive disease 17% (Table 2). The ORR was high (82%) in the small subset of patients with transformed follicular lymphoma. saaIPI was not associated with ORR or CR rates: patients with no or one adverse risk factors had an ORR of 63% and a CR rate of 43%, whereas those with 2 or 3 adverse risk factors had an ORR of 59% and a CR rate of 31%. Response to first-line therapy appeared to be associated with the outcome from second-line therapy with ofatumumab and ICE or DHAP; patients relapsing after a remission of >1 year following R-CHOP had an ORR of 83% and a CR rate of 67%, whereas patients with early relapse or primary refractory disease had an ORR of 55% and a CR rate of 30%. Of the 10 patients who had experienced primary progressive disease on R-CHOP, only one responded to study treatment.
Survival
With a median follow-up of 7 months, the median PFS of the per-protocol population was 9.5 months, which did not differ between O-ICE– and O-DHAP–treated patients (Figure 3A). Both saaIPI and response to first-line therapy predicted PFS in the Cox model (saaIPI hazard ratio [HR] = 3.5, P = .003; response HR = 5.9, P = .006) (Figure 3B-C). The median OS for PP patients was 16.7 months and Cox modeling identified saaIPI as significant (HR = 3.7, P = .02), whereas response to first-line therapy was of marginal significance (HR = 2.9, P = .11). Among those patients for whom cell-of-origin was classifiable using the Hans model,14 we observed no meaningful differences in OS or PFS according to cell-of-origin (GCB immunophenotype, non-GCB immunophenotype, or transformed follicular lymphoma).
Progression free survival. (A) PFS for O-DHAP and O-ICE. Median PFS for O-DHAP was 301 days (95% confidence interval [CI] 152, not estimable); median PFS for O-ICE was 288 days (95% CI 177, not estimable). (B) PFS according to saaIPI, 0 to 1 risk factors vs 2 to 3 risk factors. Median PFS for 0 to 1 risk factors was not reached (95% CI: 288, not estimable), median PFS for 2 to 3 risk factors was 177 days (95% CI 117, 264). (C) PFS according to response to first-line therapy, CR >12 months vs CR ≤ 12 months or failure to achieve remission. Median PFS for CR >12 months was not reached (95% CI 375, not estimable); median PFS for CR ≤ 12 months or failure to achieve remission was 261 days (95% CI 152, 296). O, ofatumumab.
Progression free survival. (A) PFS for O-DHAP and O-ICE. Median PFS for O-DHAP was 301 days (95% confidence interval [CI] 152, not estimable); median PFS for O-ICE was 288 days (95% CI 177, not estimable). (B) PFS according to saaIPI, 0 to 1 risk factors vs 2 to 3 risk factors. Median PFS for 0 to 1 risk factors was not reached (95% CI: 288, not estimable), median PFS for 2 to 3 risk factors was 177 days (95% CI 117, 264). (C) PFS according to response to first-line therapy, CR >12 months vs CR ≤ 12 months or failure to achieve remission. Median PFS for CR >12 months was not reached (95% CI 375, not estimable); median PFS for CR ≤ 12 months or failure to achieve remission was 261 days (95% CI 152, 296). O, ofatumumab.
Stem cell mobilization and consolidative therapy
Stem cell mobilization was commenced in 45 (74%) of the 61 enrolled patients, and the target number of stem cells (2 × 106 CD34+ stem cells/kg) was collected in 43 (96%) of the 45 patients. The median number of CD34+ stem cells collected was 5.78 × 106/kg. Twenty-three O-DHAP patients (88%) and 22 O-ICE patients (63%) commenced mobilization. All 23 O-DHAP patients (100%) and 20 of 22 O-ICE patients (91%) initiating mobilization were successfully mobilized. All received filgrastim for mobilization, but more O-DHAP patients (22%) than O-ICE patients (9%) received plerixafor. Of the 26 patients treated with O-DHAP per center selection, 18 (69%) went on to receive consolidative therapy (17 autotransplant and 1 allotransplant due to physician preference) and of the 35 patients treated with O-ICE, 16 (46%) received consolidation (all autotransplant).
Safety
Seventy-seven percent of patients experienced at least one AE of grade 3 or 4 severity; no deaths due to treatment-related toxicity occurred. Hematologic toxicity was common with both treatment regimens, although more pronounced in O-DHAP patients. For O-ICE and O-DHAP, grade 3/4 thrombocytopenia occurred in 49% and 73% of patients and grade 3/4 anemia in 26% and 50% of patients, respectively. Febrile neutropenia occurred in 31% of patients receiving O-DHAP but only 3% of patients receiving O-ICE. Four patients experienced grade 3/4 nephrotoxicity, of whom 3 (12%) received O-DHAP and 1 (7%) received O-ICE. Five patients (19%) crossed over from O-DHAP to O-ICE due to DHAP-specific toxicity: 4 for nephrotoxicity (grades 2-4) and 1 for ototoxicity. No O-ICE patients crossed over to O-DHAP. Six patients (10%) experienced treatment delays and 3 patients (5%) required dose reduction to ICE chemotherapy due to AEs. Three patients prematurely discontinued treatment due to AEs: one due to progressive renal dysfunction after crossover to O-ICE and 2 for central nervous system toxicity attributed to ifosfamide. Infusion reactions to ofatumumab were common (80%), with 18% experiencing a reaction of grade 3/4 severity. With the exception of one subject who was withdrawn after cycle 1 due to absence of expression of CD20 on biopsy confirming relapse or re-fractory disease, the remaining 10 patients received subsequent doses of ofatumumab. No anti-ofatumumab antibodies were detected in the 46 patients with posttreatment results.
Pharmacokinetics
The ofatumumab pharmacokinetic parameter values are summarized in Table 3. The ofatumumab maximum concentrations were higher with 1000 mg compared with 300 mg at the first infusion and were higher at cycle 3 compared with cycle 1, day 1. Ofatumumab pharmacokinetics appeared to be similar in the O-ICE and O-DHAP groups, with combined geometric mean CL values of 9.0 mL/h and t1/2 values of 26.0 days.
Discussion
For patients with DLBCL or other intermediate grade B-cell NHL who relapse after or are refractory to initial R-CHOP chemotherapy, second-line therapy followed by high-dose chemotherapy and autologous stem cell transplantation as a consolidative strategy remains the standard of care in transplant-eligible patients. The 2 most commonly used second-line cytotoxic regimens, ICE and DHAP, were compared in the CORAL trial, each given with 4 doses of rituximab. These regimens yielded similar results in rituximab-pretreated patients, with an ORR of 51%, and <1 in 4 patients were cured by rituximab combined with ICE or DHAP followed by HDT with or without posttransplant maintenance rituximab. Modifications of the consolidative regimen have been studied, including augmentation with multiple doses of pre-HDT rituximab,15 administration of radioimmunotherapy prior to HDT,16 use of alternative HDT regimens,17 and addition of maintenance therapy following HDT,18 but these have also failed to show that the outcome of these patients can be improved.
Because response to second-line therapy is required for patients to proceed to HDT and have a chance of cure, improvements in second-line therapy are certainly needed. Before rituximab was a routine component of first-line therapy, its inclusion in second-line regimens led to marked improvement in overall and CR rates.19-22 However, in the R-CHOP era, response rates of R-chemotherapy in the second-line setting more closely resemble those of salvage chemotherapy alone prior to the introduction of rituximab. Efforts at improving second-line therapy often focus on the addition of novel agents to a standard R-chemotherapy backbone, such as monoclonal antibodies against targets other than CD20 or newer compounds that are not routinely used in either first- or second-line chemotherapy regimens.23-25 The benefit of readministering rituximab in second-line regimens to patients after R-CHOP failure, particularly following early relapse or in the face of primary refractory disease, is uncertain. Thus, switching anti-CD20 therapy offers the potential of improving response in the relapse or refractory setting by overcoming or circumventing resistance to rituximab.
This study represents the first reported experience incorporating novel anti-CD20 therapy into chemoimmunotherapy for relapsed/refractory intermediate grade NHL. The findings of the current study, substituting ofatumumab for rituximab as the immunotherapy component of second-line therapy, are encouraging. The observed ORR of 61% met the a priori threshold for success of 50% and supports further investigation of the strategy. The CR rates of 37% for all patients and 42% for O-DHAP–treated patients compare favorably to the 29% CR rate in rituximab-preexposed patients in the CORAL study, although cross-trial comparisons are of limited utility.
The CORAL study found that the response to first-line treatment and saaIPI are the 2 most powerful predictors of outcome following second-line therapy. Early relapsing and refractory patients accounted for 80% of patients in the current study, but the outcome of such patients in the CORAL study was poor, with an ORR of 45% and a CR rate of 22% (Christian Gisselbrecht, MD, Hôpital Saint Louis, Paris, France; written communication, August 4, 2011). In this study, among early relapsing or primary refractory patients, the ORR and CR rates to ofatumumab combined with chemotherapy were 55% and 30%, respectively, including 64% and 36% in the O-DHAP treatment group. The response rate according to saaIPI score is not available for the rituximab-pretreated subgroup of CORAL. In the Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea study, the ORR and CR rates in the saaIPI 2-3 subgroup were 44% and 28%, respectively. In this study, the corresponding rates were 59% and 31%, respectively, with the rates being similar for both O-DHAP and O-ICE. Thus, ofatumumab combined with chemotherapy showed promising activity, particularly in the subgroups known to be unresponsive, early relapsing, refractory, and high-risk patients, according to the saaIPI.
Although 2 chemotherapy regimens, ICE and DHAP, were used in the current study, the study design does not permit comparison of the efficacy of the regimens when combined with ofatumumab. Participating centers selected 1 of the 2 regimens to be used for all accrued patients; imbalance in risk factors and the potential for unmeasured confounding preclude meaningful statistical comparison of results with the 2 regimens. Additional questions include whether ofatumumab substitution could contribute to the superiority of ICE- or DHAP-based therapy, although to answer this question would require significant resources that may be better allocated elsewhere. Data from CORAL identified rearrangement of c-myc as conferring a uniquely poor prognosis in relapsed DLBCL.26 The absence of central pathology review or uniform testing among centers for c-myc rearrangement or other cytogenetic abnormalities prevents comment on the impact of ofatumumab substitution on c-myc rearranged relapsed DLBCL. Lastly, questions regarding the differential benefit of therapies by cell-of-origin persist as well. Planned subgroup analysis of the CORAL data demonstrated differential effects of ICE- and DHAP-based therapy between GCB and non-GCB patients: for patients with non-GCB DLBCL, R-ICE and R-DHAP were equally active, whereas in GCB DLBCL, R-DHAP–treated patients experienced a superior PFS. The current study is incapable of addressing the question of whether ofatumumab substitution can augment or negate differential effects by cell-of-origin. These questions will be addressed by the ongoing phase III international randomized trial of O-DHAP and R-DHAP, which opened in March 2010 and continues to accrue patients.
The median PFS of patients treated with ofatumumab and chemotherapy was 9.5 months and the median OS was 16.7 months, comparable with the results of rituximab-pretreated patients in the CORAL and Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea studies.3,27 Analysis of PFS and baseline covariates in Cox proportional hazard models showed response to first-line treatment and saaIPI, the 2 most powerful independent prognostic markers in CORAL, retain their prognostic relevance in this study.
Although most patients experienced grade 3 or 4 AEs, treatment discontinuation due to toxicity was uncommon, and the number requiring chemotherapy dosing delay or reduction was low, suggesting that AEs were tolerable. There were no treatment-related mortalities, and no unexpected toxicity was observed. The incidence of grade 3 or 4 infusion reactions (18%) was higher than that observed in a study of rituximab-based therapy (11%),20 although the definition of infusion reactions may have differed between the studies. Here, reactions were manageable with routine supportive measures, including corticosteroids and antihistamines, and no patient was withdrawn from study due to infusion-related complications. The cytotoxic regimens used in this study cause substantial toxicity, with DHAP having previously been shown to cause greater myelosuppression and increased nephrotoxicity compared with ICE. This was again observed in this study, as 19% of O-DHAP patients crossed over to O-ICE, mostly due to nephrotoxicity. Any increase in response rates for O-DHAP compared with O-ICE suggested in this study must be considered in the context of this increased toxicity from O-DHAP. In light of ongoing work exploring the use of alternative platinum-based programs in the relapsed setting, such as substitution of oxaliplatin for cisplatin in combination with either dexamethasone and cytarabine (DHAX) or gemcitabine, the value of including cisplatin as a component of second-line therapy for aggressive lymphomas is increasingly uncertain.28,29
In summary, replacing rituximab with ofatumumab in second-line therapy, combined either with DHAP or ICE, is feasible, does not add significant toxicity, and does not negatively affect stem cell mobilization. Response rates, both ORR and CR, are encouraging, and although retreatment with rituximab should remain the current standard of care, formal investigation of the benefit accrued by this substitution is warranted.
Presented at the 2011 American Society of Hematology Annual Meeting, San Diego, CA, December 10-13, 2011.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in the study, the staff at the investigational sites, and GlaxoSmithKline, who coordinated the study and collected and analyzed the data. Editorial assistance was provided by Francesca Balordi and Jennifer Granit from Medicus International New York and funded by GlaxoSmithKline.
This work was supported by research funding from GlaxoSmithKline.
Authorship
Contribution: M.J.M., M.F., Q.L., I.S.L., M.A.K.-D., R.C.J., and C.H.M. conceived or designed research; M.J.M., M.S.C., M.A.R., T.C.S., G.S., I.S.L., M.A.K.-D., R.J., L.F., M.F., D.F., and C.H.M. provided study materials or patients; M.J.M., M.S.C., M.A.R., T.C.S., G.S., I.S.L., M.A.K.-D., R.J., L.F., K.H., M.F., Q.L., D.F., R.P.S., and C.H.M. collected and assembled data; M.J.M., M.F., Q.L., K.E., S.L., R.C.J., R.P.S., D.F., and C.H.M. analyzed and interpreted data; and all authors contributed to the manuscript writing and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.J.M. participated in advisory boards for Genentech/Roche; M.S.C. participated in advisory boards for GlaxoSmithKline and received funds for research from GlaxoSmithKline; M.A.R. received funds for clinical research activities within the last 5 years from Ortho Biotech, Amgen, Inc., Biogen IDEC, GlaxoSmithKline, Wyeth-Ayerst, Genentech, and Novartis; T.C.S. received funds for research from GlaxoSmithKline, owns stock in Amgen, and received fees for the speaker bureau from Teva Pharmaceutical Industries Ltd. and Seattle Genetics, Inc.; L.F. received funds for research from GlaxoSmithKline, Genentech/Roche, Seattle Genetics, Inc., and Pfizer, participated in advisory boards for Genentech/Roche, Seattle Genetics, Inc., and Pfizer, and received fees for the speaker bureau from Teva Pharmaceutical Industries Ltd; K.E. is an employee of GlaxoSmithKline and owns stock options in GlaxoSmithKline and Genmab A/S; M.F., Q.L., R.C.J., R.P.S., and D.F. are employees of GlaxoSmithKline and own stock options in GlaxoSmithKline; and S.L. is an employee of Genmab A/S and owns stock options in Genmab A/S. The remaining authors declare no competing financial interests.
Correspondence: Matthew Matasar, Lymphoma and Adult Bone Marrow Transplant Services, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: matasarm@mskcc.org.

![Figure 3. Progression free survival. (A) PFS for O-DHAP and O-ICE. Median PFS for O-DHAP was 301 days (95% confidence interval [CI] 152, not estimable); median PFS for O-ICE was 288 days (95% CI 177, not estimable). (B) PFS according to saaIPI, 0 to 1 risk factors vs 2 to 3 risk factors. Median PFS for 0 to 1 risk factors was not reached (95% CI: 288, not estimable), median PFS for 2 to 3 risk factors was 177 days (95% CI 117, 264). (C) PFS according to response to first-line therapy, CR >12 months vs CR ≤ 12 months or failure to achieve remission. Median PFS for CR >12 months was not reached (95% CI 375, not estimable); median PFS for CR ≤ 12 months or failure to achieve remission was 261 days (95% CI 152, 296). O, ofatumumab.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/4/10.1182_blood-2012-12-472027/4/m_499f3.jpeg?Expires=1766829843&Signature=gwPeegtwdbnKRNqLs-b6EJCfBdz877iZlrWbyXolVQDc5ZYqE-WK6uDEl1V4rs~yQnGPyIOnTQowC41M~diXOJ3QcpGS-zIzwxzsX9xRDPxDQmT5jsc2P8Gnv-rESXnoc-xcBM1RsLgXEe9CAVW2qCPHQ0yxdREk92O4xPb~nGPxSDMXNEqjzLzSS1u67D3IxLiV3vKiz-68MczxjQzyPHUuQlIQzJSYwQ8AMvKf8XvTh6luNcosHMIfyoiwzLV9jYFgaQQ4OvJYB6fW1z4hvnd2vzDjYqQ0MlkeHoCFoDHcIgWbig6GQtBy26ctnHBFXmBVtXnTVfGxOzvQkLepgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
