Key Points
Blocking STAT3 in acute myeloid leukemia cells stimulates their TLR9-induced immunogenicity and antigen-specific activation of CD8+ T cells.
Systemic delivery of CpG-Stat3 siRNA generates potent adaptive immune responses eradicating disseminated acute myeloid leukemia in vivo.
Abstract
Signal transducer and activator of transcription 3 (STAT3) is an oncogene and immune checkpoint commonly activated in cancer cells and in tumor-associated immune cells. We previously developed an immunostimulatory strategy based on targeted Stat3 silencing in Toll-like receptor 9 (TLR9)-positive hematopoietic cells using CpG-small interfering RNA (siRNA) conjugates. Here, we assessed the therapeutic effect of systemic STAT3 blocking/TLR9 triggering in disseminated acute myeloid leukemia (AML). We used mouse Cbfb-MYH11/Mpl-induced leukemia model, which mimics human inv(16) AML. Our results demonstrate that intravenously delivered CpG-Stat3 siRNA, but not control oligonucleotides, can eradicate established AML and impair leukemia-initiating potential. These antitumor effects require host’s effector T cells but not TLR9-positive antigen-presenting cells. Instead, CpG-Stat3 siRNA has direct immunogenic effect on AML cells in vivo upregulating major histocompatibility complex class-II, costimulatory and proinflammatory mediators, such as interleukin-12, while downregulating coinhibitory PD-L1 molecule. Systemic injections of CpG-Stat3 siRNA generate potent tumor antigen–specific immune responses, increase the ratio of tumor-infiltrating CD8+ T cells to regulatory T cells in various organs, and result in CD8+ T-cell–dependent regression of leukemia. Our findings underscore the potential of using targeted STAT3 inhibition/TLR9 triggering to break tumor tolerance and induce immunity against AML and potentially other TLR9-positive blood cancers.
Introduction
Acute myeloid leukemia (AML) is a genetically heterogeneous disease with poor long-term survival in the majority of patients undergoing current chemotherapies. The identification of leukemia-specific antigens and recent clinical advances in cancer immunotherapy underscore the potential for safer and more effective AML treatments.1,2 However, adoptive T-cell transfer and vaccination strategies are hampered by the immunosuppressive tumor microenvironment. Immune tolerance in AML results from the accumulation of immature dendritic cells (DCs), myeloid-derived suppressor cells, and regulatory T cells (Tregs) associated with high expression of Th2 cytokines (interleukin-4 [IL-4], IL-6, IL-10), transforming growth factor beta (TGF-β), or coinhibitory molecules such as PD-L1.3-5 In addition, the myeloid cell–specific antigen presentation and expression of proinflammatory cytokines/chemokines such as IL-12 are downregulated in leukemia.4,6
As in patients with other blood cancers, patients with AML show high frequency of signal transducer and activator of transcription 3 (STAT3) activation in leukemic blasts which correlates with decreased disease-free survival.7-9 STAT3 plays a role in promoting AML cell proliferation and survival, but whether it contributes to immune evasion has not been clearly demonstrated.7,10,11 Earlier studies indicated that STAT3 activation is also common in many tumor-associated myeloid cell populations that contribute to tumorigenesis.12 It is an attractive but challenging target for cancer therapy, because pharmacologic inhibition of nonenzymatic proteins has proved to be difficult.8,12 Targeting tyrosine kinases upstream from STAT3 by using small-molecule inhibitors of JAK, SRC, c-KIT, and FLT3 provided an alternative strategy for AML therapy, but therapeutic effects in most clinical trials were short-lived.8,13
Growing evidence suggests that to generate long-lasting effects, cancer immunotherapies need to alleviate tumor tolerance before jump-starting antitumor immune responses.2,14 We have previously shown that STAT3 activity in tumor-associated myeloid cells hampered the effect of locally administered CpG-oligodeoxyribonucleotide (ODN), a Toll-like receptor 9 (TLR9) ligand and clinically relevant immunoadjuvant.15 These results provided a possible explanation for limited clinical efficacy of TLR9 agonists against human cancers, including AML.16,17 We later demonstrated that CpG-ODNs can be used for cell-specific small interfering RNA (siRNA) delivery as CpG-siRNA conjugate to silence genes in mouse and human TLR9-positive cells.18-20 Here, we assessed whether systemically administered CpG-Stat3 siRNA would generate antitumor effects against a genetic mouse model of Cbfb-MYH11/Mpl+ (CMM+)–induced leukemia, which closely mimics human AML with inv(16)(p13:q22) gene fusion in 10% of patients.
Methods
Cells and reagents
Cbfb+/56M/Mx-Cre+ mice21 were backcrossed to wild-type C57BL/6 mice for >10 generations to generate the syngeneic AML model. Two weeks after polyinosinic-polycytidylic acid–induced (Invivogen) expression of core-binding factor β-smooth muscle myosin heavy chain, bone marrow cells from Cbfb+/56M/Mx-Cre+ mice were transduced with retroviral MIG-Mpl vector–encoding thrombopoietin receptor and GFP genes to generate transplantable Cbfb-MYH11/Mpl+ mouse AML.22
C1498 AML cells expressing SIYRYYGL model peptide antigen (C1498.SIY), recognized by CD8+ T cells in the context of MHC haplotype Kb, were generated recently.23 The CpG-siRNAs were synthesized in the DNA/RNA Synthesis Core (City of Hope [COH]) by linking CpG1668-ODN to Stat3 or luciferase (Luc) siRNAs as previously described (supplemental Data).18,20
In vivo experiments
C57BL/6 and NOD/SCID/IL-2RγKO (NSG) mice (6 to 8 weeks old) were from the National Cancer Institute (Frederick, MD) and the Jackson Laboratory, respectively. TLR9KO mice were originally from S. Akira (Osaka University, Osaka, Japan). Animal care/procedures were performed in accordance with established institutional guidance and approved protocols from the Institutional Animal Care and Use Committee (COH). The lateral tail vein of C57BL/6 mice was injected with 1 × 106Cbfb-MYH11/Mpl+ AML cells in phosphate-buffered saline. For CD8+ T-cell depletion, mice were injected intraperitoneally with anti-CD8 antibody (200 μg) on days −6, −3, and 0 before tumor challenge and then twice weekly. Blood was drawn from the retro-orbital venous sinus to monitor the circulating c-Kit+/GFP+ AML cells. After AML cell levels in blood exceeded 1%, which corresponds to 10% to 20% of bone marrow-residing AML cells (Y.-H.K., unpublished data), mice were injected intravenously 6 times with various CpG-siRNAs (5mg/kg) every other day and euthanized 1 day after the last treatment.
Flow cytometry and immunohistochemistry
Single-cell suspensions were prepared by mechanical tissue disruption and collagenase-D/DNase-I treatment as described.24 The AML cell percentages were determined by GFP and c-Kit expression. For extracellular staining, cells were incubated with fluorochrome-labeled antibodies to major histocompatibility complex (MHC) class II, CD40, CD80, CD86, PDL-1, CD3, CD4, CD8, CD69 after FcγIII/IIR blocking to prevent unspecific binding (eBioscience). For intracellular staining, cells were fixed and/or permeabilized and stained with TLR9-specific antibodies (eBioscience), Stat3P, or FoxP3 (BD) as described.18 Fluorescence data were analyzed on a BD Accuri C6 Flow Cytometer (BD) using FlowJo software (TreeStar). Immunohistochemical staining was performed on formalin-fixed/paraffin-embedded bone sections (5 μm) at the Pathology Core (COH).
Quantitative real-time polymerase chain reaction and protein assays
Total RNA isolation and complementary DNA synthesis were carried out as described previously.20 The sequences of the specific primers/probe sets are listed in the supplemental Data. Western blot to detect Stat3, Stat3P, and β-actin expression was performed as described.24 Plasma cytokines were analyzed by using Bio-Plex arrays (Bio-Rad) at the Clinical Immunobiology Core (COH).
IFN-γ ELISPOT assay
C57BL/6 mice were injected intravenously with 106Cbfb/MYH11/Mpl+ or C1498.SIY cells. Mice with established tumors were treated with CpG-siRNAs every other day (6 times) and euthanized the next day. The interferon gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assay was performed following the manufacturer’s protocol (Cell Sciences) by using 5 × 105 splenocytes and irradiated (100 Gy) CMM+ AML (5 × 104 cells per well) or SIY peptide (10 μg/mL).
Statistical analysis
One- or two-way analysis of variance plus Bonferroni post-test was applied to assess statistical significance of differences between multiple treatment groups or in tumor growth kinetics between treatment groups, respectively. Data were analyzed by using GraphPad Prism v4.0 software (GraphPad).
Results
Cbfb-MYH11/Mpl+ AML as a target for CpG-Stat3 siRNA
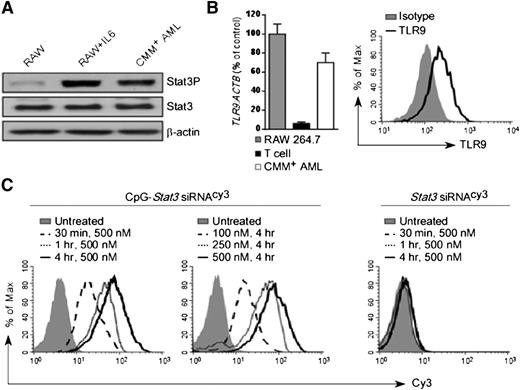
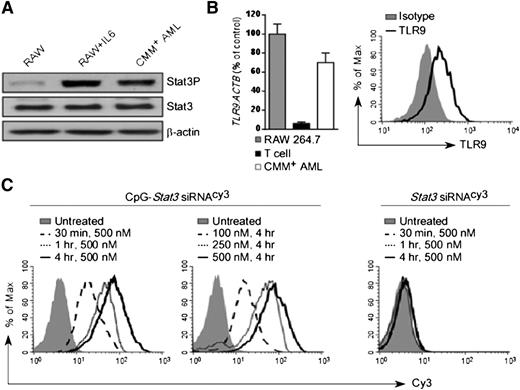
We previously demonstrated that blocking STAT3 signaling augments the efficacy of local TLR9-based immunotherapies against solid tumors.15,18 STAT3 is also commonly activated in the majority of human AML cases.25 To test the feasibility of using immunostimulatory CpG-Stat3 siRNA for AML therapy, we selected a transplantable mouse model derived from spontaneous leukemia induced by the co-expression of the Cbfb-MYH11 fusion gene and the thrombopoietin receptor Mpl.21,22 Cbfb-MYH11/Mpl+(CMM+) leukemic cells are c-Kit–positive and were engineered to express GFP. CMM+ cells freshly isolated from C57BL/6 mice showed elevated levels of constitutively active STAT3 (Figure 1A). As tumor cells of myeloid origin, CMM+ cells expressed TLR9 messenger RNA and protein at levels comparable to macrophages (Figure 1B). Next, we determined whether CMM+ cells internalized CpG-Stat3 siRNA. Freshly isolated AML cells were incubated for various times and at various doses with CpG-Stat3 siRNA or unconjugated Stat3 siRNA without any transfection reagents. Both molecules were fluorescently labeled to follow the intracellular siRNA uptake. At 30 minutes, more than 80% of CMM+ AML cells internalized CpG-Stat3 siRNA (Figure 1C, left). The conjugate uptake increased with longer incubation and peaked at 4 hours. The maximum cell internalization required 250- to 500-nM concentrations (Figure 1C, middle). In contrast, the unconjugated Stat3 siRNA was not significantly internalized, even after 4 hours of incubation at 500-nM concentration (Figure 1C, right). The rapid uptake limits exposure of the naked oligonucleotide to serum nucleases in the environment. Thus, we assessed the potential of using systemic delivery of CpG-Stat3 siRNA for leukemia immunotherapy. To assess in vivo biodistribution of CpG-Stat3 siRNA, we used mice with CMM+ AML disseminated into various organs. Mice were injected intravenously by using a single dose of CpG-Stat3 siRNACy3 (5 mg/kg) and were euthanized 3 or 18 hours later. We assessed cellular distribution of Stat3 siRNACy3 in c-Kit+/GFP+ leukemic cells and in myeloid immune cells from various organs by using flow cytometry. Our results confirmed that intravenously injected CpG-Stat3 siRNACy3 targeted both AML cells and normal myeloid immune cells in different organs (supplemental Figure 1). As expected for naked oligonucleotides, we observed the highest Stat3 siRNACy3 uptake by AML (∼40% to 50%) and nonmalignant myeloid cells (∼30% to 70%) localized in spleen and liver. In addition, Stat3 siRNACy3 was internalized by ∼15% to 20% of AML cells and ∼40% to 45% of macrophages in both bone marrow and lymph nodes. We also assessed uptake of CpG-siRNACy3 by bone marrow myeloid progenitor and hematopoietic stem cells in naïve mice. Although myeloid progenitor cells internalized low levels of siRNACy3, no siRNA uptake was detected in hematopoietic stem cells (supplemental Figure 1C). We also did not detect any effect of the CpG-Stat3 siRNA on nonmalignant bone marrow cell viability. These results suggested that immunostimulatory CpG-siRNA is suitable for targeting AML cells together with tumor-associated myeloid cells with minimal effect on normal hematopoiesis.
CpG-siRNA strategy allows for targeted delivery of Stat3 siRNA into TLR9-positive Cbfb-MYH11/Mpl+leukemic cells. (A) Western blot analysis showing constitutive activation of Stat3 in CMM+ AML cells compared with untreated or IL-6–treated RAW 264.7 macrophages; β-actin was used as an internal loading control. (B) TLR9 expression in CMM+ AML cells. Tlr9 messenger RNA and protein were assessed by using quantitative polymerase chain reaction (qPCR) (left panel) and flow cytometry (right panel). RAW 264.7 macrophages and CD3+ T cells were used as positive and negative controls, respectively. (C) Dose- and time-dependent internalization of naked CpG-Stat3 siRNA by CMM+ cells. Both molecules were labeled with Cy3 fluorochrome on the 5′ end of the siRNAs to follow their intracellular uptake. AML cells were incubated with various concentrations of fluorescently labeled CpG-Stat3 siRNACy3 conjugate or unconjugated Stat3 siRNACy3 for indicated times without any transfection reagents. Percentages of Cy3+ AML cells were assessed by fluorescence-activated cell sorter.
CpG-siRNA strategy allows for targeted delivery of Stat3 siRNA into TLR9-positive Cbfb-MYH11/Mpl+leukemic cells. (A) Western blot analysis showing constitutive activation of Stat3 in CMM+ AML cells compared with untreated or IL-6–treated RAW 264.7 macrophages; β-actin was used as an internal loading control. (B) TLR9 expression in CMM+ AML cells. Tlr9 messenger RNA and protein were assessed by using quantitative polymerase chain reaction (qPCR) (left panel) and flow cytometry (right panel). RAW 264.7 macrophages and CD3+ T cells were used as positive and negative controls, respectively. (C) Dose- and time-dependent internalization of naked CpG-Stat3 siRNA by CMM+ cells. Both molecules were labeled with Cy3 fluorochrome on the 5′ end of the siRNAs to follow their intracellular uptake. AML cells were incubated with various concentrations of fluorescently labeled CpG-Stat3 siRNACy3 conjugate or unconjugated Stat3 siRNACy3 for indicated times without any transfection reagents. Percentages of Cy3+ AML cells were assessed by fluorescence-activated cell sorter.
Systemic administration of immunostimulatory CpG-Stat3 siRNA reduces AML burden and improves survival in mice
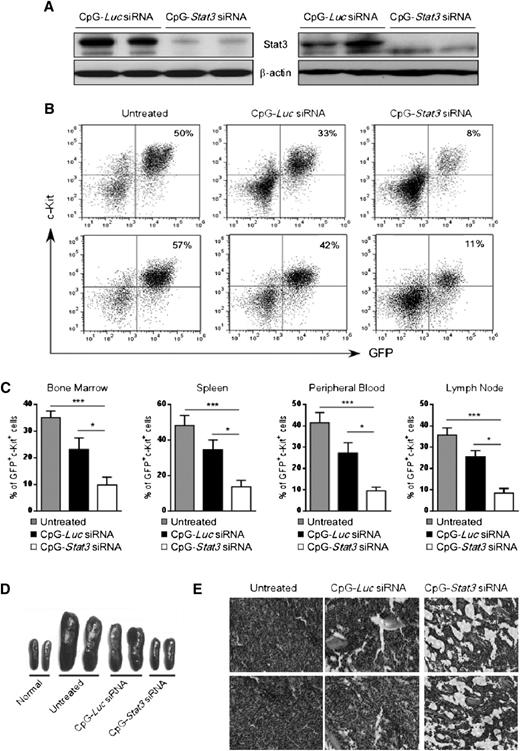
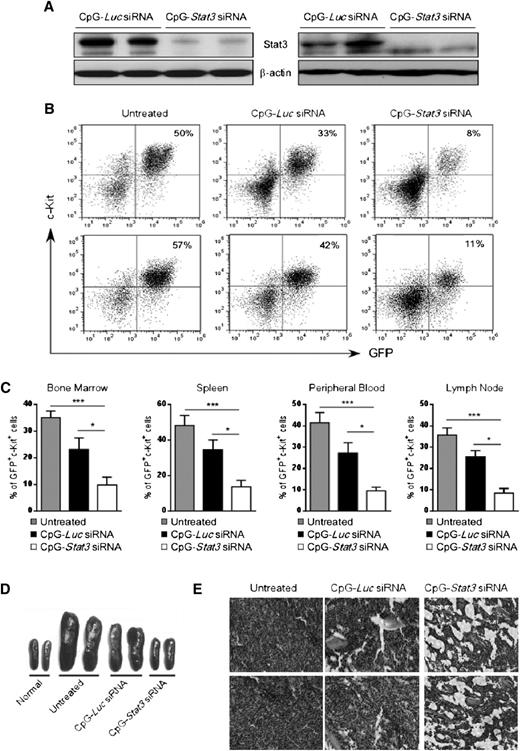
We evaluated antitumor effects of systemic CpG-Stat3 siRNA injections into Cbfb-MYH11/Mpl+ AML–bearing mice. Syngeneic recipients engrafted with CMM+ AML were injected 6 times every other day using CpG-siRNA specific for Stat3 or Luc genes starting when c-Kit+/GFP+ AML cells became detectable in peripheral blood (days 7 to 10). The CpG-Luc siRNA was used as a nontargeting control to assess immunostimulatory effects of the conjugate in the absence of STAT3 inhibition. We confirmed that STAT3 expression was reduced at messenger RNA (supplemental Figure 2A) and protein levels in c-Kit+ AML cells from spleens and/or bone marrows of CpG-Stat3 siRNA–treated mice but not in controls (Figure 2A). Finally, the in vivo CpG-Stat3 siRNA treatment reduced STAT3 activity in splenic AML cells by ∼80% as measured by flow cytometry (supplemental Figure 2B).
Systemic CpG-Stat3 siRNA treatment induces regression of disseminated AML in mice. C57BL/6 mice were injected intravenously with 1 × 106CMM+ cells. After 2 to 3 weeks when tumors were engrafted (>1%, ranging from 1% to 5% of AML cells in blood), mice were injected 6 times with CpG-Stat3 siRNA or control CpG-Luc siRNA (5 mg/kg) every other day and euthanized 1 day after the last treatment. (A) Stat3 silencing in AML cells isolated from spleen (left panel) or bone marrow (right panel) was confirmed by using western blotting. (B-C) CpG-Stat3 siRNA treatment reduced the percentage of AML cells in various organs. Flow cytometric analysis of GFP+c-Kit+ AML cells in (B, top) bone marrows or (B, bottom) spleens from various groups of mice. Shown are combined results from 6 mice per group; means ± standard error of the mean (SEM). Statistically significant differences between groups are indicated with asterisks: ***P < .001; *P < .05. (D-E) The effect of CpG-siRNA treatments on (D) spleen and (E) bone marrow cellularities. Shown are (D) representative spleen sizes or (E) histochemical analysis of bone marrow structure in indicated groups of mice. Similar results were derived from 3 independent experiments.
Systemic CpG-Stat3 siRNA treatment induces regression of disseminated AML in mice. C57BL/6 mice were injected intravenously with 1 × 106CMM+ cells. After 2 to 3 weeks when tumors were engrafted (>1%, ranging from 1% to 5% of AML cells in blood), mice were injected 6 times with CpG-Stat3 siRNA or control CpG-Luc siRNA (5 mg/kg) every other day and euthanized 1 day after the last treatment. (A) Stat3 silencing in AML cells isolated from spleen (left panel) or bone marrow (right panel) was confirmed by using western blotting. (B-C) CpG-Stat3 siRNA treatment reduced the percentage of AML cells in various organs. Flow cytometric analysis of GFP+c-Kit+ AML cells in (B, top) bone marrows or (B, bottom) spleens from various groups of mice. Shown are combined results from 6 mice per group; means ± standard error of the mean (SEM). Statistically significant differences between groups are indicated with asterisks: ***P < .001; *P < .05. (D-E) The effect of CpG-siRNA treatments on (D) spleen and (E) bone marrow cellularities. Shown are (D) representative spleen sizes or (E) histochemical analysis of bone marrow structure in indicated groups of mice. Similar results were derived from 3 independent experiments.
Next, we examined the antitumor effect of targeted Stat3 silencing in TLR9-positive cells in mice. Repeated intravenous administration of CpG-Stat3 siRNA reduced AML cells by ∼70% to 80% in bone marrow, spleen, lymph nodes, and peripheral blood while the CpG-Luc siRNA led to a reduction of ∼30% AML compared with untreated mice (Figure 2B-C). No effect was observed using nonsilencing/nonimmunostimulatory control conjugate (supplemental Figure 6).18 The injections of CpG-Stat3 siRNA but not CpG-Luc siRNA also normalized splenic (Figure 2D) and bone marrow cellularities (Figure 2E). We further confirmed that CpG-Stat3 siRNA administration induces comparable therapeutic effects against CMM+ AML tumors in mice on different genetic backgrounds (129S6) (supplemental Figure 3).
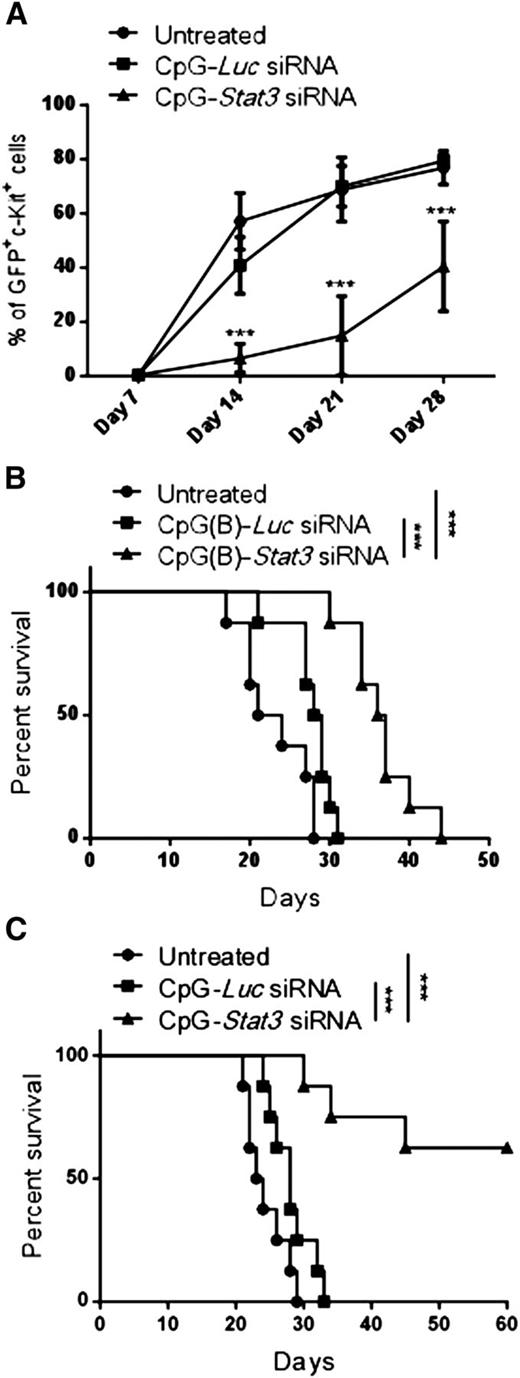
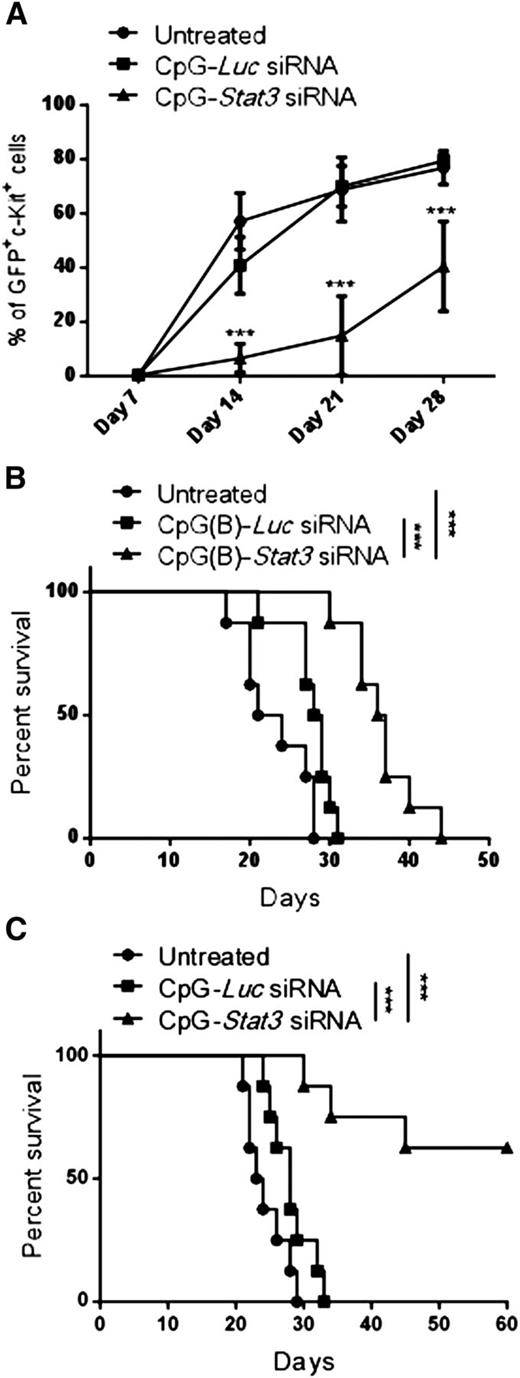
Long-term therapeutic effects in AML critically depend on the elimination of leukemia-initiating cells.26 To assess the leukemia-initiating potential of CMM+ AML after CpG-Stat3 siRNA treatment, we performed serial transplantation. Briefly, bone marrow–resident c-Kit+GFP+ AML cells were enriched from mice treated for 2 weeks with various CpG-siRNAs (as in Figure 2), were counted, and were injected into naïve secondary recipients. AML progression in secondary recipients was monitored by detecting percentages of c-Kit+GFP+ cells in peripheral blood. Mice that received AML cells from CpG-Stat3 siRNA–treated donors showed significant delay in AML engraftment (Figure 3A) and extended survival (Figure 3B) compared with both control groups. Even though control CpG-Luc siRNA partially reduced AML burden in donor mice (Figure 2), it did not reduce leukemia-initiating potential in secondary recipients (Figure 3A). These results prompted us to evaluate the effect of prolonged STAT3 inhibition/TLR9 activation. The repeated CpG-Stat3 siRNA administration twice weekly over 7 weeks resulted in survival of 60% of mice, while no mice survived in CpG-Luc siRNA–treated and untreated groups (Figure 3C). Long-term administration of CpG-Stat3 siRNA did not cause obvious adverse effects and/or immunotoxicity. Together, these data demonstrate that targeted STAT3 inhibition/TLR9 activation effectively impairs leukemia-initiating potential of CMM+ AML, thereby preventing tumor recurrence.
Targeting Stat3 reduces leukemia-initiating potential of Cbfb-MYH11/Mpl+cells. (A-B) CMM+ AML cells were isolated from bone marrows of primary recipient mice treated with CpG siRNAs (5 mg/kg) injected intravenously 6 times every other day or untreated as described in Figure 2. Magnetically enriched c-kit+ AML cells pooled from CpG-Stat3 siRNA, CpG-Luc siRNA, or untreated mice were pooled and counted, and identical cell numbers were injected into secondary recipient mice. (A) AML progression in all experimental groups was monitored weekly by using flow cytometry to detect percentages of blood GFP+c-Kit+ AML cells in the peripheral blood. (B) Survival of mice injected with CpG-Stat3 siRNA–treated AML cells was significantly improved compared with mice that received untreated or CpG-Luc siRNA–treated AML cells. (C) Survival curve showing long-term antitumor effect of systemic administration of CpG-Stat3 siRNA compared with untreated CpG-Luc siRNA–treated group. Statistically significant differences between CpG-Stat3 siRNA– and CpG-Luc siRNA–treated or untreated groups are indicated by asterisks: P = .0002 and P < .0001, respectively. Shown are means ± SEM with n = 8 per group.
Targeting Stat3 reduces leukemia-initiating potential of Cbfb-MYH11/Mpl+cells. (A-B) CMM+ AML cells were isolated from bone marrows of primary recipient mice treated with CpG siRNAs (5 mg/kg) injected intravenously 6 times every other day or untreated as described in Figure 2. Magnetically enriched c-kit+ AML cells pooled from CpG-Stat3 siRNA, CpG-Luc siRNA, or untreated mice were pooled and counted, and identical cell numbers were injected into secondary recipient mice. (A) AML progression in all experimental groups was monitored weekly by using flow cytometry to detect percentages of blood GFP+c-Kit+ AML cells in the peripheral blood. (B) Survival of mice injected with CpG-Stat3 siRNA–treated AML cells was significantly improved compared with mice that received untreated or CpG-Luc siRNA–treated AML cells. (C) Survival curve showing long-term antitumor effect of systemic administration of CpG-Stat3 siRNA compared with untreated CpG-Luc siRNA–treated group. Statistically significant differences between CpG-Stat3 siRNA– and CpG-Luc siRNA–treated or untreated groups are indicated by asterisks: P = .0002 and P < .0001, respectively. Shown are means ± SEM with n = 8 per group.
Antileukemic effects of CpG-Stat3 siRNA depend on an intact immune system
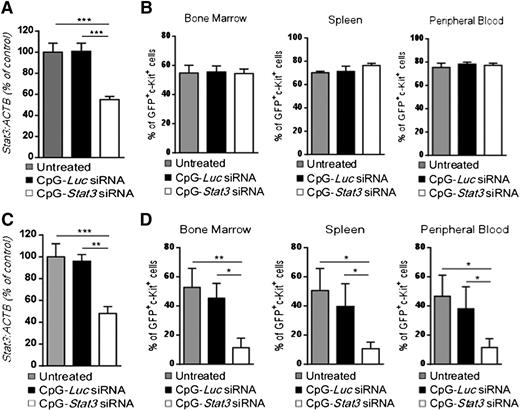
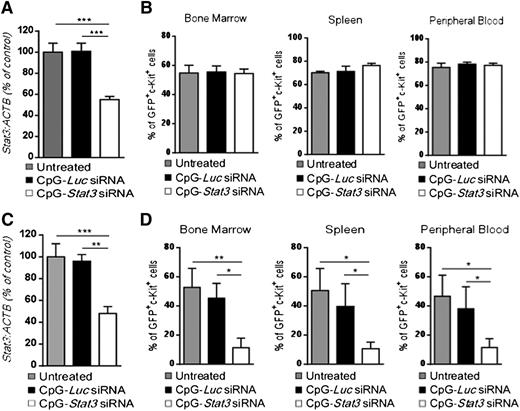
We previously demonstrated that targeting STAT3 by using CpG-siRNA can induce direct cytotoxic effects against human TLR9-positive hematologic malignancies in immunodeficient mice.20 To verify whether an intact immune system is required for the antitumor effect of CpG-Stat3 siRNA against CMM+ AML, we engrafted leukemic cells into immunodeficient NSG mice. Mice with established tumors (day 7) were treated by using 6 repeated intravenous injections of CpG-siRNAs as shown in Figure 2. Although CpG-Stat3 siRNA significantly downregulated Stat3 expression in AML cells (Figure 4A), it failed to restrain leukemia progression in NSG mice (Figure 4B). These results indicate that direct cytotoxic effect of STAT3 inhibition/TLR9 activation on CMM+ AML cells in vivo is negligible, corresponding to weak CpG-Stat3 siRNA cytotoxicity to leukemic cells in vitro (supplemental Figure 4). We previously observed that local administration of CpG-Stat3 siRNA generates immune responses against solid tumors through direct activation of the host’s antigen-presenting cells (APCs), which in turn stimulates effector T cells.27 CD8+ T lymphocytes are not directly activated by CpG-Stat3 siRNA because they poorly internalize conjugates and do not express TLR9.18,27 Therefore, CpG-Stat3 siRNA administration should not generate antileukemic effects in Tlr9-deficient mice because of impaired siRNA processing/release in myeloid and B-cell populations.18,28 To verify this assumption, we transplanted CMM+ AML tumors into Tlr9−/− mice and treated them with CpG-Stat3 siRNA or CpG-Luc siRNA (as in Figure 4A) to induce target gene silencing (Figure 4C). Unexpectedly, Tlr9 ablation in target immune cells did not prevent potent antileukemic effects of STAT3 targeting (Figure 4D). Repeated CpG-Stat3 siRNA treatments reduced tumor burden by ∼75% to 80% in bone marrow, spleen, and peripheral blood as observed before in wild-type mice (Figure 2C). Thus, the therapeutic effect of targeted STAT3 inhibition/TLR9 activation relies on effector immune cells but it does not require host’s APCs.
The antitumor effect of CpG-Stat3 siRNA is immune mediated but does not depend on activation of host’s APCs. (A-B) NOD/SCID/IL-2RγKO or (C-D) Tlr9−/− mice (n = 6) were challenged intravenously with 1 × 106CMM+ AML cells and treated by using CpG-Stat3 siRNA or CpG-Luc siRNA or left untreated as in Figure 2. (A,C) Stat3 gene silencing was confirmed by qPCR; (B,D) percentages of GFP+c-Kit+ AML cells in different organs were determined by flow cytometry. Shown are means ± SEM from two independent experiments. Statistically significant differences were indicated by asterisks: ***P < .001; **P < .01; *P < .05.
The antitumor effect of CpG-Stat3 siRNA is immune mediated but does not depend on activation of host’s APCs. (A-B) NOD/SCID/IL-2RγKO or (C-D) Tlr9−/− mice (n = 6) were challenged intravenously with 1 × 106CMM+ AML cells and treated by using CpG-Stat3 siRNA or CpG-Luc siRNA or left untreated as in Figure 2. (A,C) Stat3 gene silencing was confirmed by qPCR; (B,D) percentages of GFP+c-Kit+ AML cells in different organs were determined by flow cytometry. Shown are means ± SEM from two independent experiments. Statistically significant differences were indicated by asterisks: ***P < .001; **P < .01; *P < .05.
Targeted STAT3 inhibition/TLR9 activation augments immunogenicity of AML cells in vivo
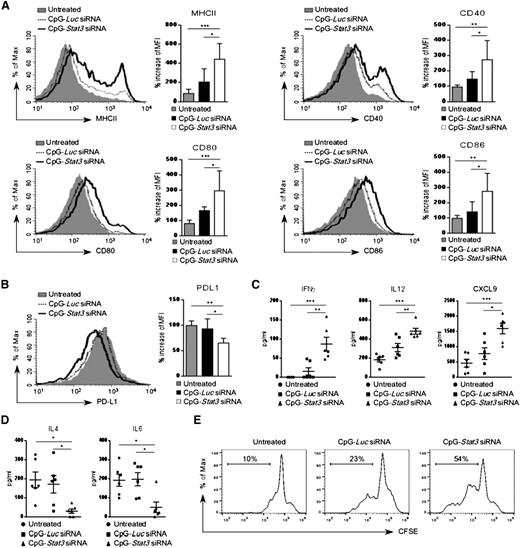
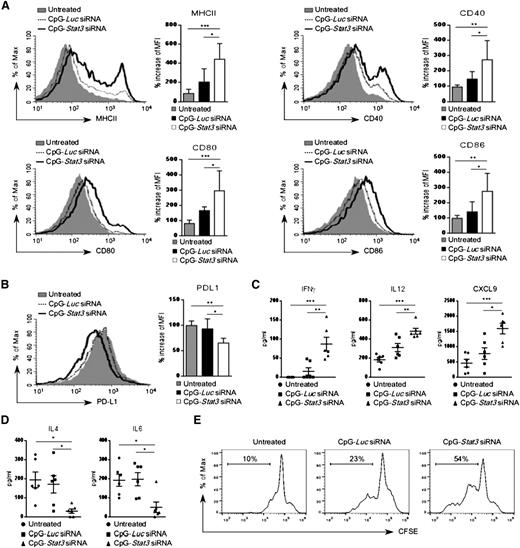
Earlier studies demonstrated that proinflammatory cytokines can induce in vitro differentiation and antigen-presenting functions of AML cells.29,30 Therefore, we analyzed surface expression of antigen-presenting molecules on leukemic cells treated in vivo. Wild-type mice with established CMM+ AML were injected by using CpG-Stat3 siRNA or CpG-Luc siRNA or were left untreated (as described in Figure 4A). CpG-Stat3 siRNA treatment induced upregulation of MHC class II, CD40, CD80, and CD86 molecules on splenic GFP+ AML cells, while the immunostimulatory effect of CpG-Luc siRNA was insignificant (Figure 5A). The levels of immunostimulatory molecules induced by STAT3 targeting were comparable between AML cells (GFP+) and normal DCs (GFP–) isolated from lymph nodes of the same mice (supplemental Figure 5A). In contrast, levels of a coinhibitory PD-L1 molecule, implicated in tumor immune tolerance, were significantly reduced in AML cells after Stat3 silencing (Figure 5B).31 We also found that mice from the CpG-Stat3 siRNA–treated group but not control animals had highly elevated plasma levels of proinflammatory mediators, such as IFN-γ, IL-12, and CXCL9/MIG (Figure 5C) with concomitant reduction of Th2 cytokines, such as IL-4 and IL-6 (Figure 5D). Finally, we evaluated whether the CpG-Stat3 siRNA–treated AML cells were capable of T-cell activation. In vitro coculture of AML cells freshly isolated from CpG-siRNA–treated mice with CD3+ T cells isolated from naïve mice demonstrated the superior potential of CpG-Stat3 siRNA to induce AML-dependent T-cell proliferation compared with CpG-Luc siRNA–treated or untreated mice (Figure 5E).
Enhanced expression of immunostimulatory molecules on CpG-Stat3 siRNA–treated AML cells in vivo. (A-B) AML-bearing C57BL/6 mice were treated with CpG-Luc siRNA or CpG-Stat3 siRNA as in Figure 2. The surface expression of MHC class II and (A) costimulatory molecules CD40, CD80, and CD86 or (B) coinhibitory PD-L1 molecules on splenic AML cells was assessed by flow cytometry. Shown are representative histogram overlays and bar graphs summarizing mean fluorescence intensity (MFI) from each group of mice (n = 6); mean ± standard deviation (SD). (C-D) Levels of different cytokines in blood plasma of mice treated as in (A) were quantified by using Luminex assays. Shown are means ± SEM (n = 6). Statistically significant P values are indicated by asterisks: ***P < .001; **P < .01; *P < .05. (E) In vivo pretreated splenic AML cells were cocultured in vitro with autologous T cells at a ratio of 1:1 for 3 days. T-cell proliferation was assessed by using carboxyfluorescein succinimidyl ester (CFSE) dilution assay. Shown are results representative for 3 independent experiments.
Enhanced expression of immunostimulatory molecules on CpG-Stat3 siRNA–treated AML cells in vivo. (A-B) AML-bearing C57BL/6 mice were treated with CpG-Luc siRNA or CpG-Stat3 siRNA as in Figure 2. The surface expression of MHC class II and (A) costimulatory molecules CD40, CD80, and CD86 or (B) coinhibitory PD-L1 molecules on splenic AML cells was assessed by flow cytometry. Shown are representative histogram overlays and bar graphs summarizing mean fluorescence intensity (MFI) from each group of mice (n = 6); mean ± standard deviation (SD). (C-D) Levels of different cytokines in blood plasma of mice treated as in (A) were quantified by using Luminex assays. Shown are means ± SEM (n = 6). Statistically significant P values are indicated by asterisks: ***P < .001; **P < .01; *P < .05. (E) In vivo pretreated splenic AML cells were cocultured in vitro with autologous T cells at a ratio of 1:1 for 3 days. T-cell proliferation was assessed by using carboxyfluorescein succinimidyl ester (CFSE) dilution assay. Shown are results representative for 3 independent experiments.
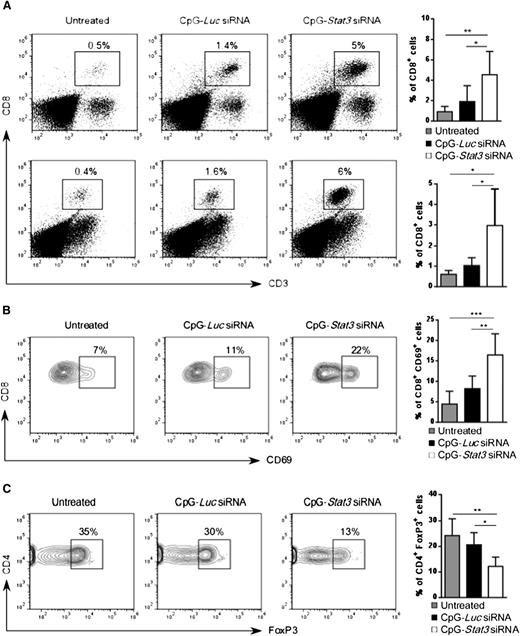
CpG-Stat3 siRNA increases the ratio of CD8+ effector to Tregs in vivo
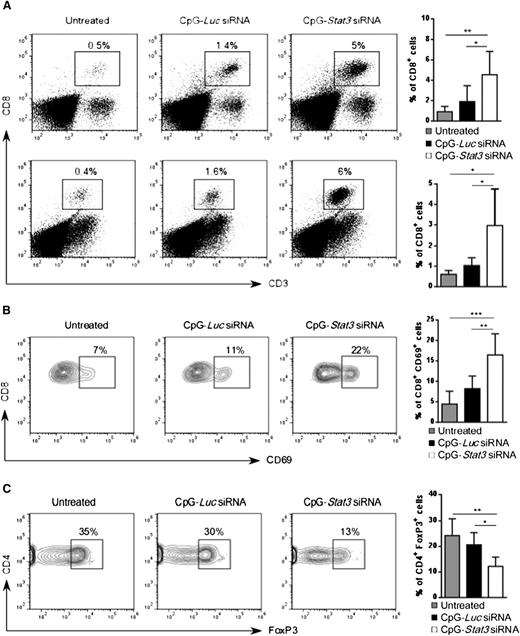
Next, we investigated whether increased AML immunogenicity in CpG-Stat3 siRNA–treated mice will induce CD8+ T-cell infiltration into major leukemia reservoirs such as bone marrow and spleen. As shown in Figure 6A, the average percentage of CD8+ T cells in spleen and bone marrow increased ∼fivefold in AML-bearing mice after 2 weeks of CpG-Stat3 siRNA treatment (Figure 2) compared with untreated controls. The immunostimulatory but nonsilencing CpG-Luc siRNA increased effector T-cell numbers only slightly. Correspondingly, CpG-Stat3 siRNA and not CpG-Luc siRNA injections triggered CD8+ T-cell activation as indicated by the CD69 upregulation (Figure 6B). The generation of adaptive anticancer immune responses is known to correlate with the increased ratio of effector to Tregs within the tumor.32 Thus, we evaluated the numbers of CD4+FoxP3+ Tregs in mice treated for 2 weeks by using CpG-siRNA as described above. Even though percentage of total CD4+ T cells did not significantly change (supplemental Figure 5B), we observed an average ∼50% reduction in splenic Tregs (CD4+/FoxP3+) in AML-bearing mice after treatment using Stat3 targeting CpG siRNA and not control conjugate compared with negative control (Figure 6C). Altogether, these findings indicate that systemic CpG-Stat3 siRNA treatment improves the ratio of effector CD8+ T cells to Tregs, thereby generating potent adaptive antitumor immunity in mice with disseminated leukemia.
CpG-Stat3 siRNA treatment modifies balance between CD8 and Treg cells in AML mice. (A) Percentages of CD3+CD8+ T cells in the spleen (upper panel) and in the bone marrow (lower panel) of mice treated with CpG-Stat3 siRNA compared with untreated and CpG-Luc siRNA–treated groups assessed by flow cytometry. Mice were treated as described in Figure 2, and spleens and bone marrows were collected and analyzed by using flow cytometry. (B-C) Representative contour plots and bar graphs showing (B) percentages of activated CD8+CD69+ T cells or (C) CD4+FoxP3+ Tregs in spleens of AML-bearing mice under the same experimental conditions. Flow cytometric analysis after (B) extracellular or (C) intracellular staining for the indicated markers by using specific antibodies. Shown are means ± SD (n = 6). Statistically significant differences were indicated by asterisks: ***P < .001; **P < .01; *P < .05.
CpG-Stat3 siRNA treatment modifies balance between CD8 and Treg cells in AML mice. (A) Percentages of CD3+CD8+ T cells in the spleen (upper panel) and in the bone marrow (lower panel) of mice treated with CpG-Stat3 siRNA compared with untreated and CpG-Luc siRNA–treated groups assessed by flow cytometry. Mice were treated as described in Figure 2, and spleens and bone marrows were collected and analyzed by using flow cytometry. (B-C) Representative contour plots and bar graphs showing (B) percentages of activated CD8+CD69+ T cells or (C) CD4+FoxP3+ Tregs in spleens of AML-bearing mice under the same experimental conditions. Flow cytometric analysis after (B) extracellular or (C) intracellular staining for the indicated markers by using specific antibodies. Shown are means ± SD (n = 6). Statistically significant differences were indicated by asterisks: ***P < .001; **P < .01; *P < .05.
Antileukemic effects of CpG-Stat3 siRNA depend on CD8+ T-cell–mediated adaptive immunity
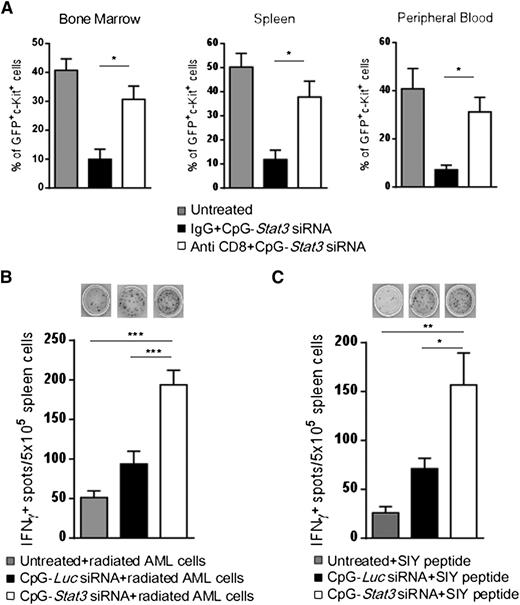
On the basis of the above results, CD8+ T cells are likely implicated in the antileukemic effects of targeted STAT3 inhibition/TLR9 activation. To corroborate these findings, we depleted CD8+ cells by using specific antibodies in AML-bearing mice and then followed with 6 repeated injections of CpG-Stat3 siRNA. The antileukemic potential of CpG-Stat3 siRNA was reduced by ∼80% in CD8+ T-cell–depleted mice compared with controls, proving that effector T cells play a key role in AML regression (Figure 7A). We further assessed whether CD8+ T-cell activation following in vivo CpG-Stat3 siRNA treatment is tumor antigen specific. First, we used the irradiated CMM+ AML cells as a source of polyvalent tumor antigens for activation of splenic T cells isolated from CpG-siRNA–treated mice. ELISPOT assay detected significantly increased production of IFN-γ in splenocytes derived from CpG-Stat3 siRNA–treated mice compared with untreated or treated control mice (Figure 7B). Next, we assessed response to defined tumor antigen by using an established AML model of C1498 cells expressing SIYRYYGL peptide.31 Preliminary analysis confirmed that C1498.SIY cells are TLR9+ and STAT3P+ similar to CMM+ AML cells (D.M.S.H. and M.K., unpublished data). Mice with established C1498.SIY tumors were treated in vivo with CpG-Stat3 siRNA or control oligonucleotides or were left untreated. Again, ELISPOT assays after recall stimulation with SIYRYYGL peptide detected increased IFN-γ production by T cells from CpG-Stat3 siRNA–treated mice compared with controls (Figure 7C). Overall, these findings provide further evidence that myeloid cell–specific STAT3 inhibition/TLR9 activation generates tumor antigen–specific adaptive immune responses in AML-bearing mice.
Antigen-specific CD8+T cells contribute to antileukemic effects of CpG-Stat3 siRNA. (A) CD8+ T-cell depletion prevents the antitumor effect of CpG-Stat3 siRNA in vivo. C57BL/6 mice were injected intraperitoneally with CD8-specific control immunoglobulin G (IgG) antibodies or left untreated and then injected intravenously with 1 × 106CMM+ AML cells. After tumors were established, both antibody-treated groups of mice were injected with CpG-Stat3 siRNA every other day 6 times. Percentages of AML cells in blood, spleen, and bone marrow were determined by flow cytometry. Shown are combined results from 1 experiment on 6 mice per group; means ± SEM (n = 6). (B) STAT3 targeting using CpG-siRNA results in tumor antigen–specific IFN-γ production. Splenocytes from untreated, CpG-Luc siRNA–, or CpG-Stat3 siRNA–treated mice were incubated overnight with irradiated (100 Gy) CMM+ AML cells. Production of IFN-γ was assessed by ELISPOT assay. Shown are representative images (top) and results combined from the group of 4 to 6 individual mice. (C) CpG-Stat3 siRNA augments recall response to SIY model tumor antigen. C57BL/6 mice were injected with 1 × 106 C1498.SIY cells intravenously and treated as described above. IFN-γ ELISPOT assay was performed by using splenocytes from 4 to 6 individual mice in each group after overnight restimulation with SIY peptide. Shown are the representative results from 2 independent experiments; means ± SEM. Statistically significant differences were indicated by asterisks: ***P < .001; **P < .01; *P < .05.
Antigen-specific CD8+T cells contribute to antileukemic effects of CpG-Stat3 siRNA. (A) CD8+ T-cell depletion prevents the antitumor effect of CpG-Stat3 siRNA in vivo. C57BL/6 mice were injected intraperitoneally with CD8-specific control immunoglobulin G (IgG) antibodies or left untreated and then injected intravenously with 1 × 106CMM+ AML cells. After tumors were established, both antibody-treated groups of mice were injected with CpG-Stat3 siRNA every other day 6 times. Percentages of AML cells in blood, spleen, and bone marrow were determined by flow cytometry. Shown are combined results from 1 experiment on 6 mice per group; means ± SEM (n = 6). (B) STAT3 targeting using CpG-siRNA results in tumor antigen–specific IFN-γ production. Splenocytes from untreated, CpG-Luc siRNA–, or CpG-Stat3 siRNA–treated mice were incubated overnight with irradiated (100 Gy) CMM+ AML cells. Production of IFN-γ was assessed by ELISPOT assay. Shown are representative images (top) and results combined from the group of 4 to 6 individual mice. (C) CpG-Stat3 siRNA augments recall response to SIY model tumor antigen. C57BL/6 mice were injected with 1 × 106 C1498.SIY cells intravenously and treated as described above. IFN-γ ELISPOT assay was performed by using splenocytes from 4 to 6 individual mice in each group after overnight restimulation with SIY peptide. Shown are the representative results from 2 independent experiments; means ± SEM. Statistically significant differences were indicated by asterisks: ***P < .001; **P < .01; *P < .05.
Discussion
We used CpG-Stat3 siRNA conjugate to test the effect of concurrent STAT3 inhibition and TLR9-dependent immunostimulation in a mouse AML model.16 Our results demonstrate that STAT3 impairs antigen-presenting functions of both nonmalignant and leukemic myeloid cells in vivo. Moreover, STAT3 inhibition/TLR9 activation in AML cells alone is sufficient for generation of antitumor immunity. Dual-function CpG-Stat3 siRNA generated antitumor immune responses in two orthotopic AML models, CMM+ and C1498, independently from mouse genetic background. The repeated systemic administration of unformulated CpG-Stat3 siRNA generated effective CD8+ T-cell–dependent immune responses, thereby leading to remission of disseminated AML in a majority of the mice. We reported previously that inhibition of STAT3 in nonmalignant tumor-associated myeloid cells triggered immune responses against solid tumors but rarely induced complete tumor regression.15,18 The elimination of leukemia after systemic CpG-Stat3 siRNA treatment underscores therapeutic potential of inducing AML cell immunogenicity and breaking tumor immune tolerance.
STAT3 gained recognition as a promising therapeutic target in AML for its oncogenic role in tumor cell proliferation and survival.8,9 It was also demonstrated that while driving AML cell proliferation, STAT3 suppresses differentiation of leukemic cells.33 This is similar to the function of STAT3 in expansion of undifferentiated myeloid cells and inhibition of DC maturation.34-37 Our results demonstrate that CpG-Stat3 siRNA–induced leukemia regression primarily depends on AML cell differentiation to APC phenotype rather than on direct tumor cell killing. The potent antitumor immunity leads to systemic AML elimination, even though penetration of leukemic cells by intravenously delivered CpG-Stat3 siRNA is incomplete. The resistance of CMM+ cells to cytotoxic effects of STAT3 inhibition is likely a result of thrombopoietin receptor–induced survival signaling, which involves Jak2/STAT5, Akt, and MAPK.22 Effective induction of direct tumoricidal effects may also require higher concentration and more frequent administration of CpG-siRNA to rapidly dividing tumor cells. We have previously demonstrated that daily intratumoral administration of CpG(A)-STAT3 siRNA into subcutaneously growing human AML tumors induced cancer cell apoptosis and inhibited tumor growth in immunodeficient NSG mice.20 Preliminary results from our in vitro studies on primary AML specimens indicate the feasibility of using CpG(A)-STAT3 siRNA strategy to trigger immunogenicity of human leukemia (D.M.S.H. and M.K., unpublished data). Further studies in humanized mouse AML models will assess whether enhanced immunogenicity of human AML cells and their decreased survival contribute to the potent therapeutic effect of CpG(A)-STAT3 siRNA.
A majority of AML cells are arrested at the early stage of myeloid cell differentiation, creating an opportunity to initiate and redirect their maturation toward DCs. Significant effort was dedicated to AML immunotherapies based on ex vivo generation of leukemic DCs with the ability to present leukemic antigens to T cells.29,30,38 However, vaccination strategies using leukemic DCs did not induce sufficient clinical responses, likely because of the strongly immunosuppressive AML microenvironment.4,5,39 Our studies support the notion that STAT3 activation in leukemic cells plays a critical role in orchestrating immunosuppression in AML, particularly through preventing leukemic cell differentiation to APCs. Blocking STAT3 together with TLR9 stimulation augmented expression of MHC class II and costimulatory molecules as previously described in nonmalignant myeloid cells such as DCs.34-37 These effects were consistent with the increase in plasma levels of proinflammatory mediators including IFN-γ, IL-12, or CXCL9/MIG, which are induced by TLR9 activation in mature immune cells.16,40 The AML cells are also the likely source of IFN-γ and IL-12, which are detectable at high levels in the plasma of CpG-Stat3 siRNA–treated mice. Our earlier studies targeting STAT3 only in tumor-associated myeloid cells resulted in mostly local upregulation of proinflammatory cytokine expression.15,18 We observed moderate but significant reduction in the surface expression of the coinhibitory PD-L1 molecule. PD-L1 is an intensively studied molecule that inhibits antitumor immunity by direct binding to a receptor (PD-1) on activated T cells.5,14,41 Studies in mouse models of AML provided compelling evidence that blocking PD-L1 can increase sensitivity of AML cells to antitumor T-cell responses.31,42-44 Disrupting PD-1/PD-L1 interaction generated promising results in initial clinical trials in hematologic malignancies, including AML.5 STAT3 was previously shown to directly control PD-L1 expression in T-cell lymphoma and later also in IL-6–induced tolerogenic APCs.45,46 In our study, the increased STAT3 activity in AML cells correlated with elevated plasma levels of IL-6, which is known as a hallmark of cancer-induced inflammations and a major STAT3 activator.47 High plasma levels of IL-6 are an unfavorable prognostic factor for survival in AML patients.48 Therefore, targeting STAT3 by the CpG-siRNA approach likely interrupts the tolerogenic feed-forward loop, reducing both STAT3 activation and IL-6 production.
In addition to tolerogenic AML cells and dysfunctional DCs, Tregs have an important role in promoting immune tolerance in leukemia.4 We demonstrate that systemic CpG-Stat3 siRNA treatment reduces Treg numbers while increasing recruitment of activated CD8 T cells into major leukemia reservoirs such as spleen and bone marrow. An increased ratio of tumor-infiltrating effector CD8 T cells to Tregs is an indicator of successful cancer immunotherapy correlated to tumor rejection.32,49 This is likely an indirect effect of CpG-Stat3 siRNA–mediated induction of tumor antigen–specific responses, as confirmed in two independent AML models in vivo using peptide antigen and whole irradiated tumor cells. Thus, our approach supports generation of polyvalent and multifaceted immune responses that are more likely to eliminate AML cells together with leukemic stem cells, which show selective expression of many leukemia-specific antigens.1
Our findings imply that systemic administration of naked CpG-siRNA conjugates avoided major pitfalls in the application of TLR agonists as immunoadjuvants, namely desensitization or tolerization of immune cells after repeated TLR stimulation.17 This is likely an effect of TLR9 triggering liberated from the negative effect of potentially tolerogenic STAT3 signaling.15 At the same time, we did not observe autoimmune effects of long-term treatment (more than 2 months) with CpG-Stat3 siRNA. Because of its role in stem cell renewal, the potential risk of systemic STAT3 inhibition could be deregulation of hematopoiesis.12 However, as verified in our control experiments, CpG-Stat3 siRNA conjugates were not internalized by mouse hematopoietic stem cells and did not significantly affect leukocytes in tumor-free mice (supplemental Figure 7). Therefore, the myeloid cell–specific CpG-Stat3 siRNA strategy seems to overcome major challenges faced by pharmacologic inhibitors aimed at this signaling pathway. Further studies using AML models in humanized mice are necessary to evaluate the bifunctional tumoricidal/immunostimulatory effect of CpG(A)-STAT3 siRNA on both leukemic and immune cells. The optimization of the CpG-siRNA serum stability by chemical modifications, conjugation to high-molecular-weight polymers, or encapsulation should further enhance their therapeutic efficacy. Altogether, these proof-of-principle studies indicate the translational potential of CpG-STAT3 siRNA strategy for treatment of hematologic malignancies that are commonly TLR9-positive such as AML and potentially also multiple myeloma, B-cell lymphoma, and certain solid cancers.20,50,51
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
We are grateful to Dr Don Diamond (City of Hope [COH]) for critical reading of the manuscript, and to the staff at the Analytical Cytometry, Pathology, and Animal Resource Cores (COH) for their support.
This work was supported in part by National Cancer Institute, National Institutes of Health award numbers R01CA155367 (M.K.), R01CA166770 (J.K.), and P30 CA033572 (COH), by STOP CANCER Allison Tovo-Dwyer Memorial Career Development Award (M.K.), STOP CANCER Career Development Award (Y.-H.K.), the V Foundation Cancer Research Scholar and Team Awards (Y.-H.K. and M.K.), the Marcus Foundation (M.K.), the American Cancer Society Research Scholar Grant 123278-RSG-12-140-01-CSM (Y.-H.K), and by the Kosciuszko Foundation (A.K.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: D.M.S.H., C.D.S., Q.Z., A.K., H.L., C.G., D.M., Y.-H.K., and M.K. performed research; P.S. and A.J. were involved in conjugate design/testing; M.K. and Y.-H.K. designed experiments; J.K. contributed vital reagents; M.K., Y.-H.K., S.F., and R.B. analyzed the data; and M.K. and D.M.S.H. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcin Kortylewski, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: mkortylewski@coh.org; and Ya-Huei Kuo, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: ykuo@coh.org.