Key Points
eBL was positively associated with anti–HRP-II antibodies and inversely associated with anti-SE36 antibodies.
Anti–HRP-II antibodies suggest that recent malaria infection triggers the onset of eBL; anti-SE36 antibodies suggest long-term infection and immunity.
Abstract
Endemic Burkitt lymphoma (eBL) is linked to Plasmodium falciparum (Pf) infection geographically, but evidence from individual-level studies is limited. We investigated this issue among 354 childhood eBL cases and 384 age-, sex-, and location-matched controls enrolled in Ghana from 1965 to 1994. Immunoglobulin G1 (IgG1) and immunoglobulin G3 (IgG3) antibodies to antigens diagnostic of recent infection Pf histidine-rich protein-II (HRP-II) and 6NANP, Pf-vaccine candidates SE36 and 42-kDa region of the 3D7 Pf merozoite surface protein-1 (MSP-1), and tetanus toxoid were measured by indirect enzyme-linked immunoassay. Odds ratios (ORs) and 95% confidence intervals (CIs) for association with eBL were estimated using unconditional logistic regression. After adjustments, eBL was positively associated with HRP-IIIgG3 seropositivity (adjusted OR: 1.60; 95% CI 1.08-2.36) and inversely associated with SE36IgG1 seropositivity (adjusted OR: 0.37; 95% CI 0.21-0.64) and with tetanus toxoidIgG3 levels equal or higher than the mean (adjusted OR: 0.46; 95% CI 0.32-0.66). Anti–MSP-1IgG3 and anti-6NANPIgG3 were indeterminate. eBL risk was potentially 21 times higher (95% CI 5.8-74) in HRP-IIIgG3–seropositive and SE36IgG1-seronegative responders compared with HRP-IIIgG3–seronegative and SE36IgG1-seropositive responders. Our results suggest that recent malaria may be associated with risk of eBL but long-term infection may be protective.
Introduction
Endemic Burkitt Lymphoma (eBL) is a non-Hodgkin lymphoma with high-incidence clusters in many countries in equatorial Africa and Papua New Guinea, where it accounts for 50% to 75% of all childhood cancers.1 Plasmodium falciparum (Pf) malaria is suspected to play an etiologic role in eBL2 based on the geographical coclustering of high eBL incidence and high incidence of Pf malaria.3 Chronic polyclonal stimulation of B cells by recurrent Pf infection is thought to increase the risk for stochastic translocation of the Myc proto-oncogene on human chromosome 8 into the vicinity of the regulatory elements of immunoglobulin heavy or light chain loci on human chromosome 14, 2, or 22 in B cells, the earliest event in eBL tumorigenesis.4 Alternatively, recurrent Pf infection could influence eBL risk indirectly through impairment of T-cell immunity leading to uncontrolled proliferation of Epstein Barr virus (EBV),5 which is linked to eBL.6
However, direct evidence for a role of Pf malaria infection in eBL in individual-level studies is limited.7 Case-control studies conducted in the 1970s8,9 failed to show significant differences in malaria antibodies in sera of eBL cases and controls, but the small sample size (<60 cases and controls in the largest study), nonrepresentative controls, and use of early-generation antibody tests were limitations. Two case-control studies conducted recently including more cases and better selection of controls have shown a 5 to 12 times higher risk of eBL in children with high titers of whole schizont extract (WSE) antibodies compared with controls.10,11 In both studies, children with eBL were less likely to report using household insecticides or mosquito bed nets at night compared with controls, implying recent exposure to Pf in eBL cases. However, these studies are limited by a focus on antibodies to 1 Pf antigen, which does not fully capture the immunobiology of Pf malaria in eBL.12 Immunity to Pf develops slowly, with the antibody repertoire to various Pf epitopes increasing unevenly in children with comparable levels of repeated or chronic Pf infection.13,14 Moreover, the antibody responses may be transient and may wane quickly in the absence of frequent exposure to Pf parasites.15 A new interest in vaccine development for malaria16 is providing a new conceptual framework to study the link between immunity to Pf and eBL. We reported recently, in a proof-of-concept study, that Ghanaian eBL cases had significantly lower anti-SE36 antibodies than age- and location-matched controls.17 SE36 is a subunit of Pf (Honduras 1) serine repeat antigen-5 protein, which is thought to be vital for completion of the erythrocytic phase of the malaria life cycle and has emerged as a vaccine target.18 In humans, high anti-SE36 titers are associated with a significantly lower risk for severe clinical malaria in children.19 In vitro studies have shown that high anti-SE36 titers inhibit Pf parasite growth in culture,16 and animal trials have shown that Aotus monkeys immunized with SE36 protein are protected against challenge with Pf.17,18
Here, we report results from our investigation expanded to include 4 malaria antigens, namely, SE36 and 1 additional Pf vaccine candidate antigen, the 42-kDa region of the 3D7 Pf merozoite surface protein-1 (MSP-1),16,20 and 2 diagnostic antigens, namely, histidine-rich protein-II (HRP-II)21 and 6 tetrapeptide (6NANP) repeats of circumsporozoite protein (CSP).22
Methods
Study population
Cases were children (0-15 years) with histologically or cytologically confirmed (92% of cases) eBL treated at Korle-Bu Teaching Hospital, Accra, Ghana, from 1965 to 1994.23,24 The cases were mostly from southern areas of Ghana, where malaria transmission intensity is moderate to high (mesoendemic).25 Most controls were apparently healthy children of a similar age and sex to the case enrolled from the nearest neighboring house to the case.26 About one-third of the controls were children referred to the Korle-Bu Teaching Hospital with suspected eBL but were diagnosed subsequently with a benign or a nonlymphoid malignancy. These controls were representative of the cases in terms of referral patterns, so they were included to increase the statistical power of the study.
Cases and controls were enrolled after obtaining permission from a parent or guardian. Children older than 8 years gave assent. Age, sex, and date of enrollment were recorded on structured forms. Malaria status was evaluated by light microscopy at the time of the study and children who were positive were treated, but these results were not recorded consistently. A pretreatment venous blood sample was drawn and separated into aliquots, which were stored at −70°C at the U.S. National Cancer Institute repository until testing. Previously unthawed samples or aliquots of samples that were thawed only once were used in this study. Ethical approval for the current study was obtained from the Office of Human Subject Research at the National Institutes of Health and The George Washington University institutional review board. No identifying subject data were used in this study.
Serological methods
Subclass-specific immunoglobulin G (IgG) subclasses IgG1 and IgG3 against 4 recombinant Pf antigens were measured by an indirect enzyme-linked immunosorbent assay as described in detail in the supplemental Methods. The antigens included SE3618 and the 42-kDa MSP-1 region of 3D7 Pf parasites,20 which are blood-stage vaccine candidates, and HRP-II21 and 6NANP proteins, which are included in kits used to measure current or recent malaria infection.21,22 HRP-II is expressed during the posthepatic blood stage of infection, while 6NANP is part of CSP and it is expressed in the prehepatic stage of infection.27 Tetanus toxoid was included as an antigen irrelevant for eBL to assess general humoral response. Laboratory staff were blinded to the status (case or control) of the samples to minimize bias. Replicate samples (n = 100) were embedded within the same plate and also in different test plates to measure within- and between-plate assay reproducibility. Well-characterized serum from 10 Pf-exposed individuals from Uganda19 was included in all plates as a standard reference sera to enable homologous interpolation of the optical density readings of experimental sera into arbitrary units (AUs) onto a 4-parameter standard calibration curve using the methods proposed by Miura et al28 for measuring antibodies induced by experimental malaria vaccines. This method allows a limit of quantification (LOQ) for each assay to be determined (see supplemental Methods). Samples with AU values below LOQ are considered antibody negative, while samples with AU values equal or above LOQ are considered antibody positive (see supplemental Methods).
Statistical methods
Statistical analyses were performed using STATA (version 11; STATA, College Station, TX). The main analyses were performed using the full set of controls. However, sensitivity analyses were performed using healthy controls only. Within- and between-plate coefficients of variation (CVs), calculated by dividing assay mean by the assay standard deviation of replicate samples, were used to evaluate the reliability of IgG assays. Assay results with within- and between-plate CVs <30% passed and were included in analyses. Assay results with CVs ≥30% failed and were excluded from the analyses. Correlations between log-transformed AU antigen-specific antibody results and between antigen-specific antibody results with age were evaluated using Spearman’s correlation coefficient. The Student t test was used to analyze continuous variables and the χ2 test to analyze categorical variables. Crude and adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for association between eBL and antigen-specific antibody results were estimated using unconditional logistic regression. A trend in the odds ratios (ORs) for eBL with different antigen-specific antibody responder categories of low, medium, or high compared with negative was evaluated using the χ2 test for trend. In this analysis, negative category was defined as AU values less than the LOQ. The low, medium, and high categories were defined using tertile cutoff points for the specific antibody among healthy controls with results equal or greater than the LOQ. The antigen-specific antibody ORs were adjusted for sex, age group (3-year bands), calendar year of enrollment, inclusion in the previous SE36 study,17 and each other. The antigen-specific antibody results were considered a priori to be both exposures and potential confounders of each other. The independent contribution of each antigen-specific antibody result to the full model was evaluated using the likelihood ratio test. Two-sided P values < .05 were considered statistically significant.
Results
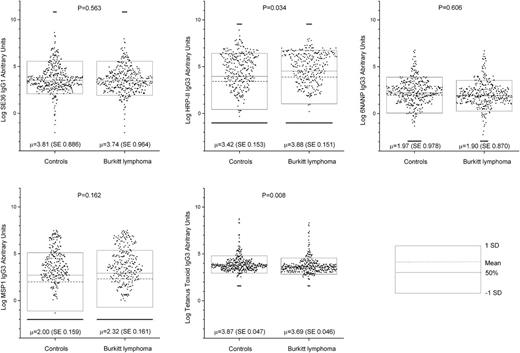
We studied 354 cases and 384 controls (Table 1), including 269 healthy location-matched controls. The gender distribution was similar for the cases and controls. Compared with controls, cases were overrepresented in ages 3 to 8 years and underrepresented from 1965 to 1974, the early years of the study. Five assays passed reliability criteria of within- and between-plate CVs <30% (anti-SE36IgG1, anti–MSP-1IgG3, anti–HRP-IIIgG3, anti-6NANPIgG3, and anti–tetanus toxoid IgG3), while 3 assays failed (CVs ≥30%: anti-SE36IgG3, anti–MSP-1IgG1, and anti–HRP-IIIgG1) and were excluded (supplemental Figure 2). The correlations between antibody results for the 5 assays with acceptable CVs were generally small (correlation coefficient range 0.14-0.31 in all pairs), although some were statistically significant (not shown). Anti–tetanus toxoidIgG3 antibodies were detected in 99% of the cases and 97% of the controls (P = .252; Table 1), suggesting comparable overall access to tetanus vaccination and immune seroconversion among the cases and controls. However, the proportion of children with anti–tetanus toxoidIgG3 response less than the mean titer observed in healthy controls was higher in eBL cases than in controls (78% vs 67%, P = .001, not shown). The proportion of children with antibodies to at least 1 Pf antigen was similar in the cases and the controls (95% in each group, P = .664; Table 1). Antibody positivity was 91% for anti-SE36IgG1, 53% for anti–MSP-1IgG3, 66% for anti–HRP-IIIgG3, and 84% for anti-6NANPIgG3 (Table 2). The mean antibody level (AU) for anti–HRP-IIIgG3 was 0.46-log higher in eBL cases than in controls (P = .034). AU was 0.18-log lower in eBL cases than in controls for anti–tetanus toxoidIgG3 (P = .008). AUs were similar for the remaining antibodies (Figure 1; supplemental Table 1).
Dot plot showing log-transformed (base 2) antibody titers (AUs) to Pf antigens SE36IgG1, HRP-IIIgG3, 6NANPIgG3, MSP-1IgG3, and tetanus toxoidIgG3 for children with eBL and location-matched controls. Each dot represents a single subject. The dotted line inside the box represents the mean, the solid line inside the box represents the median, and the outer lines of the boxes represent values equal to the mean ± 1 standard deviation. The P values are the probability that the mean results in the controls are equal to the mean results in the cases, based on the Student t test. SD, standard deviation; SE, standard error.
Dot plot showing log-transformed (base 2) antibody titers (AUs) to Pf antigens SE36IgG1, HRP-IIIgG3, 6NANPIgG3, MSP-1IgG3, and tetanus toxoidIgG3 for children with eBL and location-matched controls. Each dot represents a single subject. The dotted line inside the box represents the mean, the solid line inside the box represents the median, and the outer lines of the boxes represent values equal to the mean ± 1 standard deviation. The P values are the probability that the mean results in the controls are equal to the mean results in the cases, based on the Student t test. SD, standard deviation; SE, standard error.
After adjusting for sex, age, calendar-year period, inclusion in the previous SE36 study,17 and all antibody results, eBL was inversely associated with anti-SE36IgG1 seropositivity (aOR: 0.37; 95% CI 0.21-0.64; P < .001) and positively associated with anti–HRP-II IgG3 seropositivity (aOR 1.60; 95% CI 1.08-2.36; P = .019; Table 2). eBL was unrelated to anti–MSP-1IgG3 seropositivity (P = .982) and unrelated to anti-6NANPIgG3 seropositivity (P = .844). Consistent with the finding that anti–tetanus toxoid response was lower in eBL cases than in controls, having anti–tetanus toxoidIgG3 levels equal to or higher than the mean was inversely associated with eBL (aOR: 0.46; 95% CI 0.31-0.66; P < .001; Table 2). We therefore evaluated the statistical correlation between anti-SE36IgG1 seropositivity and mean levels for anti–tetanus toxoidIgG3. Among the controls, anti-SE36IgG1 seropositives were more likely than anti-SE36IgG1 seronegatives to have anti–tetanus toxoidIgG3 levels equal to or greater than the mean (35% vs 18%, P = .053). A similar but nonsignificant difference was seen among the cases (23% vs 15%, P = .164). We therefore repeated the analysis looking at association between eBL with anti-SE36 and anti–HRP-II antibodies stratified according to anti–tetanus toxoidIgG3 response less than, or equal to or above, the mean. The inverse association between eBL anti-SE36IgG1 seropositivity was observed in children with anti–tetanus toxoid antibody response less than the mean (OR 0.45; 95% CI 0.26-0.77) and in those with anti–tetanus toxoid antibody response equal to or higher than the mean (OR 0.39; 95% CI 0.12-1.26). Likewise, the positive association between eBL and anti–HRP-IIIgG3 seropositivity was observed in children with anti–tetanus toxoid antibody response less than the mean (OR 1.94; 95% CI 1.30-2.91) and those with anti–tetanus toxoid antibody response equal to or higher than the mean (OR 1.17; 95% CI 0.61-2.24), but the OR was not statistically significant in the latter responder group.
After adjustment, the OR of eBL decreased with increasing tertiles of tetanus toxoidIgG3 responder categories (Figure 2A). The ORs for eBL were unrelated to anti–MSP-1 responder categories (Figure 2B). In contrast with the qualitative results, the ORs for association between eBL with different anti-6NANP responder categories were mixed. The association was positive among the low anti-6NANP antibody responder group but then became negative in the medium and high antibody responder group, with a statistically significant aOR in the high anti-6NANP antibody responder category (Figure 2C).
Forest plot showing crude and adjusted ORs and 95% CIs of association between eBL and subclass-specific antibodies to tetanus toxoid and Pf malaria antigens MSP-1 and 6NANP. Top: tetanus toxoid; middle: MSP-1; bottom: 6NANP. Open circles are crude ORs and filled squares are aORs. Adjusted models included age group, sex, enrollment year, and inclusion or not in the first study of anti-SE36 seroreactivity and antimalaria and anti–tetanus toxoid antibodies (see Methods). The x-axis scales in the first 2 panels are from 0.1 to 10.
Forest plot showing crude and adjusted ORs and 95% CIs of association between eBL and subclass-specific antibodies to tetanus toxoid and Pf malaria antigens MSP-1 and 6NANP. Top: tetanus toxoid; middle: MSP-1; bottom: 6NANP. Open circles are crude ORs and filled squares are aORs. Adjusted models included age group, sex, enrollment year, and inclusion or not in the first study of anti-SE36 seroreactivity and antimalaria and anti–tetanus toxoid antibodies (see Methods). The x-axis scales in the first 2 panels are from 0.1 to 10.
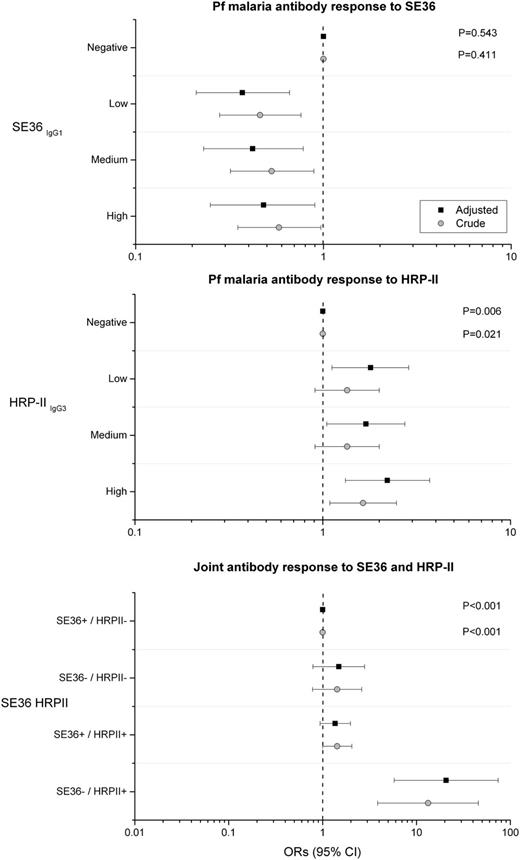
The ORs for association between eBL and anti-SE36 was negative for low, medium, and high vs negative responder categories, but without showing a statistical trend (Ptrend = .543; Figure 3A). Conversely, the ORs for association between eBL with anti–HRP-II were positive for low, medium, and high vs negative responder categories and showed a statistical trend with increasing titers (Ptrend = .006; Figure 3B). The associations between eBL and both anti-SE36 and anti–HRP-II persisted and became stronger with adjustment for baseline characteristics and antibody responses to other malaria antigens (Figure 3A-B). We examined the joint effects of anti-SE36IgG1 and anti–HRP-IIIgG3 reactivity by grouping subjects into 4 responder categories based on a priori biological postulation. The crude OR for eBL was 13 (95% CI 3.85-45.6) in the anti-SE36 seronegative and anti–HRP-II seropositive responder category compared with the anti-SE36 seropositive and anti–HRP-II seronegative responder category (Figure 3C). The association with eBL persisted and became stronger (aOR 21; 95% CI 5.78-74) after adjustment of demographical risk factors, other anti-malaria antibodies, and anti–tetanus toxoid, but the association was imprecise because of relatively small numbers. The aOR for eBL was 1.49 (95% CI 0.79-2.80) in the anti-SE36IgG1 seronegative and anti–HRP-IIIgG3 seronegative responder group and 1.36 (95% CI 0.94-1.98; Figure 3C) in the anti–HRP-IIIgG3 seropositive and anti-SE36IgG1 seropositive responder group.
Forest plot showing crude and adjusted ORs and 95% CIs of association between eBL and subclass-specific antibodies to Pf malaria antigens SE36 and HRP-IIIgG3 and joint effects of detection of antibodies to SE36 and HRP-II. Top: SE36; middle: HRP-IIIgG3; bottom: joint effects of detection of antibodies to SE36 and HRP-II. Open circles are crude ORs and filled squares are aORs. Adjusted models included age group, sex, enrollment year, and inclusion or not in the first study of anti-SE36 seroreactivity and antimalaria and anti–tetanus toxoid antibodies (see Methods). The x-axis scales in the first 2 panels are from 0.1 to 10, while the x-axis scale in the third panel is 0.01 to 100.
Forest plot showing crude and adjusted ORs and 95% CIs of association between eBL and subclass-specific antibodies to Pf malaria antigens SE36 and HRP-IIIgG3 and joint effects of detection of antibodies to SE36 and HRP-II. Top: SE36; middle: HRP-IIIgG3; bottom: joint effects of detection of antibodies to SE36 and HRP-II. Open circles are crude ORs and filled squares are aORs. Adjusted models included age group, sex, enrollment year, and inclusion or not in the first study of anti-SE36 seroreactivity and antimalaria and anti–tetanus toxoid antibodies (see Methods). The x-axis scales in the first 2 panels are from 0.1 to 10, while the x-axis scale in the third panel is 0.01 to 100.
Discussion
We showed positive associations between eBL and anti–HRP-IIIgG3 positivity, considered to indicate recent or past malaria infection, and an inverse association between eBL and anti-SE36IgG1 positivity, considered a vaccine candidate antigen. The HRP-II results confirm the positive associations between eBL and Pf malaria WSE reported in 2 prior case-control studies.10,11 Conversely, the SE36 results confirm our previous anti-SE36 antibody findings17 using results obtained from an independent laboratory. Using joint HRP-IIIgG3 and SE36IgG1 results to define extreme responder groups, the risk of eBL was potentially 13 to 20 times higher in children with a recent infection but not a protected responder profile compared with the children with a protected or no recent infection profile. Possibly, anti-SE36 antibodies indicate long-term exposure to Pf, which could be suggestive of immunity, while anti–HRP-II antibodies suggest recent infection with Pf, which might suggest that recent exposure to malaria might trigger eBL onset and/or progression.
Biologically, Pf malaria infection induces B-cell proliferation, which might trigger chromosomal translocation of c-MYC, the earliest event in eBL pathophysiology. In addition, temporary immunosuppression by recurrent malaria infection may predispose children to EBV infection or trigger reactivation of latent EBV infection, which may lead also lead to eBL onset.29,30 Our results highlight recent malaria as a trigger for eBL and the need for future research directed at understanding the relationship between humoral immune response to a wide array of Pf antigens that are associated with risk for eBL. Follow-up studies should take advantage of the novel proteomic technologies to fully characterize the repertoire of antibodies to Pf that may be predictive of eBL.10,11
The inverse association between eBL and anti–tetanus toxoid antibodies was unexpected. The results could be due to chance, given that we studied many antibody outcomes. Chance, however, is unlikely because previous studies have reported impairment of total serum IgG, immunoglobulin M, and immunoglobulin A levels and malaria-specific immunoglobulin M antibodies among eBL cases when compared with controls in Ghana,24 Nigeria,31 and Uganda,32 and anti-measles antibodies were also low in cases in the Uganda study.32 The pathogenesis of immunoglobulin impairment is unknown, but various mechanisms, including filtration loss into urine,31 immunosuppression due to disease,33 and increased antibody catabolism,24 have been suggested. These mechanisms would lead to general impairment of immunoglobulin levels, but 3 exceptions suggest that immunoglobulins are not globally impaired in eBL. Immunoglobulin levels are higher in eBL cases than in controls against EBV virus capsid antigen,6 Pf WSE,10,11 and, based on our results, HRP-II protein. The associations of eBL with low anti-SE36IgG1 and with high anti–HRP-IIIgG3 are likely valid because the overall associations continued to be in same direction and of similar magnitude in the anti–tetanus toxoid–stratified analyses, suggesting that the results were not confounded. We found a positive correlation between high anti–tetanus toxoid antibody response and anti-SE36 antibody responses, which might suggest that low antibodies to SE36 and tetanus toxoid are due to a common factor, but the nature of such a factor is unknown. If such humoral deficits precede eBL, they could potentially influence the successful establishment of EBV infection and subsequent oncogenic transformation.
We found a null association between eBL and antibodies to the 42-kDa region of 3D7 MSP-1 antigen, thereby confirming null results from a small study (32 eBL cases and 25 age- and residence-matched controls) conducted in Kenya.34 Although the 42-kDa MSP-1 region of 3D7 Pf parasites includes protection-bearing epitopes,16 a recent vaccine trial conducted in Kenya using the same antigen failed to demonstrate protection against severe Pf malaria.20 Our findings for anti-6NANP reactivity are mixed and could be due to chance. However, follow-up studies are warranted given that RTS,S/AS01,35 the most successful malaria vaccine to date, is based on protection-inducing epitopes of the central repeat region fused to the C-terminal region of the CSP.16
Our study has some limitations. Our results are based on case-control data, so temporality of association cannot be determined and reverse causality is a concern.36 However, our choice of Pf antigens according to whether or not they possess protection-inducing epitopes and from different Pf life stages allows biologically plausible associations to be evaluated. Moreover, our analysis included only results from assays measured to a high degree of reliability, which minimizes null associations generated by substantial measurement error. We used both community- and hospital-based controls, which may have introduced some biases.37 However, the finding of similar associations in analyses restricted to community controls was reassuring. Although malaria positivity was evaluated by light microscopy at the time of the study, these results were not recorded completely and cannot be included in the current analysis. Finally, the samples had been stored for long periods. However, sample storage period was comparable for cases and controls and the samples were stored at −70°C, which we believe preserved antibodies adequately.38
In conclusion, we report a positive association between eBL and anti–HRP-IIIgG3 antibodies and a negative association between eBL and anti-SE36IgG1 antibodies. Our results provide further support for the hypothesis that recent malaria infection may be associated with risk of eBL but long-term infection may be protective.
Presented in abstract form at a late-breaker abstract session at the 54th annual meeting and exposition of the American Society of Hematology, Atlanta, GA, December 11, 2012.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr James J. Goedert at the Infections and Immunoepidemiology Branch at the National Cancer Institute (Bethesda, MD) for editorial comments, Dr Ruth Parsons at Information Management Services (Rockville, MD) for preparing data analysis files, and Mr David Check at the Biostatistics Branch at the National Cancer Institute (Rockville, MD) for help drawing the graphs.
This work was supported by a grant from the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services (grant number N01-CO-12400) and by The Biomedical Kansai project supported by the Regional Innovation Cluster Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan and The George Washington University.
Authorship
Contribution: P.A. analyzed and interpreted data and drafted the manuscript; M.C.V., A.J., M.V.P., T.T., M.Y., N.M.Q.P., T.H., and J.M.B. conducted laboratory tests, interpreted data, and edited the manuscript; F.N., J.N., P.H.L., and R.J.B. conducted field work and interpreted data; B.E. and C.K. helped with the analysis and interpretation of data, and R.M.P. advised on the analysis and interpretation of data; K.B. interpreted data; S.M.M. conceived the idea, guided data analysis, interpreted data, and edited the manuscript; and all authors had access to data and commented on and contributed to the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sam M. Mbulaiteye, National Institutes of Health/NCI, 9600 Medical Center Dr, Rm 6E118, MSC 9704, Bethesda, MD 20892-9704; e-mail: mbulaits@mail.nih.gov.