Key Points
Licensed NK cells based on the donor MHC-I haplotype show greater anti-MCMV resistance than unlicensed cells in allogeneic HSCT.
Ly49H+ licensed NK-cell expansion based on donor MHC-I with greater IFNγ production than unlicensed NK cells is seen after MCMV infection.
Abstract
Natural killer (NK) cells express inhibitory receptors with varied binding affinities to specific major histocompatibility complex class I (MHC-I) haplotypes. NK cells can be classified as licensed or unlicensed based on their ability or inability to bind MHC-I, respectively. The role of donor vs host MHC on their development after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is not known. Following reciprocal MHC-disparate allogeneic transplants and during de novo NK-cell recovery, depletion of the licensed and not unlicensed population of NK cells as determined by the licensing patterns of donor MHC-I haplotypes, resulted in significantly increased susceptibility to murine cytomegalovirus (MCMV) infection. A corresponding expansion of the licensed Ly49H+ NK cells occurred with greater interferon γ production by these cells than unlicensed NK cells in the context of donor MHC-I. Thus, NK licensing behavior to MCMV corresponds to the donor, and not recipient, MHC haplotype after allo-HSCT in mice.
Introduction
Natural killer (NK) cells possess inhibitory receptors to self that are thought to be involved in the determination of intrinsic functional capabilities in a process known as “licensing.”1-3 NK cells with receptors that can bind to self–major histocompatibility complex class I (MHC-I) are considered licensed and functional, whereas those with receptors that cannot bind to the MHC-I haplotype present are unlicensed and hyporesponsive.2,4-6 The process of licensing is currently not well understood. In allogeneic hematopoietic stem cell transplantation (allo-HSCT), the process of licensing is complicated further due to differences between donor and host MHC-I.7 A previous study demonstrated that in a killer cell immunoglobulin-like receptor (KIR) ligand-mismatched HSCT, NK cells showed a licensing pattern consistent with the donor’s KIR ligands.8
We aim to expand upon these findings and show a similar pattern of licensing in mice in a pathologic setting of murine cytomegalovirus (MCMV). We hypothesized that post–allo-HSCT, the developing NK cells will be licensed based on the donor MHC-I haplotype. Although a previous study showed unlicensed NK cells resulted in greater MCMV protection than licensed cells,9 we have demonstrated the regulation of licensed NK cells by regulatory T cells (Tregs) and the importance of licensed NK cells postsyngeneic HSCT, where Tregs are depleted, in MCMV responses.10 Others have similarly shown evidence of NK regulation by Tregs.11,12 In this current article, we observe significant differences in viral resistance, expansion, and interferon γ (IFNγ) production based on expression of Ly49 inhibitory receptors that can bind to donor MHC-I in mice post–allo-HSCT in MHC-disparate strains of mice in concordance with previous human findings.8
Study design
Mice
Female B10.D2 (H-2d), B10 (H-2b), C57BL/6 (H-2b), and CB6F1 (H-2b × H-2d) Jackson Laboratory mice were 6 to 8 weeks old and housed in Association for Assessment and Accreditation of Laboratory Animal Care–approved specific pathogen-free facilities under Institutional Animal Care and Use Committee–approved protocols.
HSCT
Bone marrow cells (BMCs) were extracted from donors depleted of NK cells (anti-NK1.1 [PK136] in vivo) and T cells (in vitro Thy1.2 [30H12] and rabbit complement13 ). Recipients were exposed to a lethal dose of 950 cGy γ-irradiation from a 137Cs source and injected intravenously with syngeneic or allogeneic (full MHC-mismatch H2d vs H2b) BMCs (5 × 106 BMCs).
MCMV infection
MCMV Smith strain was obtained from American Type Culture Collection. MCMV (5 × 104 plaque-forming units) was administered intraperitoneally at 10 or 17 days post-HSCT. Two days prior to infection, mice were depleted of specific cells with either 300 µg of purified anti-Ly49G2 (4D11), anti-Ly49C/I (5E6), anti-NK1.1 (PK136), or control rat/mouse immunoglobulin G (IgG) in 0.2 mL of phosphate-buffered saline (PBS) intraperitoneally. Mice were sacrificed at day 7 postinfection.
Flow cytometry
Single-cell suspensions were prepared from the harvested spleens in PBS with 1% fetal bovine serum. Intracellular staining was performed using the BD Cytofix/Cytoperm Fixation/Permeabilization kit with phorbol 12-myristate 13-acetate/ionomycin treatment 4 hours beforehand. Antibodies used are listed in supplemental Methods (available on the Blood website).
Viral titer determination
Quantification of MCMV from DNA extracted from livers using real-time–polymerase chain reaction was performed as previously described.14
Statistical analysis
Statistical significance was determined by using 1-way analysis of variance (ANOVA) with Tukey posttest analysis or 2-tailed Student t test. P values were considered statistically significant when P < .05.
Results and discussion
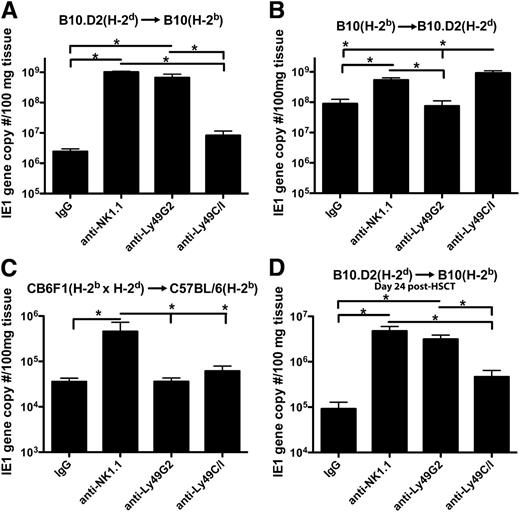
We first compared the ability of NK subsets to mediate MCMV resistance by in vivo depletion of subsets following syngeneic or allo-HSCT. After syngeneic HSCT, B10 (H-2b) mice depleted of their licensed population of NK cells (Ly49C/I+ cells which bind to H-2b) showed greater viral loads in the liver as compared with mice depleted of the unlicensed (Ly49G2+, which bind H-2d) cells along with expansion of licensed cells in nondepleted mice (supplemental Figure 1). However, when an allo-HSCT, devoid of NK and T cells to avoid graft-versus-host-disease, was performed using donor hematopoietic stem cells from B10.D2 mice into host B10 mice, a licensing pattern consistent with the donor H-2d MHC-I haplotype was observed with depletion of the Ly49G2+ population resulting in greater viral loads (Figure 1A). Depletions of the subsets are shown to be effective in B10 (supplemental Figure 2) and B10.D2 (supplemental Figure 3) mice.
Increased MCMV viral loads upon depletion of licensed Ly49 subsets based on donor MHC-I in allo-HSCT. Livers of mice that underwent allogeneic HSCT 10 days prior to infection and depleted of various NK subsets were compared in terms of viral load 7 days postinfection in (A) B10 (H-2b) and (B) B10.D2 (H-2d) host mice using real-time–polymerase chain reaction for MCMV IE1 gene sequences. (C) CB6F1 mice that are a cross between C57BL/6 (H-2b) and Balb/c (H-2d) mice were used as donors in an HSCT for C57BL/6 (H-2b) host mice, and livers were harvested 7 days postinfection for viral load determination. (D) Allo-HSCT was performed on B10 host mice, mice were infected 17 days post-HSCT, and livers were harvested 7 days postinfection for viral load determination. Mice were given 300 μg of rat/mouse IgG, anti-NK1.1, anti-Ly49G2, or anti-Ly49C/I 2 days prior to infection. Data are representative of 2 to 3 experiments with 4 mice per group. Error bars represent the standard error of the mean. Statistical analysis was performed using 1-way ANOVA and Tukey posttest. *P < .05.
Increased MCMV viral loads upon depletion of licensed Ly49 subsets based on donor MHC-I in allo-HSCT. Livers of mice that underwent allogeneic HSCT 10 days prior to infection and depleted of various NK subsets were compared in terms of viral load 7 days postinfection in (A) B10 (H-2b) and (B) B10.D2 (H-2d) host mice using real-time–polymerase chain reaction for MCMV IE1 gene sequences. (C) CB6F1 mice that are a cross between C57BL/6 (H-2b) and Balb/c (H-2d) mice were used as donors in an HSCT for C57BL/6 (H-2b) host mice, and livers were harvested 7 days postinfection for viral load determination. (D) Allo-HSCT was performed on B10 host mice, mice were infected 17 days post-HSCT, and livers were harvested 7 days postinfection for viral load determination. Mice were given 300 μg of rat/mouse IgG, anti-NK1.1, anti-Ly49G2, or anti-Ly49C/I 2 days prior to infection. Data are representative of 2 to 3 experiments with 4 mice per group. Error bars represent the standard error of the mean. Statistical analysis was performed using 1-way ANOVA and Tukey posttest. *P < .05.
The reciprocal transplant model using B10 donors into B10.D2 host mice showed an identical pattern of licensing based on donor MHC-I, suggesting the findings are intrinsic to licensing itself and not due to a specific strain of mouse or one of the NK subsets (Figure 1B). Additionally, utilization of CB6F1 mice that express both H-2d and H-2b as donors to C57BL/6 recipient mice (which were shown to have equivalent responses to MCMV as B10 mice10 ) in a HSCT resulted in equivalent viral loads upon depletion of either Ly49G2+ or Ly49C/I+ subsets, suggesting equivalent licensing of both populations (Figure 1C).
The viral loads after allo-HSCT were greater than after syngeneic HSCT, implying potential differences in overall recovery and bone marrow reconstitution of the mice. However, a greater fold difference between licensed and unlicensed NK depletion in viral load between allo-HSCT and syngeneic HSCT is apparent (81.1 ± 6.7 vs 8.6 ± 2.3 in B10 host mice, respectively, and 12.9 ± 2.12 vs 4.5 ± 1.1 in B10.D2 host mice, P < .05 in both by t test), suggesting not only a licensing effect but a lack of inhibition of these cells by host MHC-I resulting in greater activity as suggested previously.15
A significant expansion of licensed NK cells, based on the donor MHC-I, was observed that were also Ly49H+ (activating receptor that binds to MCMV glycoprotein m15716,17 ). The NK cells observed were all donor-derived (Figure 2A) with expansion of the donor-licensed population post-MCMV infection in terms of percentage of cells (Figure 2B-D) and total numbers (supplemental Figure 4).
Expansion of licensed but not unlicensed Ly49H+ NK cells with increased IFNγ production post-HSCT in MHC-disparate recipients following MCMV infection. (A) Example flow plots of CD3-NK1.1+ NK cells gated on H-2d vs H-2b MHC-I haplotypes from splenocytes of B10.D2 (H-2d), B10 (H-2b), and C57BL/6 (H-2b) host mice 7 days post-MCMV infection and that underwent allo-HSCT 10 or 17 days prior to MCMV infection. (B-D) Splenocytes were stained for CD3, NK1.1, Ly49H, Ly49C/I, and Ly49G2 7 days after infection post-HSCT and compared with noninfected mice. Percentage of CD3− NK1.1+ Ly49C/I+/H+ and Ly49G2+/H+ cells in (B) B10, (C) B10.D2, (D) C57BL/6 infected and noninfected host mice are illustrated. (E-G) The percentage of CD3− NK1.1+ Ly49H+ IFNγ+ Ly49G2+ or Ly49C/I+ NK cells was determined from infected and noninfected mice after allo-HSCT in (E) B10, (F) B10.D2, and (G) C57BL/6 mice. Data are representative of 2 to 3 experiments with 4 mice per group. The error bars represent the standard error of the mean. Statistical analysis was performed using 1-way ANOVA and Tukey posttest.*P < .05; **P < .01.
Expansion of licensed but not unlicensed Ly49H+ NK cells with increased IFNγ production post-HSCT in MHC-disparate recipients following MCMV infection. (A) Example flow plots of CD3-NK1.1+ NK cells gated on H-2d vs H-2b MHC-I haplotypes from splenocytes of B10.D2 (H-2d), B10 (H-2b), and C57BL/6 (H-2b) host mice 7 days post-MCMV infection and that underwent allo-HSCT 10 or 17 days prior to MCMV infection. (B-D) Splenocytes were stained for CD3, NK1.1, Ly49H, Ly49C/I, and Ly49G2 7 days after infection post-HSCT and compared with noninfected mice. Percentage of CD3− NK1.1+ Ly49C/I+/H+ and Ly49G2+/H+ cells in (B) B10, (C) B10.D2, (D) C57BL/6 infected and noninfected host mice are illustrated. (E-G) The percentage of CD3− NK1.1+ Ly49H+ IFNγ+ Ly49G2+ or Ly49C/I+ NK cells was determined from infected and noninfected mice after allo-HSCT in (E) B10, (F) B10.D2, and (G) C57BL/6 mice. Data are representative of 2 to 3 experiments with 4 mice per group. The error bars represent the standard error of the mean. Statistical analysis was performed using 1-way ANOVA and Tukey posttest.*P < .05; **P < .01.
To determine whether there were differences in functional capabilities between the donor licensed and unlicensed cells beyond cell expansion differences, IFNγ production by the different subsets was examined. Based on donor MHC-I, the frequency of licensed, Ly49H+ NK cells producing IFNγ was significantly greater than the unlicensed population (Figure 2E-G). Potentially, increased exposure time to host MHC-I could result in licensing to host MHC-I, but infecting mice at a later time point post–allo-HSCT (day 17) resulted in MCMV-resistance patterns and activity consistent with licensing based on donor MHC-I (Figure 1D; supplemental Figure 5).
The data demonstrate that NK licensing post-HSCT does indeed occur based on donor MHC-I in terms of IFNγ production, expansion, and MCMV resistance in newly developing NK cells. Although the cytokine milieu and lymphodepletion post-HSCT and MCMV infection may alter the development and activation of NK cells, the pattern of licensing in anti-MCMV responses was still apparent in all mouse strains tested. These findings may appear to differ from previous work showing adoptive transfer of MHC-I–deficient NK cells into wild-type mice resulting in licensing of NK cells based on host MHC-I expression.18 However, because our model used HSCT and we see a pattern of licensing consistent with donor MHC-I regardless of exposure time to host MHC-I, our data indicate the cells involved with licensing seem to be of hematopoietic origin and donor-derived.
Our findings are in concordance with human studies regarding NK licensing being based on donor KIR ligands8 and show that mice also follow this pattern of licensing in a pathological model and reinforce the validity of preclinical mouse models. These data are of significant clinical relevance as recent studies have attempted to use NK cells after HSCT to reduce tumor relapse and viral reactivation.19-21 Having allogeneic NK cells that are licensed in the donor could provide significant antiviral protection in these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant R01 HL089905.
Authorship
Contribution: C.M.S. designed and performed experiments, analyzed data, and wrote the paper; Y.J.T.-F. maintained MCMV and helped perform experiments; A.E.Z. helped perform experiments and reviewed the paper; M.A. helped in experiment design and review of the paper; C.P. provided insight into MCMV experiments, designed experiments, and reviewed the paper; and W.J.M. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr William J. Murphy, Departments of Dermatology and Internal Medicine, UC Davis School of Medicine, University of California, Davis, 2921 Stockton Blvd, IRC Building, Suite 1630, Sacramento, CA 95817; e-mail: wmjmurphy@ucdavis.edu.