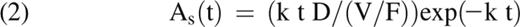Key Points
Erwinia asparaginase was granted FDA approval in November 2011 for use in patients with allergic reactions to E coli-derived asparaginase.
Erwinia asparaginase 25 000-IU/m2 for 6 intramuscular doses M/W/F can be substituted for a single dose of pegaspargase.
Abstract
AALL07P2 evaluated whether substitution of Erwinia asparaginase 25 000 IU/m2 for 6 doses given intramuscularly Monday/Wednesday/Friday (M/W/F) to children and young adults with acute lymphoblastic leukemia and clinical allergy to pegaspargase would provide a 48-hour nadir serum asparaginase activity (NSAA) ≥0.10 IU/mL. AALL07P2 enrolled 55 eligible/evaluable patients. NSAA ≥0.1 IU/mL was achieved in 38 of 41 patients (92.7%) with acceptable samples 48 hours and in 38 of 43 patients (88.4%) 72 hours after dosing during course 1. Among samples obtained during all courses, 95.8% (252 of 263) of 48-hour samples and 84.5% (125 of 148) of 72-hour samples had NSAA ≥0.10-IU/mL. Pharmacokinetic parameters were estimated by fitting the serum asparaginase activity-time course for all 6 doses given during course 1 to a 1-compartment open model with first order absorption. Erwinia asparaginase administered with this schedule achieved therapeutic NSAA at both 48 and 72 hours and was well tolerated with no reports of hemorrhage, thrombosis, or death, and few cases of grade 2 to 3 allergic reaction (n = 6), grade 1 to 3 hyperglycemia (n = 6), or grade 1 pancreatitis (n = 1). Following allergy to pegaspargase, Erwinia asparaginase 25 000 IU/m2 × 6 intramuscularly M/W/F can be substituted for a single dose of pegaspargase.
Introduction
Following the first reported use of asparaginase in an 8-year-old boy with relapsed acute lymphoblastic leukemia (ALL) in 1966,1 single-agent studies in the early 1970s of asparaginase for the treatment of children diagnosed with ALL demonstrated response rates of 20% to 68%.2-7 Since that time, asparaginase has become an essential component of multiagent chemotherapy for childhood ALL.3,4,7-16
In the United States prior to November 2011, two asparaginase preparations received approval for use by the US Food and Drug Administration (FDA): native Escherichia coli asparaginase and pegaspargase. Pegaspargase has been the more commonly used product because it requires less frequent administration than E coli asparaginase as a consequence of its longer biological half-life and because of its lower immunogenicity.17-21 A third preparation, Erwinia asparaginase, derived from the bacterium Erwinia chrysanthemi, was not commercially available in the United States during the conduct of this study but was available on a compassionate use basis.
Pegaspargase has been the sole asparaginase preparation used in Children’s Oncology Group (COG) trials for newly diagnosed patients with ALL since 2005 (excluding infants ≤1 year old). The overall aim of COG AALL07P2, undertaken to support FDA approval of Erwinia asparaginase, was to evaluate the utility of Erwinia asparaginase as an alternative in cases of hypersensitivity to pegaspargase by determining whether intramuscular administration of Erwinia asparaginase 25 000 IU/m2 on a Monday/Wednesday/Friday (M/W/F) schedule for 2 weeks would achieve a nadir serum asparaginase activity (NSAA) ≥0.10 IU/mL, which has been associated with complete asparagine depletion.22,23
Patients and methods
Patients
Eligible patients on COG AALL07P2 were >1 to <30 years of age, currently enrolled on a frontline COG ALL treatment study, had documented grade ≥2 allergy (according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events [CTCAE] v3.0) to pegaspargase, and had ≥1 remaining scheduled doses of pegaspargase. Patients who had previously received Erwinia asparaginase or had a history of grade ≥2 pancreatitis were excluded.24
The study was approved by the National Cancer Institute and by institutional review boards at the individual institutions prior to patient enrollment. Informed consent was obtained according to Department of Health and Human Services Guidelines and in accordance with the Declaration of Helsinki.
Treatment plan
Erwinia asparaginase was provided by EUSA Pharma, Inc. (Langhorne, PA). Patients received Erwinia asparaginase 25 000 IU/m2 × 6 intramuscular doses on a M/W/F schedule for 2 weeks as a replacement for each remaining scheduled dose of pegaspargase. Because all other chemotherapy continued according to the original treatment protocol, patients were permitted to begin receiving Erwinia asparaginase on Monday, Wednesday, or Friday, so that their schedules are defined as M/W/F, W/F/M, or F/M/W. Adverse events (all grades) related to Erwinia asparaginase were reported according to the CTCAE v3.0.
Determination of serum asparaginase activity
Twelve blood samples (2 mL) were scheduled for collection from each patient during course 1, prior to each Erwinia asparaginase dose, on days 15 and 22 postadministration, and at 2 and 24 hours following doses 1 and 4 for patients beginning treatment on Monday or Wednesday or doses 2 and 5 for patients beginning treatment on Friday. Additional samples were collected before administering doses 1 and 6 and on day 15 during all subsequent courses of therapy. Blood was allowed to clot for 1 to 2 hours over ice before centrifuging (1300 g, 10 minutes, 4°C). Serum was removed and stored at −70°C until packaged for shipment to a central laboratory for analysis.
Asparaginase activity was determined by a coupled enzymatic assay with minor modifications, as previously reported.17,25 Briefly, aspartic acid formed from the asparaginase catalyzed deamination of added asparagine reacts with α-ketoglutaric acid in the presence of glutamic oxaloacetic transaminase yielding oxaloacetic acid, which oxidizes reduced β-nicotinamide adenine dinucleotide in the presence of malic dehydrogenase, resulting in a decrease in absorbance at 340 nm. The rate of reaction at 37°C is a linear function of enzyme activity. E coli asparaginase purchased from Sigma (St. Louis, MO) was used as the analytical reference standard to prepare a series of seven calibration standards in normal human serum with activities of 0.025 to 0.25 IU/mL. Samples with activities exceeding the upper range of the calibration curve were reanalyzed after diluting with normal human serum. Back-calculated asparaginase activities from a total of 81 calibration curves were used to assess the between-day accuracy and precision of the assay during its application to samples from this study. Accuracy was 97.0% of the nominal activity and the precision was 6.0% at the 0.025 IU/mL lower limit of quantitation. Quality control samples with asparaginase in serum at activities of 0.035, 0.120, and 0.220 IU/mL were assayed with a between-day accuracy of 99.1% to 102.1% and a precision of 4.7% to 5.8%.
Statistical analysis of serum asparaginase activity data
This study was powered to test the hypothesis that the 48-hour NSAA would be ≥0.1 IU/mL in at least 70% of patients vs meeting this target activity in 50% of patients. A Simon minimax two-stage design was used with a total sample size of 50 to distinguish between a true null response probability of 70% vs a true alternative response probability of 50% with a significance level of 5% and 90% power. A total of 31 patients were to be accrued in the first stage. The study would be closed to further accrual if the 48-hour NSAA was ≥0.1 IU/mL in ≤15 patients. Otherwise, the study would continue to the second stage of accrual. A total of 19 more patients would be accrued in the second stage. Acceptance of the null hypothesis required achieving a 48-hour NSAA ≥0.1 IU/mL in at least 30 of the 50 total patients.
Actual dosing and sample collection times were calculated relative to the time of the prior Erwinia asparaginase dose and to the initial dose for course 1. Samples obtained on the scheduled day relative to the starting dose of the course, prior to the successive dose, and within 5% of the scheduled time relative to administration of the prior dose were considered to be acceptable for assessing the primary and secondary end points of the clinical trial. Additional criteria for excluding assay results were failure to record the actual dosing or sample collection times, a dose that differed from 25 000 IU/m2 by more than ± 5%, the prior dose was not administered on the correct day, or samples were thawed upon receipt from the study site.
Results from the analysis of 48- and 72-hour NSAA samples found to be unacceptable for any of the above reasons were excluded from statistical analyses. The primary end point of the study was evaluated from a single 48-hour NSAA determination for each patient during course 1, which included samples obtained before giving dose 6 for patients receiving dose 1 on a Monday or a Friday and prior to dose 5 for patients beginning treatment on a Wednesday. A secondary end point of the study was evaluated from a single 72-hour NSAA determination during course 1, which included samples collected before dose 4 for patients beginning treatment on a Monday, before dose 6 for patients starting on a Wednesday, and before dose 5 for patients starting on a Friday. Descriptive statistics for all NSAA data were calculated by using Microsoft Office Excel 2003 SP3 (Microsoft Corp, Redmond, WA).
Pharmacokinetic modeling
Serum asparaginase activity-time data for the 6 intramuscular doses of Erwinia asparaginase given during the first course of therapy were fit to a one-compartment open model with first-order absorption by unweighted nonlinear regression using WinNonlin Professional 5.0 (Pharsight Corp, Cary, NC). Data for each patient were initially fit to the equation,
with the repeated dosing option, where As(t) is the serum asparaginase activity at time t relative to the first dose, ka is the apparent rate constant for absorption of enzyme from the extravascular administration site into serum, D is the dose, V/F is the extravascular apparent volume of distribution, and ke is the apparent rate constant for the loss of serum enzyme activity. In cases where the estimated values of ka and ke were within 10% of each other, the data were refit using the form of the equation for which ka = ke = k,26
which is also included in the WinNonlin library of pharmacokinetic models. Pharmacokinetic variables (ie, half-lives, apparent extravascular clearance, maximum serum asparaginase activity, and time) were calculated by the program using final values of the iterated parameters (ie, V/F, ka, ke). The two-tailed Student t test was used to compare mean pharmacokinetic variables between groups of patients after logarithmic transformation of the data. A P value < .05 was the criterion for statistical significance.
Results
Patient characteristics
Fifty-nine patients were enrolled on AALL07P2 (Figure 1). Patients were also enrolled on one of six upfront studies, AALL0232, AALL0331, AALL0434, AALL07P4, AALL0622, and AALL08P1, in which planned doses of pegaspargase varied by study arm and ranged from 1 to 13. Fifty-eight enrolled patients received at least one dose of Erwinia asparaginase and are included in the safety analysis (Table 1). The mean age at study entry was 9.7 years (range, 2 to 18 years) and the majority of patients were male (34 [58.6%]). Fifty-one patients (87.9%) had B-precursor ALL and 7 (12.1%) had T-ALL. All patients were within 9 months of diagnosis (50%, 0 to 3 months; 44.8%, 4 to 6 months; 5.2%, 7 to 9 months). Patients received a median of 3 doses (range, 1 to 5 doses) of pegaspargase prior to enrollment on AALL07P2 and received a median of 3 (range, 1 to 9) courses of Erwinia asparaginase. Fifty-five patients were eligible and evaluable for AALL07P2, and 44 (80%) of 55 patients were able to complete all remaining courses of planned asparaginase therapy.
Demographic and baseline characteristics for the safety population (n = 58)
| Characteristic . | No. . | % . |
|---|---|---|
| Age, y | ||
| Mean | 9.7 | |
| Standard deviation | 5.20 | |
| Median | 10.5 | |
| Range | 2-18 | |
| Gender | ||
| Male | 34 | 58.6 |
| Female | 24 | 41.4 |
| Race | ||
| White | 45 | 77.6 |
| Black or African American | 6 | 10.3 |
| Other | 7 | 12.1 |
| Ethnicity | ||
| Hispanic or Latino | 20 | 34.5 |
| Not Hispanic or Latino | 38 | 65.5 |
| Primary Disease | ||
| Precursor B-cell ALL | 51 | 88 |
| T-cell ALL | 7 | 12 |
| Characteristic . | No. . | % . |
|---|---|---|
| Age, y | ||
| Mean | 9.7 | |
| Standard deviation | 5.20 | |
| Median | 10.5 | |
| Range | 2-18 | |
| Gender | ||
| Male | 34 | 58.6 |
| Female | 24 | 41.4 |
| Race | ||
| White | 45 | 77.6 |
| Black or African American | 6 | 10.3 |
| Other | 7 | 12.1 |
| Ethnicity | ||
| Hispanic or Latino | 20 | 34.5 |
| Not Hispanic or Latino | 38 | 65.5 |
| Primary Disease | ||
| Precursor B-cell ALL | 51 | 88 |
| T-cell ALL | 7 | 12 |
Toxicity
Grade 2 to 3 allergic reaction, grade 1 to 3 hyperglycemia, and grade 1 pancreatitis related to Erwinia asparaginase were reported in 6, 6, and 1 patients, respectively (Table 2). There were no reports of hemorrhage, thrombosis, hyperlipidemia, ketoacidosis, or death.
Targeted toxicities (n = 55)
| Toxicity . | No. . | % . |
|---|---|---|
| Allergy (grade 2, n = 4; grade 3, n = 2) | 6 | 10.9 |
| Hyperglycemia (grade 1, n = 3; grade 2, n = 2; grade 3, n = 1) | 6 | 10.9 |
| Pancreatitis (grade 1, n = 1) | 1 | 1.8 |
| Hemorrhage/thrombosis (grade 3 to 4, n = 0) | 0 |
| Toxicity . | No. . | % . |
|---|---|---|
| Allergy (grade 2, n = 4; grade 3, n = 2) | 6 | 10.9 |
| Hyperglycemia (grade 1, n = 3; grade 2, n = 2; grade 3, n = 1) | 6 | 10.9 |
| Pancreatitis (grade 1, n = 1) | 1 | 1.8 |
| Hemorrhage/thrombosis (grade 3 to 4, n = 0) | 0 |
NSAA
The primary end point was based on a single 48-hour NSAA determination in 41 patients during course 1. Samples from 14 patients were excluded from statistical analysis because they were not obtained, were collected at a time differing from 48 hours by more than 5%, had dosing or sample collection times that were not recorded, or because they had dosing inconsistencies. The median asparaginase activity 48 hours after the prior dose of Erwinia asparaginase was 0.684 IU/mL (range, less than the limit of quantitation to 2.884 IU/mL), and 38 patients (92.7%) had an enzyme activity ≥0.100 IU/mL. Forty-three patients had acceptable 72-hour NSAA determinations during course 1 for assessing the secondary end point. The median asparaginase activity in these samples was 0.327 IU/mL (range, 0.043 to 1.026 IU/mL), and the enzyme activity was ≥0.100 IU/mL in samples from 38 of the patients (88.4%).
Data pertaining to all acceptable 48-hour and 72-hour NSAA determinations for each course of therapy are summarized in Table 3. Four 48-hour NSAA samples and two 72-hour samples were scheduled for collection from each patient during course 1, regardless of the day on which therapy was initiated. During each subsequent course, single 48-hour and 72-hour NSAA samples were obtained from patients beginning therapy on a Monday or a Wednesday, whereas two 48-hour NSAA samples were obtained from patients starting on a Friday. The number of patients with NSAA samples that met the criteria for acceptability decreased from 50 (90.9%) of 55 for both trough times in course 1 to 16 (66.7%) of 24 for the 48-hour NSAA and 7 (53.8%) of 13 for the 72-hour NSAA in course 4. Overall, 94.5% of the patients had at least one acceptable NSAA sample, and 69.8% of the 48-hour NSAA samples (n = 263) and 72.9% (n = 148) of the 72-hour NSAA samples were acceptable. The median 48-hour NSAA decreased progressively from 0.715 IU/mL during course 1 to 0.418 IU/mL in course 4, with an overall median of 0.645 IU/mL for all acceptable samples collected in all courses. The median 72-hour NSAA did not show evidence of a course-dependent trend, and the overall median was 0.248 IU/mL. The percentage of patients with acceptable 48-hour NSAA samples ≥0.10 IU/mL ranged from 92.0% to 100% during the first 4 courses, with 92.9% to 97.4% of the acceptable samples being ≥0.10 IU/mL. With regard to the 72-hour NSAA, 78.9% to 98.0% of the patients had samples with an enzyme activity ≥0.10 IU/mL, and 78.9% to 88.9% of the acceptable samples were ≥0.10 IU/mL.
Summary statistics for 48- and 72-h NSAA data for each course of therapy
| Trough time (h) . | Cycle . | Acceptable data . | NSAA (IU/mL) . | NSAA ≥0.10 IU/mL . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Samples . | Patients . | Samples . | ||||||||
| No. . | % . | No. . | % . | Median . | Range . | No. . | % . | No. . | % . | ||
| 48 | 1 | 50 | 90.9 | 155 | 72.4 | 0.715 | <LOQ-2.884 | 50 | 100 | 151 | 97.4 |
| 48 | 2 | 25 | 58.1 | 28 | 56.0 | 0.654 | <LOQ-1.294 | 23 | 92.0 | 26 | 92.9 |
| 48 | 3 | 29 | 90.6 | 37 | 88.1 | 0.551 | 0.099-1.429 | 28 | 96.6 | 36 | 97.3 |
| 48 | 4 | 16 | 66.7 | 24 | 70.6 | 0.418 | 0.080-1.635 | 16 | 100 | 23 | 95.8 |
| 48 | ≥5 | 12 | 75.0 | 19 | 51.4 | 0.669 | <LOQ-1.114 | 10 | 83.3 | 16 | 84.2 |
| 48 | All | 52 | 94.5 | 263 | 69.8 | 0.645 | <LOQ-2.884 | 52 | 100 | 252 | 95.8 |
| 72 | 1 | 50 | 90.9 | 86 | 79.6 | 0.251 | 0.043-1-612 | 49 | 98.0 | 73 | 84.9 |
| 72 | 2 | 19 | 57.6 | 19 | 57.6 | 0.248 | <LOQ-0.873 | 15 | 78.9 | 15 | 78.9 |
| 72 | 3 | 18 | 90.0 | 18 | 90.0 | 0.163 | 0.030-0.741 | 16 | 88.9 | 16 | 88.9 |
| 72 | 4 | 7 | 53.8 | 7 | 53.8 | 0.475 | <LOQ-0.622 | 6 | 85.7 | 6 | 85.7 |
| 72 | ≥5 | 11 | 78.6 | 18 | 62.1 | 0.245 | 0.087-0.717 | 10 | 90.9 | 15 | 83.3 |
| 72 | All | 52 | 94.5 | 148 | 72.9 | 0.248 | <LOQ-1.612 | 52 | 100 | 125 | 84.5 |
| Trough time (h) . | Cycle . | Acceptable data . | NSAA (IU/mL) . | NSAA ≥0.10 IU/mL . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Samples . | Patients . | Samples . | ||||||||
| No. . | % . | No. . | % . | Median . | Range . | No. . | % . | No. . | % . | ||
| 48 | 1 | 50 | 90.9 | 155 | 72.4 | 0.715 | <LOQ-2.884 | 50 | 100 | 151 | 97.4 |
| 48 | 2 | 25 | 58.1 | 28 | 56.0 | 0.654 | <LOQ-1.294 | 23 | 92.0 | 26 | 92.9 |
| 48 | 3 | 29 | 90.6 | 37 | 88.1 | 0.551 | 0.099-1.429 | 28 | 96.6 | 36 | 97.3 |
| 48 | 4 | 16 | 66.7 | 24 | 70.6 | 0.418 | 0.080-1.635 | 16 | 100 | 23 | 95.8 |
| 48 | ≥5 | 12 | 75.0 | 19 | 51.4 | 0.669 | <LOQ-1.114 | 10 | 83.3 | 16 | 84.2 |
| 48 | All | 52 | 94.5 | 263 | 69.8 | 0.645 | <LOQ-2.884 | 52 | 100 | 252 | 95.8 |
| 72 | 1 | 50 | 90.9 | 86 | 79.6 | 0.251 | 0.043-1-612 | 49 | 98.0 | 73 | 84.9 |
| 72 | 2 | 19 | 57.6 | 19 | 57.6 | 0.248 | <LOQ-0.873 | 15 | 78.9 | 15 | 78.9 |
| 72 | 3 | 18 | 90.0 | 18 | 90.0 | 0.163 | 0.030-0.741 | 16 | 88.9 | 16 | 88.9 |
| 72 | 4 | 7 | 53.8 | 7 | 53.8 | 0.475 | <LOQ-0.622 | 6 | 85.7 | 6 | 85.7 |
| 72 | ≥5 | 11 | 78.6 | 18 | 62.1 | 0.245 | 0.087-0.717 | 10 | 90.9 | 15 | 83.3 |
| 72 | All | 52 | 94.5 | 148 | 72.9 | 0.248 | <LOQ-1.612 | 52 | 100 | 125 | 84.5 |
LOQ, limit of quantitation.
Pharmacokinetics of serum asparaginase activity
Serum asparaginase activity-time data obtained during treatment with the 6 doses of Erwinia asparaginase given in the first course of therapy were amenable to pharmacokinetic analysis for a total of 54 patients. A sparse sampling schedule was used in which asparaginase activity was monitored at 10 time points over a time interval of 14 days to enable patients to be treated with minimal inconvenience on an outpatient basis. Although it is not possible to estimate any pharmacokinetic parameters by noncompartmental methods of analysis, simultaneously fitting the entire data set by nonlinear regression to a one-compartment open model with first-order absorption, using the repeated dosing option for the model, yielded an acceptable fit of the experimental data for all patients.
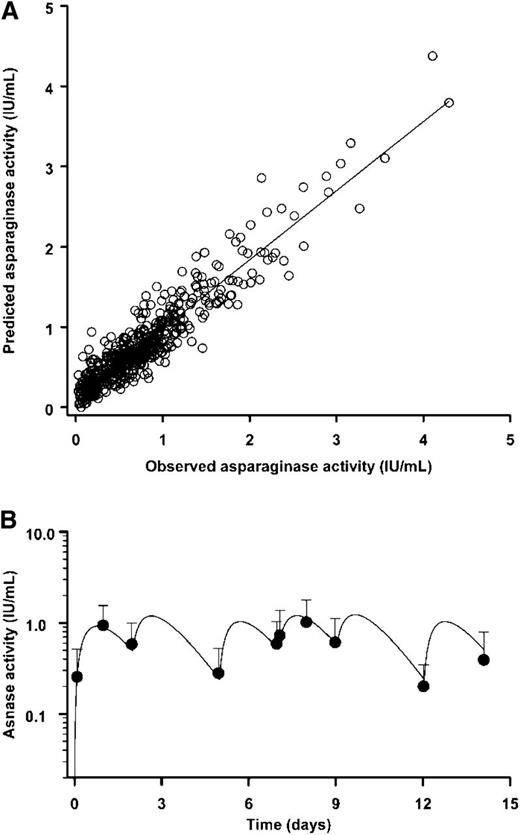
The existence of two distinct populations of patients was discerned for which the estimated values of ka and ke were either well differentiated (group A) or kinetically indistinguishable (group B). Specifically, the average ka/ke ratio was 6.6 (range, 1.8 to 26.8) for 26 categorical group A patients and 0.98 (range, 0.9 to 1.1) for 28 group B patients when initially fit to equation 1. Pharmacokinetic data for group B patients were refit to equation 2. The goodness of fit of the appropriate form of the equation for the pharmacokinetic model to the observed serum asparaginase activity-time data for each patient is illustrated by the correlation plot shown in Figure 2A. Linear regression analysis of the relationship between the observed and model-predicted asparaginase activity for the 510 acceptable data points yielded a correlation coefficient of 0.94 with a slope of 0.86 for the best-fit line.
Pharmacokinetics of Erwinia Asparaginase. (A) Correlation between the observed and model-predicted asparaginase activity in 510 serum samples obtained from 54 patients during the initial course of therapy. The overall goodness of fit of the one-compartment open model with first-order absorption to the experimental data for individual patients is indicated by the closeness of the correlation coefficient (0.94) and slope of the best-fit line (0.86) to unity. (B) Mean serum asparaginase activity-time profile for the six 25 000 IU/m2 intramuscular doses of Erwinia asparaginase given during the first course of therapy for 24 patients receiving the initial dose on a Wednesday. Data points are the geometric mean values of the observed asparaginase activity at each sample time shown together with 1-standard deviation unit error bars. The continuous line is the best-fit curve determined by nonlinear regression analysis of the mean profile.
Pharmacokinetics of Erwinia Asparaginase. (A) Correlation between the observed and model-predicted asparaginase activity in 510 serum samples obtained from 54 patients during the initial course of therapy. The overall goodness of fit of the one-compartment open model with first-order absorption to the experimental data for individual patients is indicated by the closeness of the correlation coefficient (0.94) and slope of the best-fit line (0.86) to unity. (B) Mean serum asparaginase activity-time profile for the six 25 000 IU/m2 intramuscular doses of Erwinia asparaginase given during the first course of therapy for 24 patients receiving the initial dose on a Wednesday. Data points are the geometric mean values of the observed asparaginase activity at each sample time shown together with 1-standard deviation unit error bars. The continuous line is the best-fit curve determined by nonlinear regression analysis of the mean profile.
Mean values of the parameters describing the best-fit equations and derived variables are presented separately in Table 4 for both groups of patients. The mean CL/F was not significantly different (P = .33) for the two groups, whereas V/F was approximately 50% greater for group A than group B (P = .0045). Consequently, there was a difference of similar magnitude in the mean apparent biological half-life of serum asparaginase activity between the two groups, which was 22.1 ± 7.7 hours for group A compared with 12.6 ± 2.1 hours for group B (P < .001). The maximum predicted asparaginase activity for the first dose of Erwinia asparaginase was not significantly different (P = .41) for the two pharmacokinetic groups, although the time that it occurred was earlier (P < .001) for group A patients (13.0 ± 5.3 hours) than for group B patients (18.2 ± 3.1 hours).
Mean pharmacokinetic parameters for serum asparaginase activity
| Parameter . | Group A (n = 26) . | Group B (n = 28) . | ||
|---|---|---|---|---|
| . | % coefficient of variation . | . | % coefficient of variation . | |
| V/F (liters/m2) | 12.8 | 55 | 8.3 | 50 |
| ka (h−1) | 0.149 | 62 | —* | |
| ke (h−1) | 0.031 | 35 | 0.055 | 17 |
| CL/F (L/h/m2) | 0.40 | 53 | 0.46 | 49 |
| t1/2,z (h) | 22.1 | 35 | 12.6 | 17 |
| tmax (h)† | 14.0 | 38 | 18.4 | 17 |
| Amax (IU/mL)† | 1.25 | 57 | 1.10 | 49 |
| Parameter . | Group A (n = 26) . | Group B (n = 28) . | ||
|---|---|---|---|---|
| . | % coefficient of variation . | . | % coefficient of variation . | |
| V/F (liters/m2) | 12.8 | 55 | 8.3 | 50 |
| ka (h−1) | 0.149 | 62 | —* | |
| ke (h−1) | 0.031 | 35 | 0.055 | 17 |
| CL/F (L/h/m2) | 0.40 | 53 | 0.46 | 49 |
| t1/2,z (h) | 22.1 | 35 | 12.6 | 17 |
| tmax (h)† | 14.0 | 38 | 18.4 | 17 |
| Amax (IU/mL)† | 1.25 | 57 | 1.10 | 49 |
Amax, maximum serum enzyme activity; CL/F, apparent extravascular clearance of enzyme activity; t1/2,z, apparent biological half-life of enzyme activity; tmax, time of maximum serum enzyme activity.
For group B, ka = ke = k.
Predicted values for the initial dose of the drug.
More of the patients started therapy on a Wednesday (44%) than either a Monday (26%) or a Friday (28%). The mean asparaginase activity-time course for the 24 patients with a Wednesday starting day is shown in Figure 2B. The predicted maximum asparaginase activity achieved after each dose of Erwinia asparaginase was relatively constant, and the mean trough asparaginase activity remained well above the 0.10 IU/mL threshold for at least 72 hours after all doses.
Discussion
Leukemic lymphoblasts are deficient in asparagine synthetase and are therefore thought to be dependent upon extracellular sources of asparagine for protein synthesis.27-29 Asparaginase is an exogenous enzyme that catalyzes the hydrolysis of asparagine to aspartic acid and ammonia. Studies in both Europe and the United States have concluded that maintaining serum asparaginase activity above 0.10 IU/mL is adequate to deplete asparagine.22,23,30,31 This therapeutic threshold was established from a number of independent investigations which revealed that the pharmacodynamic effects of asparaginase are best demonstrated by monitoring the concentration of asparagine in cerebrospinal fluid. Accurately measuring the concentration of asparagine in either plasma or serum is complicated by the rapid ex vivo hydrolysis of asparagine that occurs in the presence of asparaginase, even at very low subtherapeutic activity levels during the time required to harvest plasma from blood samples and inactivate the enzyme by acidification.30-38
Asparaginase has become a critical component of multiagent chemotherapy for the treatment of ALL.3,4,7,9-13 Current chemotherapy regimens for pediatric ALL typically include a postinduction intensification phase during which asparaginase is routinely administered.25,39-42 All COG trials for newly diagnosed patients with ALL, with the exception of infant ALL, administer pegaspargase starting during induction therapy and as the sole asparaginase product. However, allergic reactions develop in many patients treated with pegaspargase. In addition, immunologic responses to all asparaginase preparations are associated with formation of neutralizing antibodies against the enzyme that may or may not be associated with a symptomatic allergy.19,21,22,42,43
The primary role of Erwinia asparaginase in the treatment of ALL has been to replace native E coli asparaginase or pegaspargase after patients exhibit allergic reactions to either or both preparations.25,44,45 In large randomized clinical trials, administration of Erwinia asparaginase once or twice a week was associated with inferior outcomes as compared with E coli asparaginase40,46 when administered on identical dosing schedules. It is likely that the NSAA in patients receiving Erwinia asparaginase would not have been maintained above 0.10 IU/mL because Erwinia asparaginase has a biological half-life that is much shorter than that of E coli asparaginase.17,23 It has been suggested that treatment with lower doses of Erwinia asparaginase given daily or on alternating days could be more effective than higher but less frequent doses in producing complete and sustained asparagine depletion.37 Specifically, the M/W/F schedule was recommended when substituting Erwinia asparaginase for weekly E coli asparaginase.47 The dose of Erwinia asparaginase is also a critical consideration, since only 33% of the patients receiving a 10 000-IU/m2 intramuscular dose once every 3 days had mean trough levels ≥0.10 IU/mL.48
The results of COG AALL07P2 showed that of the 55 eligible/evaluable patients, NSAA ≥0.1 IU/mL was achieved in 38 (92.7%) of 41 patients, with samples meeting acceptability criteria 48 hours after dosing; hence, it was concluded that >70% of patients achieved the required trough serum asparaginase activity. Further, intramuscular administration of Erwinia asparaginase 25 000 IU/m2 M/W/F for 2 weeks achieved serum enzyme activity ≥0.10 IU/mL in 97.4% of the 48-hour trough samples and 84.9% of the 72-hour trough samples during the initial course of therapy. These percentages did not change significantly during successive courses of therapy, since 95.8% of all 48-hour trough samples (median activity, 0.645 IU/mL) and 84.5% of all 72-hour trough samples (median activity, 0.248 IU/mL) had a serum enzyme activity ≥0.10 IU/mL. Moreover, the serum asparaginase activity was maintained continuously above 0.10 IU/mL for 14 days during course 1 in 34 (68%) of 50 patients. These findings are in excellent agreement with data previously reported for Erwinia asparaginase 25 000 IU/m2 given intramuscularly twice weekly at 3- and 4-day intervals to patients who experienced allergic reactions to weekly E coli asparaginase.25 After switching to Erwinia asparaginase, the median 72-hour NSAA was found to be 0.247 IU/mL, and the asparaginase activity was ≥0.10 IU/mL in 83% of evaluable samples.
AALL07P2 also provides the most comprehensive assessment of the pharmacokinetics of intramuscular Erwinia asparaginase undertaken to date. Although Erwinia asparaginase was first introduced into clinical trials in the early 1970s,49 prior clinical pharmacokinetic studies of this preparation of the enzyme are limited to only two investigations involving relatively small patient numbers.17,18 The time course of serum asparaginase activity resulting from intramuscular injection of a single 25 000-IU/m2 dose of Erwinia asparaginase to pediatric ALL patients was first reported by Asselin et al.17 They found that the mean terminal phase half-life of asparaginase activity was 15.6 ± 3.1 hours for intramuscular Erwinia asparaginase in 10 patients. In our study, the overall mean apparent biological half-life of asparaginase activity for the entire group of 54 patients with evaluable course 1 pharmacokinetic data was 16.5 ± 6.4 hours, in excellent agreement with this value.
In another study, the plasma pharmacokinetics of asparaginase activity was characterized in 13 pediatric ALL patients receiving daily doses of 30 000 IU/m2Erwinia asparaginase given either as a 3-hour intravenous infusion or intramuscular injection.18 Asparaginase activity decayed with a mean half-life of 6.4 ± 1.9 hours when the enzyme was given by the intravenous route. The mean total body clearance of Erwinia asparaginase was 0.16 ± 0.06 L/hour/m2, and it had a mean apparent volume of distribution of 1.35 ± 0.31 L/m2. The time course of asparaginase activity in plasma following intramuscular administration was best described by a one-compartment open model with first-order absorption. However, the much shorter half-life of the enzyme when given intravenously as compared with intramuscularly demonstrated that Erwinia asparaginase exhibits absorption rate-limiting pharmacokinetics, also commonly known as the “flip-flop phenomenon,” when given by the intramuscular route. Under these circumstances, the smaller rate constant derived from the terminal region of decreasing drug levels actually corresponds to ka, whereas the larger rate constant derived from the early region of increasing drug levels corresponds to ke.50 This association is strictly true only if the ratio between the larger and smaller rate constants is sufficiently large (ie, >3); otherwise, estimated values of the rate constants do not approximate either the true ka or the ke.
Results obtained from our study, which involves considerably more patients than previously evaluated, confirms the absorption rate-limiting pharmacokinetics of intramuscular Erwinia asparaginase. Two subpopulations of patients, each of which comprised approximately 50% of the total cohort, were identified by the relative magnitude of ka and ke. Patients designated as group A had kinetically distinguishable apparent rate constants and those designated as group B did not. The mean ka for group A patients (0.15 ± 0.09 hours−1) is in very good agreement with the mean ke previously reported for the loss of asparaginase activity following intravenous administration of Erwinia asparaginase in 13 patients (0.12 ± 0.04 hours−1).18 In this earlier study, the apparent ke for intramuscular Erwinia asparaginase could be estimated for only 7 (44%) of the 16 patients evaluated. It is perhaps more than coincidental that this is similar to the proportion of patients in our study whose enzyme activity-time data were described by equation 1 of the pharmacokinetic model (ie, 48%). In any event, the mean ke for group A patients (0.031 ± 0.013 hours−1) was very similar to the apparent ke reported for the 7 patients receiving intramuscular Erwinia asparaginase in the study by Albertsen et al (0.034 ± 0.005 hours−1).
The mean apparent half-life of serum asparaginase activity is almost twofold shorter for patients in group B as compared with group A. Enzyme absorption from the intramuscular injection site remains rate-limiting for group B patients because the mean apparent half-life in these patients is still two times larger than the mean half-life reported for intravenous administration of the enzyme. It follows that patient-specific factors, which remain to be identified, are probably responsible for influencing the rate of absorption of the enzyme from the intramuscular injection site into systemic circulation, resulting in the observed differences in the pharmacokinetic behavior of asparaginase activity between the two subgroups. The very low bioavailability of intramuscular Erwinia asparaginase, which was reported to be only 27.1% on average and highly variable between patients, as indicated by a 57.7% coefficient of variation, lends additional plausibility to this hypothesis.18 Relatively minor changes in the rate or extent of absorption of the enzyme would have a marked effect on the time course of serum asparaginase activity under these circumstances. Most importantly, the effect does not appear to be clinically relevant for the Erwinia asparaginase dosing regimen evaluated in this study because serum asparaginase activity remained above the 0.10 IU/mL therapeutic threshold for up to 72 hours after dosing in 84.5% of acceptable samples.
The Dana-Farber Cancer Institute ALL Consortium Protocol 95-0140 and European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group 5888146 studies suggested that there were fewer toxicities associated with Erwinia asparaginase therapy than were associated with E coli asparaginase. These studies, however, failed to dose-adjust Erwinia asparaginase based on its shorter half-life, raising concern that suboptimal dosing may have resulted in fewer toxicities. The AALL07P2 dosing schedule of 25 000 IU/m2 on a M/W/F schedule resulted in few clinically significant toxicities. Because of the sample size of this study, firm conclusions cannot be made on the incidence of these targeted toxicities.
In conclusion, Erwinia asparaginase administered intramuscularly using the M/W/F AALL07P2 regimen was well tolerated and achieved a therapeutic NSAA at both 48 and 72 hours after dosing in a high percentage of patients. Although intramuscular administration of Erwinia asparaginase on an every-other-day schedule would theoretically provide greater consistency in the NSAA, the ability to dose on the M/W/F schedule and maintain NSAA ≥0.10 IU/mL with a periodic 72-hour dosing interval enabled the drug to be given during normal operating hours of most outpatient clinics, thereby avoiding the problems and inconvenience associated with weekend dosing. This study provided the basis for the FDA approval, granted in November 2011, to use Erwinia asparaginase in patients with allergic reactions to pegaspargase, substituting a single dose of pegaspargase with Erwinia asparaginase 25 000 IU/m2 × 6 intramuscular doses on a M/W/F schedule.44 Future studies should investigate the pharmacokinetics and toxicity profile of intramuscular Erwinia asparaginase given according to this M/W/F schedule in young adults who are at least 18 years old, since all patients enrolled in AALL07P2 were younger than 18 years of age. In addition, the dose and schedule of intravenous Erwinia asparaginase required to achieve therapeutic NSAA on a continuous basis has yet to be established.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants CA13539 and CA98543 from the National Institutes of Health and a research agreement with Jazz Pharma, Inc (Langhorne, PA), formerly EUSA Pharma, Inc. S.P.H. is the Ergen Family Chair in Pediatric Cancer.
The opinions and assertions contained herein are the private views of the author(s) and are not to be construed as the official policy or position of the US Government, the Department of Defense, or the Department of the Air Force.
Authorship
Contribution: W.L.S., B.A., J.G.S., N.A.K., N.J.W., G.H.R., E.R., W.L.C., and S.P.H. designed research, performed research, collected data, analyzed and interpreted data, and wrote the manuscript; M.D. and P.P. designed research, collected data, analyzed and interpreted data, performed statistical analyses, and wrote the manuscript
Conflict-of-interest disclosure: P.P. is the Senior Vice President of Clinical Oncology for Jazz Pharmaceuticals, Inc. The remaining authors declare no competing financial interests.
Correspondence: Wanda L. Salzer, US Army Medical Research and Materiel Command, Combat Casualty Care Research Program, 504 Scott St, Ft Detrick, MD 21702-5012; e-mail: wanda.l.salzer.mil@mail.mil.