Key Points
Reduction in ADAMTS13 function and complement dysregulation coexist in a significant number of patients with aHUS.
Variations in the ADAMTS13 gene (polymorphisms and rare variants) are partly responsible for the reduced ADAMTS13 function in aHUS.
Abstract
Complement dysregulation leads to atypical hemolytic uremic syndrome (aHUS), while ADAMTS13 deficiency causes thrombotic thrombocytopenic purpura. We investigated whether genetic variations in the ADAMTS13 gene partially explain the reduced activity known to occur in some patients with aHUS. We measured complement activity and ADAMTS13 function, and completed mutation screening of multiple complement genes and ADAMTS13 in a large cohort of aHUS patients. In over 50% of patients we identified complement gene mutations. Surprisingly, 80% of patients also carried at least 1 nonsynonymous change in ADAMTS13, and in 38% of patients, multiple ADAMTS13 variations were found. Six of the 9 amino acid substitutions in ADAMTS13 were common single nucleotide polymorphisms; however, 3 variants—A747V, V832M, and R1096H— were rare, with minor allele frequencies of 0.0094%, 0.5%, and 0.32%, respectively. Reduced complement and ADAMTS13 activity (<60% of normal activity) were found in over 60% and 50% of patients, respectively. We concluded that partial ADAMTS13 deficiency is a common finding in aHUS patients and that genetic screening and functional tests of ADAMTS13 should be considered in these patients.
Introduction
Thrombotic thrombocytopenia purpura (TTP) and hemolytic uremic syndrome (HUS) are thrombotic microangiopathies (TMAs) with overlapping clinical phenotypes. TTP is characterized by the pentad of neurological symptoms: fever, microangiopathic hemolysis, thrombocytopenia, and renal failure; whereas in HUS the presenting triad is typically limited to microangiopathic hemolysis, thrombocytopenia, and renal failure. In spite of this overlap, distinctly different etiologies define these diseases.
Most cases of TTP are due to a deficiency in the activity of the Von Willebrand factor (VWF)–cleaving protease, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), caused by either inherited mutations or inhibitory autoantibodies. Severe deficiency of ADAMTS13 activity to <5% as measured by cleavage assays is considered diagnostic for TTP, although ADAMTS13 deficiency does not inevitably result in TTP. In addition to several reports of healthy individuals with severely reduced ADAMTS13 activity (usually family members of TTP patients), many TTP patients have persistently low levels of ADAMTS13 during disease remission.
The inciting event in the pathogenesis of typical or endemic HUS is infection with Shiga-toxin-producing bacteria, but in about 10% of patients with HUS there is no history of antecedent exposure to Shiga toxin. These atypical cases (aHUS), characterized by relapsing thrombotic microangiopathy that often leads to end-stage renal failure, have been linked to genetic abnormalities in the alternative pathway of the complement cascade, with mutations reported in complement factor H (CFH), CD46, complement factor I (CFI), complement factor B (CFB), complement component 3 (C3), complement factor H-related 5 (CFHR5), and thrombomodulin (THBD). In about 5% to 10% of aHUS patients, the disease is triggered by autoantibodies to factor H that are usually associated with homozygosity for a copy number variation, the tandem deletion of CFHR3 and CFHR1, 2 genes in the complement factor H gene family that lie immediately 3′ of CFH.
Normal ADAMTS13 activity has been reported in patients with typical HUS; however, in one study about 25% of aHUS patients had reduced ADAMTS13 activity.1 This observation suggests that in aHUS reduced ADAMTS13 activity may contribute to disease pathogenesis. To test this hypothesis, we measured ADAMTS13 activity in sera of 29 aHUS patients. In 26 patients, DNA samples were available for mutation screening of ADAMTS13 and multiple complement genes.
Methods
Atypical HUS patients
Twenty-nine patients diagnosed with aHUS by standard clinical criteria were included in this study. Patients with positive assays for Shiga-toxin assay were excluded. All studies were approved by the Institutional Review Boards of the University of Iowa and the University of Texas MD Anderson Cancer Center. Informed consent was obtained in accordance with the Declaration of Helsinki.
Serum samples, collection, and storage
Patient serum samples were collected from 6 mL of whole blood drawn in a serum Vacutainer tube and allowed to clot at 37°C for 45 minutes. Clotted blood was centrifuged at room temperature (1000 × g) for 10 minutes. Cell-free serum was removed and immediately placed at −80°C. All serum samples requiring shipping were shipped frozen on dry ice. Patient serum samples were collected at least 2 weeks after any plasma exchange or eculizumab treatment. Information about other plasma infusions prior to collection of serum samples was not available in these patients. Serum was collected after the acute presentation at varying intervals from the time of disease diagnosis. Clinical data are available for review in supplemental Table 1, found on the Blood Website.
Reagents
The ADAMTS13 activity assay kit (Hologic Gen-probe, Inc., San Diego, CA), pooled normal human serum (Complement Technology, Inc., Tyler, TX), normal human serum samples (Bioreclamation, Westbury, NY), PGEX-2T vector (GE Healthcare, Piscataway, NJ), rabbit anti-glutathione S-transferase (GST) antibody (Invitrogen, Grand Island, NY), and secondary anti-rabbit horseradish peroxidase-conjugated IgG antibody (GE Healthcare) were purchased from the indicated sources.
Recombinant GST-VWF-A2 fusion protein
The human complementary DNA (cDNA) sequence encoding the VWF-A2 domain (amino acids P1480-G1672) was polymerase chain reaction (PCR)–amplified from VWF cDNA (5′ primer, ACTGGATCCCCGGGGCTCTTGGGG; 3′ primer, CAGGAATTCTCCGGAGCAGCACCT), digested with BamH I and EcoR I, and ligated into the pGEX-2T GST vector. The GST-A2 recombinant fusion protein was expressed in Escherichia coli, and purified by GSTrap FF columns and the GST buffer kit (GE Healthcare). The eluted protein was concentrated to 100 µg/mL and dialyzed in phosphate-buffered saline (pH 7.6) with mini dialysis units (Thermo Scientific, Rochester, NY).
Generating recombinant ADAMTS13 variants
Full length of human ADAMTS13 cDNA in pSecTag2B expression vector (Invitrogen) was obtained from Dr Jing-Fei Dong’s laboratory (Puget Sound Blood Center, Seattle, WA). Single-nucleotide ADAMTS13 mutations (Q448E, 1342C>G; P618A, 1852C>G; A732V, 2195C>T; A747V, 2240C>T; V832M, 2494G>A; A900V, 2699C>T; A1033T, 3097G>A; R1060W, 3178C>T; R1096H, 3287G>A) were introduced into wild-type (WT) ADAMTS13 cDNA by PCR-based mutagenesis using the QuikChange lightning site–directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). The accuracy of generated constructs was confirmed by DNA sequencing. The sequences of mutagenesis primers were provided in supplemental Table 2.
PSecTag2B vectors carrying the cDNA encoding ADAMTS13 (WT and variants) were transfected into human embryonic kidney (HEK) 293 cells purchased from the American Type Culture Collection (CRL-1573). HEK 293 cells were cultivated in Dulbecco’s modified Eagle medium (DMEM) medium (Invitrogen) containing 10% fetal calf serum. Subconfluent cells grown in 150-mm dishes were washed 3 times with serum-free DMEM medium and kept in 10-mL Opti-MEM (minimum essential medium). The cells were transfected with 10 µg plasmid DNA and 50 µL lipofectamine 2000 (Invitrogen). Six hours after transfection, Opti-MEM medium was replaced by DMEM medium containing 10% fetal calf serum. Transfected cells were selected by Hygromycine B (Invitrogen) at a final concentration of 200 µg/mL. Conditioned media from stably transfected cells were collected and processed by affinity purification using Ni-His GraviTrap column (GE Healthcare). The concentration of each purified protein was determined with the BCA protein assay kit (Pierce, Rockford, IL), using bovine serum albumin (Bio-Rad, Hercules, CA) as a standard.
To compare secretion of ADAMTS13 variants by transfected HEK 293 cells, we collected serum-free conditioned media 48 hours after transfection, from which proteins were purified using Ni-NTA Fast Start Kit (Qiagen, Venlo, The Netherlands). Transfected cells were lysed with sample buffer to obtain total cell lysate. Proteins obtained from cell media and cell lysates were separated and immunoblotted using an antibody against ADAMTS13 (Immunogen affinity purified Goat Sera; Novus Biologicals, Littleton, CO).
ADAMTS13 activity
We measured ADAMTS13 activity in 30 serum samples from normal controls and in all patient serum samples using fluorescence resonance energy transfers (FRETS) VWF73 and VWF-A2 as substrates.
FRETS-VWF73 cleavage assay.
ADAMTS13 activity was measured as the rate of cleavage of a fluorogenic substrate that contains 73 amino acids of the VWF-A2 domain with FRET tags on either side of the ADAMTS13 cleavage site (FRETS-VWF73) following the manufacturer’s protocol (Hologic Gen-probe, Inc., San Diego, CA). Briefly, 5 µL of each plasma or serum sample—or in some experiments, 1 µg of purified recombinant ADAMTS13—was diluted with supplied buffer to a total volume of 50 µL, and incubated for 30 minutes at room temperature with 50 µL of substrate (FRETS-VWF73) at a final concentration of 1 µM. Fluorescence was measured at 440–460 nm using a BioTek fluorometer (BioTek, Winooski, VT), measuring ADAMTS13 activity as the increase in fluorescence at 30 minutes in comparison with base line. This value was used to calculate ADAMTS13 activity in each sample using the calibration curve obtained from standards provided with each kit. In those samples showing reduced ADAMTS13 activity, different concentrations of FRET-VWF73 (0, 1, 2, 3, and 4 µM) were used to determine the relative rate of substrate cleavage. Data were fitted to a Michaelis–Menten equation, calculating Vmax, Km, and kcat using a Lineweaver–Burk plot.
Recombinant VWF-A2 cleavage assay.
ADAMTS13 activity was measured using recombinant GST-conjugated VWF-A2 as a substrate. Twenty microliters of VWF-A2 (100 µg/mL) was diluted with 20 µL of cleavage buffer (100 mM Tris-HCl, pH 8.5, 4 mM calcium chloride, 0.02% Brij-35) and incubated with 1 μL of normal or aHUS sera for 2 hours at 37°C. Cleavage activity was terminated by adding 40 µL of reduced sample buffer. GST VWF-A2 cleavage products were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (10% gels), transferred to immobilon-P membranes (Millipore, Billerica, MA), blotted with rabbit anti-GST antibody, visualized by chemiluminescence, and quantified by densitometry using National Institutes of Health (NIH) Image J software (rsb.info.nih.gov/nih-image). Cleavage of VWF-A2 was determined by measuring the band density ratio of cleaved products/(cleaved products + noncleaved VWF-A2). Results were normalized to ADAMTS13 activity of normal pooled sera and presented as the mean ± standard deviation (SD).
DNA analysis
Genomic DNA was extracted from blood samples using a QIAamp DNA Blood Kit (Qiagen, Valencia, CA). Coding exons and intron–exon boundary junctions for ADAMTS13 (NM_139025.3), CFH (NM_000186.3), CFI (NM_000204.3), CD46 (NM_002389.4), CFB (NM_001710.5), C3 (NM_000064.2), and THBD (NM_000361.2) were amplified by PCR using gene-specific primers and standard conditions. All PCR amplifications cycles were performed on Applied Biosystems 2720 thermal cyclers (Life Technologies, Carlsbad, CA). Amplified PCR products were run on 2% agarose gels and imaged using an α Innotech Fluor Chem SP imager (ProteinSimple, Santa Clara, CA). PCR products were then bidirectionally sequenced with using standard Big Dye Terminator version 3.1 protocols (Life Technologies), followed by analysis on an ABI 3130×l Capillary Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequence analysis and mutation detection were performed using Sequencher version 5.0 software (Gene Codes, Ann Arbor, MI).
Copy number variation was assessed by multiplex ligation-dependent probe amplification (MLPA) across the CFH–CFHR5 region. MLPA probes were designed according to MRC-Holland’s guidelines (MRC-Holland, Amsterdam, The Netherlands). MLPA was performed following the manufacturer’s instructions. Amplified PCR fragments were separated by capillary electrophoresis on a 3130×l genetic analyzer, 50-cm array, and POP7 polymer using the GeneScan 500 LIZ size standard (Applied Biosystems). Peak areas were analyzed using GeneMapper version 4.0 software (Applied Biosystems).
Complement alternative pathway functional assays
Hemolytic assay.
A hemolytic assay using sheep erythrocytes was completed as previously described.2 This assay measures complement-mediated lysis of sheep erythrocytes caused by activation of the alternative pathway. Sheep erythrocytes are nonactivators of complement-mediated lysis in human serum. Observed lysis was graded as follows: normal (<3%); 1+ (3% to 20%); 2+ (20% to 40%); 3+ (40% to 60%); 4+ (60% to 80%); and 5+ (80% to 100%, complete hemolysis).
Alternative pathway functional activity (APFA).
The Wieslab complement alternative pathway assay kit was used to evaluate alternative pathway complement activity as described.2 This method induces alternative pathway activation and uses labeled antibodies specific for terminal complement components to quantitate the result of complement activation, with the amount of neoantigen generated being proportional to the functional activity of the alternative pathway. On-going activation of complement causes consumption of complement components and lowers their concentration. Thus, low levels of terminal complement components suggest that complement has been activated by pathologic or immunological mechanisms prior to testing. Results were expressed as a percentage of normal (normal reference range, 65% to 130% based on 50 normal sera samples).
CFH autoantibody assay
Factor H autoantibodies were analyzed by enzyme-linked immunosorbent assay as described.2 An optical density of 2 SDs above normal (based on 50 healthy controls) was considered positive (normal reference range: <300 units).
Results
Atypical aHUS patients
Demographic and clinical data are summarized in supplemental Table 1. DNA samples were available for genetic studies on 26 patients.
DNA analysis
Mutations in complement genes were identified in 13 patients (Table 1). CFH was the most frequently mutated gene (6 mutations), with mutations also identified in CFI (2 mutations), C3 (2 mutations), CFB (2 mutations), and THBD (3 mutations). Of the 6 mutations seen in CFH, 4 are predicted to cause early truncation of the CFH transcript (E625X, C915X, E1071fsX1089, and E1195X), and another is located in the last short consensus repeat (SCR) of CFH (S1191L). The CFB mutation, p.F286L, is located within the von Willebrand type A domain and has been reported to be a gain-of-function mutation of CFB resulting in increased alternative pathway activation.3 No patients were homozygous for the ΔCFHR3-CFHR1 by MLPA, although 1 patient carried an additional copy number of CFHR3 and a hybrid CFHR1/CFH fusion gene comprising CFHR1 SCRs 1–3 and CFH SCRs 19–20.
Sequence analysis of ADAMTS13 identified 9 amino acid changes in 26 patients; 21 patients (80%) patients had at least 1 variation, and 10 patients (38%) had multiple variations (Table 2). Six of the 9 amino acid substitutions—R7W, Q448E, P618A, A732V, A900V, and A1033T—were common single nucleotide polymorphisms (SNPs) with minor allele frequencies >1%; however, the remaining 3 missense variants—A747V, V832M, and R1096H—were rare variants with minor allele frequencies of 0.0094%, 0.5%, and 032%, respectively (1000 Genome Projects Consortium).
Complement functional assays and CFH autoantibodies
APFA was below normal (<65%) in 19 patients (Table 1), a finding consistent with excessive complement activation and consumption of complement proteins. Genetic mutations in complement genes were present in 14 of these 19 patients (Table 1). The hemolytic assay demonstrated excessive hemolysis in 7 patients with abnormal APFA results; in the remaining 11 patients it was normal (normal hemolytic assays in the presence of an abnormal APFA are likely to be false-negative results secondary to depletion of complement components). Three patients had elevated titers of anti-CFH antibodies (>1/50 titration).
ADAMTS13 activity
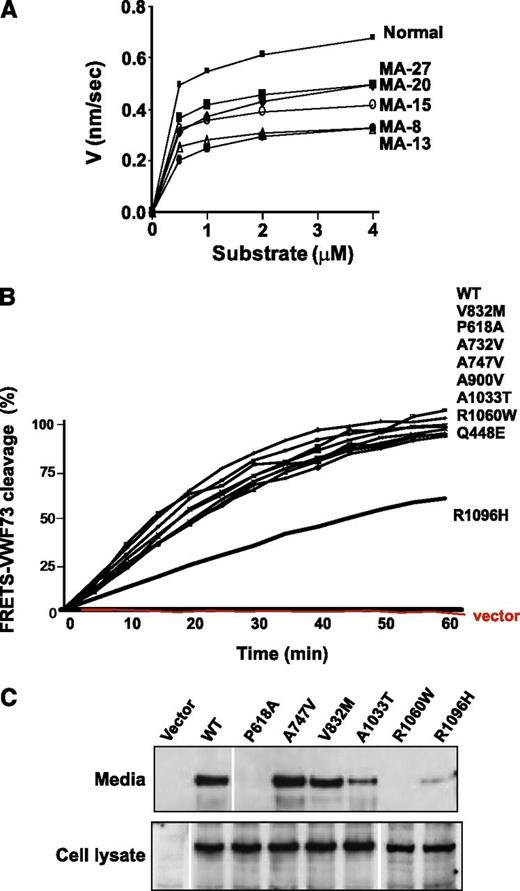
In control normal serum samples (n = 30), ADAMTS13 activity was measured using FRETS VWF73 and VWF-A2 as substrates, and found to be 119% ± 10% (mean ± SD) and 97% ± 3%, respectively. In the aHUS cohort, 15 patients (50%) had reduced ADAMTS13 activity (≤60%) as measured by either the FRETS-VWF73 cleavage assay or the recombinant VWF-A2 cleavage assay (Table 2). Six patients (20%) had reduced ADAMTS13 activity on both assays (Table 2). We completed kinetic studies on sera from 5 patients with reduced ADAMTS13 activity by monitoring the amount of fluorescence generated every 2 minutes for 30 minutes and estimated ADAMTS13 cleavage activity to be about half of normal (average, 44%; range, 21% to 57%) (Figure 1A).
ADAMTS13 activity in aHUS (serum and recombinant). (A) Different concentrations of FRETS-VWF73 substrate were used to determine the kinetics of ADAMTS13 activity in serum samples of aHUS patients with reduced ADAMTS13 activity (patient identification numbers MA-8, MA-13, MA-15, MA-20, and MA-27) and in normal pooled serum. The results are shown as a linear graph summarizing 3 separate experiments. The generated fluorescence was correlated with the concentration of cleaved FRETS-VWF73, using data obtained through a calibration standard with 100% activity. (B) Activity of purified recombinant ADAMTS13 molecules (WT and mutants) was measured by monitoring fluorescence generated as a result of cleavage of FRETS-VWF73 substrate after incubation with 1 µg of recombinant ADAMTS13. (C) Comparison of secretion of recombinant ADAMTS13 variants to cell culture media. Forty-eight hours after transfection of HEK293 cells with cDNAs encoding ADAMTS13 (WT and mutants), supernatants and cell lysates were collected and used for western and immunoblotting using polyclonal ADAMTS13 antibody. ADAMTS13 in media is secreted from the cells, and ADAMTS13 in the cell lysates is synthesized inside the cells.
ADAMTS13 activity in aHUS (serum and recombinant). (A) Different concentrations of FRETS-VWF73 substrate were used to determine the kinetics of ADAMTS13 activity in serum samples of aHUS patients with reduced ADAMTS13 activity (patient identification numbers MA-8, MA-13, MA-15, MA-20, and MA-27) and in normal pooled serum. The results are shown as a linear graph summarizing 3 separate experiments. The generated fluorescence was correlated with the concentration of cleaved FRETS-VWF73, using data obtained through a calibration standard with 100% activity. (B) Activity of purified recombinant ADAMTS13 molecules (WT and mutants) was measured by monitoring fluorescence generated as a result of cleavage of FRETS-VWF73 substrate after incubation with 1 µg of recombinant ADAMTS13. (C) Comparison of secretion of recombinant ADAMTS13 variants to cell culture media. Forty-eight hours after transfection of HEK293 cells with cDNAs encoding ADAMTS13 (WT and mutants), supernatants and cell lysates were collected and used for western and immunoblotting using polyclonal ADAMTS13 antibody. ADAMTS13 in media is secreted from the cells, and ADAMTS13 in the cell lysates is synthesized inside the cells.
Secretion and activity of recombinant ADAMTS13 variants
We generated recombinant ADAMTS13 molecules carrying the variations identified in this study and used the FRETS-VWF73 cleavage assay to measure the functional significance of these changes. Only the R1096H showed a significant difference in activity in comparison with normal recombinant ADAMTS13 (Figure 1B). This variant, detected in 1 aHUS patient in combination with the A747V, was associated with 50% reduced cleavage activity. Consistent with this result, sera samples from this patient had less than 40% activity in both ADAMTS13 activity assays. No complement mutations were identified in this patient (MA-13).
To determine whether any of these variants affect synthesis and secretion of ADAMTS13, we compared amounts of ADAMTS13 in the conditioned media and in whole cell lysates prepared from HEK293 cells transfected with WT or mutant ADAMTS13 cDNAs (Figure 1C). P618A and R1096H variations reduced secretion of ADAMTS13 in comparison with WT, as was evident by the reduced amount of ADAMTS13 in the media of cells transiently transfected with P618A and R1096H cDNAs. The R1060W variant of ADAMTS13 has been known to be associated with intracellular retention of ADAMTS134,5 and was included as a control. P618A was identified in 4 of our aHUS patients with <60% ADAMTS13 activity and 1 patient with >60% activity. R1096H was present in 1 patient with reduced ADAMTS13 activity and was not found in patients with normal ADAMTS13 activity.
Discussion
TMAs can be diagnosed in different clinical settings, including sepsis, TTP, HUS, malignancy, medications, solid-organ transplantation, hematopoietic stem-cell transplantation, and pregnancy related. Although the genetic basis for many of these conditions is unknown, a deficiency of ADAMTS13 and decreased complement regulation underlie the pathogenesis of TTP and aHUS, respectively. Several aspects of the TTP and aHUS phenotypes overlap, suggesting the possibility of commonalities in the pathogenesis of these 2 diseases. In an illustrative case report published by Noris and colleagues, 2 members of a family presented with TMAs, one with end-stage renal failure, suggesting the diagnosis of aHUS, and the other with CNS involvement and cerebrovascular accidents, suggesting TTP.6 Both patients shared a known aHUS-associated CFH mutation, although in the patient with TTP, a mutation was also found in ADAMTS13.
ADAMTS13 activity has been measured in patients with HUS and found to be normal,7 although in patients with aHUS, there are 2 reports implicating partial to severe deficiency of ADAMTS13 cleavage activity in familial disease.1,8 Remuzzi et al and Veyradier et al also noted that it can be very difficult to clinically differentiate congenital TTP (Upshaw-Schulman syndrome due to deficiency of ADAMTS13) from infantile-onset aHUS.1,8 Partial deficiency of ADAMTS13 also has been reported in sepsis9,10 and pregnancy-associated HELLP syndrome (hemolysis, elevated liver enzyme, and low platelet),11 both diseases associated with TMA.
We investigated the possibility that reduced ADAMTS13 activity and complement dysregulation coexist in aHUS by studying a cohort of patients diagnosed with aHUS. We measured ADAMTS13 activity using both FRETS-VWF73 and the recombinant VWF-A2 peptide as substrates. Both methods have been validated for quantitating ADAMTS13 activity,12,13 although we found that the FRETS-VWF73 cleavage assay had a larger intrasample variability (±5.6%) in comparison with the VWF-A2 cleavage assay (±2.8%).
Consistent with the clinical diagnosis in these patients, none had a severe deficiency of ADAMTS13. We found, however, that about 50% of patients had ADAMTS13 cleavage activity below 60% of normal on at least 1 assay, which is more than 2 SDs below the normal reference range in our control samples. In 6 patients (20%), ADAMTS13 cleavage activity was reduced in both assays. These results are consistent with several studies evaluating ADAMTS13 activity assays, which show significant interassay and interlaboratory concordance between assays when ADAMTS13 activity is severely reduced but less accuracy and reproducibility when the reduction in ADAMTS13 activity is mild to moderate.14,15 An additional important consideration in measuring ADAMTS13 activity in patients with aHUS (or other disorders associated with intravascular hemolysis) is that free hemoglobin inhibits ADAMTS13 activity.16 Of note, we did not identify any significant correlation between hemoglobin values and ADAMTS13 activity in our patient cohort.
In aHUS, excessive activation of the alternative complement system is the primary pathogenic mechanism of disease. Complement activation results in endothelial cell damage, leukocyte recruitment, and platelet activation, which cause microvascular thrombosis leading to TMA. Autoantibodies and genetic abnormalities in alterative pathway regulators and activators are found in about 60% of patients.17 Consistent with such data, we identified factor H autoantibodies and genetic mutations in 14 of 26 patients tested (54%). In 73% of our patients, we also documented excessive activity of the alternative complement system by functional assays.
Sequence analysis of ADAMTS13 identified both common and rare variants. Mutations of ADAMTS13 that cause severe deficiency in either the expression or function of this protein are well documented with TTP, but there is a growing body of evidence that several relatively common polymorphisms within the coding region impact the activity and secretion of ADAMTS13 (Table 3). The first report on this type of functional polymorphism showed that the P475S variant (c.1423C>T) had reduced activity,18 and the magnitude of this reduction depended on the method used to measure ADAMTS13 activity.19 P475S has been reported in Japanese,18 Korean,20 and Chinese21 populations but not among Caucasians.22 Plaimauer et al expressed several constructs of recombinant ADAMTS13 and observed that both the A732V and P618A polymorphisms reduced secretion (60% and 27%, respectively) and activity (72% and 14%, respectively) in comparison with WT.23 In addition, they discovered that specific combinations of SNPs modify ADAMTS13 expression and activity. For example, though either R7W or Q448E had little impact on ADAMTS13 secretion and activity, their combination reversed the reduced activity and secretion of the ADAMTS13 phenotype associated with P618A and A732V. In a separate study, Schettert et al discovered an association between the A900V variant and increased risk of death by cardiovascular events.24 Although the exact mechanism by which the A900V variant contributes to cardiovascular pathology is unknown, an increase in platelet thrombi formation as a result of reduced ADAMTS13 activity or secretion is a plausible mechanism.
Because caution must be used in attributing a functional consequence to a detected variation in ADAMTS13, we generated recombinant mutant ADAMTS13 and completed additional studies that showed that the R1096H variant reduced protein activity, and the P618A and R1096H variants reduced protein secretion, providing a probable mechanistic basis for low ADAMTS13 activity in 27% of our cohort. Although the cause of reduced ADAMTS13 activity in the remaining patients remains to be determined, our data strongly suggest that reduced ADAMTS13 activity contributes to the TMA phenotype in some aHUS patients. Partial deficiency of ADAMTS13 alone will not induce a TMA phenotype but may act together or even synergistically with a primary pathophysiologic trigger, such as an inherited mutation in a complement gene or overproduction and secretion of VWF following endothelial damage, to generate the pathologic state. Consistent with this scenario, 50% of our patients had both excessive complement activation and partially decreased ADAMTS13 activity. Whether the functional consequence of mutations in complement proteins has a direct role in reducing ADAMTS13 function is not known, but it is reasonable to speculate that low ADAMTS13 activity reduces the threshold for a florid TMA phenotype in patients with complement mutations. This relationship may also help to explain the issue of incomplete genetic penetrance in aHUS, because only 50% of individuals with complement mutations present with disease by the age of 45.25
In summary, our data implicate ADAMTS13 in the pathogenesis of aHUS and suggest that there exists a subgroup of patients with aHUS in whom complement is dysregulated and ADAMTS13 function is reduced. If additional studies confirm our results, by including genetic tests and functional assays of ADAMTS13 in the evaluation of aHUS patients, we may better define genotype–phenotype relationships, thus improving our understanding of the disease. The simultaneous presence of complement dysregulation and ADAMTS13 deficiency may also blur the distinction between aHUS and TTP in some patients with TMA. Lastly, additional insight into the pathophysiology of aHUS may facilitate the identification of novel targets for disease treatment tailored to a given patient’s genetic and functional abnormalities. Thus, for example, patients with coexisting ADAMTS13 deficiency and complement abnormalities may benefit from combined anticomplement therapy and plasma exchange.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Doris Duke Clinical Research Foundation (to S.J.E.), the Foundation for Children with Atypical HUS (to R.J.S.), and E.A. Smith Foundation (to S.F. and M.H.K.). This study also received funding from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (1R21AI101932 to V.A.-K.).
Authorship
Contribution: S.F., S.J.E., R.J.S., and V.A.-K. designed the experiments; S.F., S.J.E., Y.Z., T.M., and C.M.N. performed the experiments; and S.F., S.J.E., C.M.N., M.H.K., R.J.S., and V.A.-K. interpreted the data and wrote the paper.
Conflict-of-interest disclosure: M.H.K.: Optimer (Consulting), and Aplagon (Scientific Advisory Board). The remaining authors declare no competing financial interests.
Correspondence: Vahid Afshar-Kharghan, Division of Internal Medicine, Benign Hematology, University of Texas MD Anderson Cancer Center, 1400 Pressler St, Unit 1464, Houston, TX 77030; e-mail: vakharghan@mdanderson.org; or Richard J. Smith, Molecular Otolaryngology and Renal Research Laboratories, 5270 CBRB, University of Iowa, Iowa City, IA 52242; e-mail: richard-smith@uiowa.edu.
References
Author notes
S.F., S.J.E., and Y.Z. contributed equally to this study.