Key Points
Histologically aggressive CLL differs from histologically indolent CLL. Patients with different CLL phases but similar SUVmax have similar outcomes.
FDG/PET is a useful diagnostic tool for patients with CLL and suspected transformation.
Abstract
Richter syndrome (RS) is associated with poor outcome. The prognosis of patients with histologically aggressive chronic lymphocytic leukemia (CLL), or HAC, has not been studied. We aimed to correlate 2-deoxy-2-[18F]fluoroglucose/positron emission tomography (FDG/PET) data, histological diagnosis, clinical characteristics, and survival in patients with CLL. A total of 332 patients with CLL were histologically classified as: 95 RS, 117 HAC, and 120 histologically indolent CLL (HIC). HAC and RS patients had higher maximum standardized uptake value (SUVmax), more frequent constitutional symptoms, poorer performance status (PS), lower hemoglobin and platelets, and higher lactate dehydrogenase and β-2-microglobulin. An SUVmax ≥10 strongly correlated with mortality (overall survival [OS], 56.7 vs 6.9 months in patients with SUVmax <10 vs ≥10). Survival of patients with RS and HAC was similar among patients with SUVmax <10 or ≥10. SUVmax ≥10, PS ≥2, bulky disease, and age ≥65 were independently associated with shorter OS. In patients undergoing both fine-needle aspiration and biopsy, the former proved diagnostically inadequate in 23%, 29%, and 53% of HIC, HAC, and RS, respectively. FDG/PET is a useful diagnostic tool in patients with CLL and suspected transformation. Patients with HAC show different characteristics and worse prognosis compared with those with HIC. Patients with different CLL phases, but similar SUVmax have similar outcome. Tissue biopsy should be preferred for diagnosing RS.
Introduction
Richter syndrome (RS) is a rare clinical entity defined by the development of diffuse large B-cell lymphoma (DLBCL) or, much less frequently, classical Hodgkin lymphoma in patients with chronic lymphocytic leukemia (CLL).1 RS occurs cumulatively in approximately 8% of patients with CLL and its incidence is influenced by several factors such as clinical and biological disease features and quality and duration of response to prior treatments.2 Recently published evidence suggests that RS typically arises from the predominant CLL clone through the acquisition of multiple genetic lesions, such as disruption of TP53, NOTCH1 activation, and disruption of CDKN2A/B.3 Clinically, RS is often accompanied by constitutional symptoms, increasing lactate dehydrogenase (LDH), and rapidly enlarging lymph nodes.4 RS carries a dismal prognosis. In the historical experience of our institution, disease control can only be obtained in a fraction of patients and is of brief duration (complete response rates of 0% to 25%, failure-free survival of 1.3 to 5 months, and overall survival [OS] of 2.2 to 8.5 months).5-8 Allogeneic stem cell transplant (SCT) is a promising approach for patients with RS.9,10 However, it can be performed only in a minority of cases because of multiple factors such as inability to obtain sufficient disease control before SCT, uncontrolled infectious complications, and severe comorbidities.
Patients with CLL often present with clinical and laboratory findings highly suggestive of RS. Although histologic evaluation of their disease frequently shows features of increased aggressiveness, it does not meet the criteria for RS diagnosis. Currently, information regarding these patients with clinical and histologic “intermediate features” is scarce and limited data have been published11 describing these patients’ characteristics, response to therapy, and prognosis.
2-Deoxy-2-[18F]fluoroglucose (FDG) positron emission tomography (PET), is a noninvasive radionuclide-based imaging technique that evaluates glucose metabolism. Because tumor cells tend to be more metabolically active compared with their normal counterparts, FDG/PET is increasingly used in the diagnosis and staging of patients with solid or hematologic malignancies.12 Standardized uptake value (SUV) is a FDG/PET-derived semiquantitative measure of cell metabolic rate. In patients with lymphoma, SUV can distinguish between indolent and aggressive forms.13 SUV has also been used for the assessment of response to therapy and prognosis in patients with DLBCL.14-17 Furthermore, Bruzzi and colleagues showed that SUV is low in patients with CLL, but can increase in RS.18
Tsimberidou and colleagues identified prognostic factors for OS in patients with RS.9 Their study did not include patients with CLL showing “intermediate features.” Furthermore, FDG/PET was not available in most cases, and its potential prognostic value could not be addressed.
We report on the experience with FDG/PET in the diagnosis and management of patients with CLL or RS seen at our institution. The aims of the present analysis were to evaluate how FDG/PET data correlate with disease histology and clinical outcomes in patients with CLL.
Methods
Patients
We reviewed the electronic medical records of patients with CLL who were evaluated at our institution and assessed by both FDG/PET and concurrent (within 3 months) tissue biopsy performed at referral or during the course of disease. Patients with a history of solid tumor were excluded unless this was pathologically confined to the primary site, in complete remission for ≥5 years after treatment, or presenting ≥1 year after the index FDG/PET. This study was approved by the internal review board and conducted in accordance with the institutional guidelines and the Declaration of Helsinki.
The following were analyzed: (1) patient characteristics: demographics, physical examination findings (bulky disease = presence of palpable nodal or extranodal mass with maximum diameter ≥5 cm), performance status (PS), and prior therapies; (2) laboratory data: complete blood count with differential, LDH, β-2-microglobulin (β2-m), immunoglobulin heavy chain–variable region gene mutational status, genomic analysis by fluorescence in situ hybridization, and CD38 and ZAP70 expression; (3) staging procedures: FDG/PET, tissue diagnosis obtained from fine-needle aspiration (FNA), and core or excisional biopsy and bone marrow biopsy; and (4) therapy and outcome: type of treatment, response, and survival status at last follow-up.
Histology
All specimens, including those obtained at outside facilities, were reviewed; diagnoses were rendered within the Division of Pathology (hematopathology and/or cytopathology) at our institution. For the purposes of the study, the diagnoses were further classified into 1 of 3 groups: (1) histologically indolent CLL (HIC), defined as morphologically typical CLL with no histologic features of progression or transformation such as increased large cells, large confluent proliferation centers, or high proliferation rate; (2) CLL with histological features of intermediate aggressiveness (histologically aggressive CLL [HAC]), defined as CLL with one of the following features—increased large cells, large confluent proliferation centers, or high proliferation rate assessed by Ki67 expression using immunohistochemistry and reported as an estimate of the overall percentage of positive lymphoid cells; or (3) RS, defined by the identification of areas histologically consistent with DLBCL or classical Hodgkin lymphoma.
Imaging
A total of 313 (94% of the study population) patients underwent FDG/PET examinations at our institution (integrated PET-CT19 in 306 patients and dedicated FDG/PET in 7). Nineteen examinations (17 PET-CT and 2 FDG/PET) were performed at outside facilities and considered fully evaluable after internal review. Attenuation-corrected and non–attenuation-corrected datasets were reconstructed.
The maximum SUV (SUVmax) within the volume of interest in the FDG/PET examinations was calculated as previously described.18 A cutoff SUVmax ≥5 was considered indicative of ongoing CLL transformation, as previously suggested by various groups.18,20,21 To test the prognostic impact of the extent of disease by FDG/PET, we defined PET-limited (PET-lim) disease as hypermetabolic sites with SUVmax ≥5 above or below the diaphragm and PET-extensive (PET-ex) disease as hypermetabolic sites with SUVmax ≥5 on both sides of the diaphragm. Posttherapy FDG/PET examinations were analyzed when available.
Statistical analysis
Patient characteristics were summarized using median (range) for continuous variables and frequency (percentage) for categorical variables. Kruskal-Wallis test and χ-squared test were used to assess the association between continuous and categorical variables and patient histology (ie, RS, HAC, and HIC). OS was calculated from biopsy until death or last follow-up, whichever occurred first. The analysis of the FDG/PET data was performed retrospectively and not used for pretreatment patient stratification. Kaplan-Meier22 method was used to estimate probabilities of OS, and log-rank test23 was used to compare subgroups of patients. Receiver operating characteristic analysis was performed to derive the optimal SUVmax cutoff. The criterion we used was based on maximizing the sum of the sensitivity and specificity, which is equivalent to maximizing (sensitivity + specificity − 1), also known as the Youden’s index. Univariable and multiple Cox proportional hazards regression models24 were fit to assess the association between patient characteristics and OS. All statistical analyses were conducted using SAS 9.3 or Splus 8.2.
Results
Patients
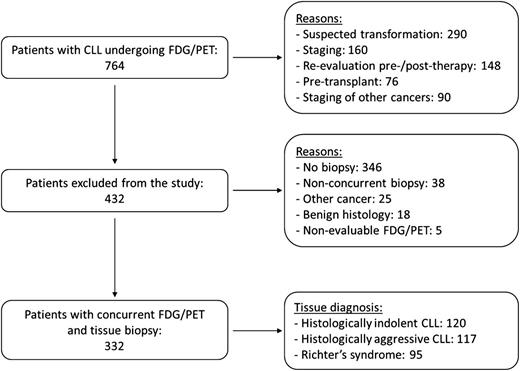
Between October 2001 and July 2012, 764 patients with CLL underwent FDG/PET for one of the following reasons: suspected transformation (290), staging (160), re-evaluation after or before therapy (148), pre-SCT (76), or staging of other cancers (90). A total of 384 patients either had no tissue diagnosis or biopsy performed at a different time than FDG/PET, 25 had other cancers, 18 had benign histology, and 5 had nonevaluable FDG/PET examinations. The remaining 332 patients had concurrent FDG/PET and tissue biopsy and are the focus of this report. Ninety-five were histologically classified as RS, 117 as HAC, and 120 as HIC. The selection process of the study population is summarized in Figure 1. The 3 histological groups had a similar median age and gender distribution. Compared with patients with HIC, both patients with HAC and RS had received more lines of therapy, had a higher frequency of constitutional symptoms, poorer PS, lower hemoglobin levels and platelet counts, and higher LDH and β2-m levels. There were no significant differences between patients with HAC and RS (Table 1). Patients with HAC had a higher proportion of cases with expression of ZAP70 and with deletion of chromosome 17p (del17p), although the latter difference was of borderline significance (P = .053). The proportion of patients who had been refractory to the last treatment before the concurrent FDG/PET and biopsy was similar across the 3 groups.
Study population selection process. The figure illustrates the 2-step process leading to the identification of patients with CLL and concurrent FDG/PET and tissue specimens.
Study population selection process. The figure illustrates the 2-step process leading to the identification of patients with CLL and concurrent FDG/PET and tissue specimens.
Tissue biopsy is the preferred approach for diagnosing RS
Tissue diagnosis was obtained by core or excisional biopsy in 149 patients, FNA alone in 95 patients, and simultaneous FNA and biopsy of the same lesion in 88 patients. Among patients who had both procedures, FNA proved to be diagnostically inadequate (ie, absence of malignant cells or histologic downgrading in FNA compared with biopsy) in 53%, 29%, and 23% of patients with RS, HAC, and HIC, respectively. Ki67 antigen expression was available from 88 samples. Higher Ki67 expression strongly correlated with disease histology (median expression 70%, 32%, and 10% in patients with RS, HAC, and HIC, respectively) (Table 2).
Patients with RS have higher SUVmax and are more likely to have PET-ex disease
Median SUVmax was significantly higher in patients with RS compared with HAC and HIC. Median SUVmax was 17.6, 6.8, and 3.7 in patients with RS, HAC, and HIC, respectively. Compared with patients with HIC, those with RS showed a higher percentage of cases with SUVmax ≥5 (88% vs 34%; P < .0001), and cases with PET-ex disease (60% vs 14%; P < .0001). Among patients with HAC, the percentage of cases with SUVmax ≥5 and PET-ex disease were 72% and 38%, respectively (Table 2). The presence of SUVmax ≥5 had a sensitivity of 88%, specificity of 47%, positive predictive value (PPV) of 38%, and negative predictive value (NPV) of 92% for the detection of histologically confirmed RS.
SUVmax ≥10, PS ≥2, and bulky disease correlate with inferior survival
At the time of this analysis, 21 patients (22%) with RS, 37 (32%) with HAC, and 88 (73%) with HIC were alive. Median survival was 7.7 months for patients with RS, 17.6 months for patients with HAC, and not reached (median follow-up 77.2 months) for patients with HIC (P < .0001). Similar differences in survival were observed in patients who underwent FNA only (6.9, 17.3, and 67.4 months for patients with RS, HAC, and HIC, respectively, P < .0001). Causes of death included progressive disease or disease-related complications (76%, 63%, and 37% in patients with RS, HAC, and HIC, respectively), treatment-related complications (16%, 16%, and 22%, respectively), other cancers (1%, 7%, and 16%, respectively), or unrelated events (0%, 4%, and 6%, respectively). The cause of death was unknown in 4%, 5%, and 8% of patients with RS, HAC, and HIC, respectively. Interestingly, among patients with treatment-refractory disease or del17p, patients with HAC had an inferior survival compared with patients with HIC (11 vs 71 months, P = .02, and 9 vs 32 months, P = .006, respectively).
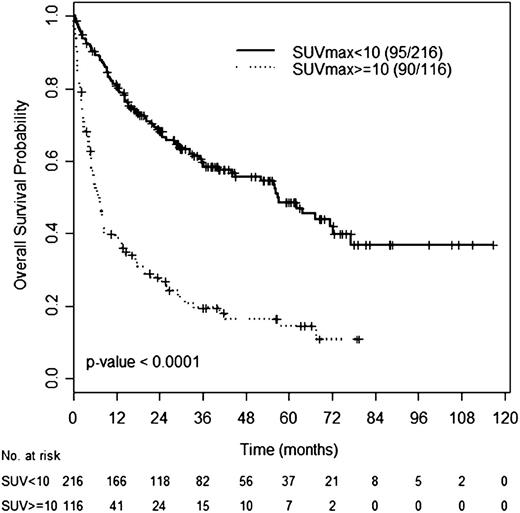
An SUVmax cutoff of 10 had an optimal discriminatory power for OS by receiver operating characteristic analysis (data not shown). Median OS was 56.7 months (95% CI: 41.1-72.4) for patients with SUVmax <10 and 6.9 months (95% CI: 4.8-9.0) for patients with SUVmax ≥10 (Figure 2). Similar, differences in OS were observed between patients with SUVmax <10 and ≥10 within the HIC (not reached vs 42 months, P = .017), HAC (29 months vs 7 months, P = .001), or RS group (21 months vs 6 months, P = .015).
OS according to SUVmax. OS of patients with SUVmax ≥10 or SUVmax <10 across CLL histologies.
OS according to SUVmax. OS of patients with SUVmax ≥10 or SUVmax <10 across CLL histologies.
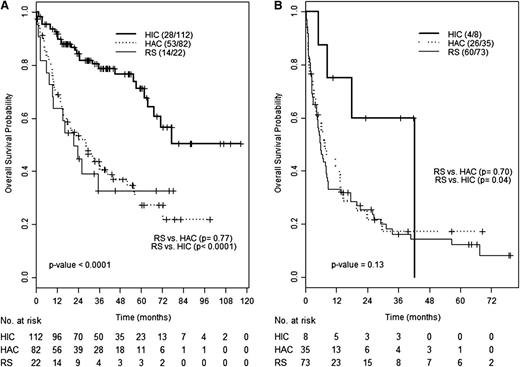
To determine whether SUVmax or histology has a greater impact on survival, we compared the outcome of patients with RS, HAC or HIC, and SUVmax ≥10 or <10. For patients with SUVmax <10, survival of patients with RS and HAC was similar (median of 28.6 vs 21.3 months, P = .62) and shorter compared with that of HIC patients (not reached, median follow-up 30.3 months) (Figure 3A). Similarly, the OS of patients with RS and HAC and SUVmax ≥10 were superimposable (7.6 vs 5.7 months, P = .71). Although only 8 patients with HIC had an SUVmax ≥10, their outcome did not significantly differ from that of HAC or RS patients (Figure 3B). Of these, 5 were biopsied from sites with SUV ≥10, 2 from a site with lower SUV (8 and 4), and 1 from a brain mass.
OS according to histology and SUVmax. OS of patients with RS, HAC, or HIC among patients with SUVmax <10 (A) or ≥10 (B).
OS according to histology and SUVmax. OS of patients with RS, HAC, or HIC among patients with SUVmax <10 (A) or ≥10 (B).
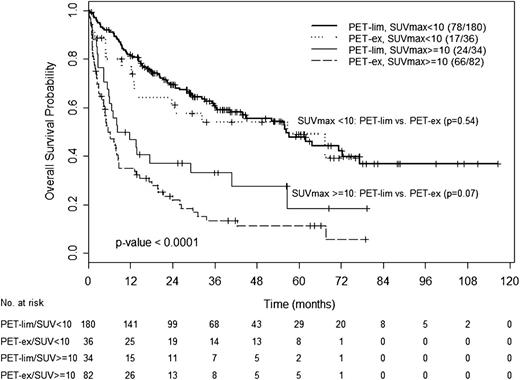
We analyzed the impact of the extent of disease on the survival of patients with SUVmax <10 or ≥10. For this analysis, we stratified patients into 4 groups: PET-lim, SUVmax <10; PET-lim, SUVmax ≥10; PET-ex, SUVmax <10; or PET-ex, SUVmax ≥10. For patients with SUVmax <10, median survival of patients with PET-lim or PET-ex disease was similar (56.3 and 56.7 months, P = .54). Likewise, for those with SUVmax ≥10, survival of patients with PET-lim or PET-ex disease was not significantly different (8.2 and 5.6 months, respectively, P = .07). These findings suggest that the prognostic value of SUVmax may overcome that of the extent of disease (Figure 4). Within the RS group, patients with PET-ex disease had a shorter survival time compared with patients with PET-lim disease (5.1 vs 13.8 months, P = .04)
OS according to SUVmax and disease extent by FDG/PET. OS of patients with PET-lim disease and SUVmax <10, PET-ex disease and SUVmax <10, PET-lim disease and SUVmax ≥10, or PET-ex disease and SUVmax ≥10.
OS according to SUVmax and disease extent by FDG/PET. OS of patients with PET-lim disease and SUVmax <10, PET-ex disease and SUVmax <10, PET-lim disease and SUVmax ≥10, or PET-ex disease and SUVmax ≥10.
For univariable and multivariable analyses of factors associated with survival, we excluded patients with de novo RS. Age ≥65 years, constitutional symptoms, PS ≥2, bulky disease, LDH at or above the upper limit of normal (618 U/L in our laboratory), higher β2-m, del17p, SUVmax ≥10, PET-ex disease, and higher Ki67 expression were significantly associated with shorter survival in univariable analysis. In multivariable analysis, age ≥65, PS ≥2, bulky disease, and SUVmax ≥10 were independently associated with shorter OS. Because histology (RS vs others) was not an independent prognostic factor for survival, we performed the same analysis including only patients with HAC and RS. In this latter group, older age was no longer significant, whereas SUVmax ≥10, PS ≥2, and bulky disease remained of independent prognostic value (Table 3).
Response to therapy by FDG/PET is associated with superior survival in patients with HAC and RS
Of the 212 patients with HAC or RS, follow-up was available in 152 patients because 60 of them (27 with HAC and 33 with RS) died before treatment initiation, lacked follow-up studies, or immediately transitioned to SCT. Among 152 evaluable patients, 34 with HAC (38%) and 32 with RS (52%) received dose-dense, dose-intense chemoimmunotherapy after histologic diagnosis. Other treatment regimens used included standard-dose chemoimmunotherapy, immunotherapy with or without steroids, immunomodulating agents, or radiation therapy. By CT scan, overall response rates were 42% and 43% and complete response rates were 24% and 27% in patients with RS and HAC, respectively. We next analyzed response to therapy by FDG/PET. Based on published data in patients with DLBCL,14-17 we chose an SUVmax reduction of ≥66% compared with the pretherapy examination as indicative of response and marker of chemosensitivity. Among 98 patients with available re-evaluation FDG/PET scans, 36 (37%) patients showed a response. In 48 (31%) patients, therapy was followed by SCT. At the time of this analysis, the median OS is 15.3 months for chemoresistant patients and has not been reached (median follow-up 35.9 months) for chemosensitive patients.
Discussion
To our knowledge, this is the largest series of fully characterized patients with CLL evaluated with FDG/PET imaging and concurrent biopsy. Newer treatments, such as the B-cell receptor pathway inhibitors and pro-apoptotic agents targeting the bcl-2 pathway, are showing promising results in patients with HIC. However, the limited experience so far indicates that these strategies are not as effective in patients with RS. Furthermore, progression to RS has been reported in patients undergoing treatment with B-cell receptor pathway inhibitors. The management of patients with transformed CLL is challenging because of clinical aggressiveness and chemotherapy-refractoriness of the disease. Prompt identification of transformation is therefore a key element in the management of these patients.
Radionuclide-based imaging has been proposed as a tool to detect CLL transformation.18,25 Our center reported on the usefulness of FDG/PET in diagnosing RS. A cutoff SUVmax ≥5 showed sensitivity, specificity, NPV, and PPV of 91%, 80%, 53%, and 97%, respectively.18 In the present study, conducted on a larger number of patients, we confirmed excellent sensitivity and NPV of FDG/PET, but found lower specificity and PPV. This is likely because of the large proportion of patients with biopsy-proven HAC in this series. With biopsy remaining the standard diagnostic procedure to ascertain CLL transformation, the high sensitivity and NVP of FDG/PET found in our study may be particularly important to identify the sites most likely to be diagnostically informative. Therefore, in patients with suspected CLL transformation, we recommend that biopsy of a lymph node with SUVmax ≥5 be performed. Importantly, in our experience, FNA resulted in a high rate of false negatives, particularly in diagnosing RS. Core or excisional biopsy should therefore be the recommended diagnostic procedure in patients with suspected CLL transformation. In patients who only underwent FNA for tissue diagnosis, misclassification of a number of cases is a possibility given the relatively low diagnostic yield of this technique. However, differences in survival among patients with HIC, HAC, and RS within the FNA-only population were similar to those of the entire study population.
Interestingly, in the population we studied, patients with HAC or RS with similar clinical and FDG/PET characteristics received comparable treatments and had similar outcome. This observation emphasizes the importance of FDG/PET in aiding the diagnosis of CLL transformation, particularly when biopsy cannot be performed. Tissue biopsy, however, should be obtained when feasible because it will exclude other neoplastic or nonneoplastic (reactive, infectious, autoimmune) conditions that can clinically mimic RS.
We found that the presenting clinical and laboratory features of patients with HAC were similar to those of patients with RS, whereas their survival (median 17.6 months) was intermediate between that of patients with HIC (77.2 months) and RS (7.7 months). Notably, the survival of HAC patients was shorter compared with that of HIC patients even in very high-risk groups, such as refractory disease or presence of del17p, suggesting that HAC CLL reflects a different state of the disease. Therapeutic decision-making based solely on histologic findings may be difficult for patients with HAC because they do not meet a formal definition of RS, yet their disease clearly behaves more aggressively than HIC. FDG/PET, therefore, is an important tool to assist with designing an appropriate treatment approach for this group of patients. Giné and colleagues reported the histological features of a retrospective series of 100 patients with CLL. They defined “accelerated” CLL by the presence of expanded proliferation centers or a high proliferation rate.11 In their report, 22 patients met the criteria for RS. Among the remaining 78, 55 had chronic phase CLL, whereas 23 patients showed “accelerated” CLL. “Accelerated” patients had higher LDH and β2-m compared with the “nonaccelerated” ones. Median survival was 4.3, 34, and 76 months for patients with true RS, “accelerated” CLL, and “nonaccelerated” CLL, respectively. Acceleration was not a criterion used for treatment intensification.
Suspicion of transformation was the reason for ordering a FDG/PET in 55% of the 332 patients. Regardless of the reason behind obtaining a FDG/PET study, an SUVmax ≥10 strongly correlated with shorter OS. In our analysis, SUVmax ≥10, PS, and the presence of bulky disease were the only variables independently associated with shorter survival. Prognostic stratification of patients with transformed CLL based on these 3 factors may be relevant in clinical decision-making (ie, choosing the timing and type of treatment or consolidation therapy with SCT). The distinction between PET-lim and PET-ex disease in this study was modeled on the Cotswolds limited- and advanced-stage disease definition used in the International Prognostic Index for DLBCL.26 Interestingly, although the presence of PET-ex disease per se correlated with shorter OS in our patients with RS, the extent of disease did not appear to separate survival of patients with SUVmax <10 or ≥10 and was not an independent prognostic factor in the multivariable analysis.
Response to therapy is regarded as a strong predictor of survival in cancer patients. In patients with DLBCL, a reduction in SUVmax of 66% after 2 cycles or 70% to 73% after 4 cycles of chemoimmunotherapy has been shown to correlate with improved event-free survival, progression-free survival, and OS.14-17 Here we report a similar observation in patients with CLL, in whom improvement documented by FDG/PET correlated with longer survival.
Our analysis has limitations. First, although the diagnostic criteria for HIC and RS are relatively uniform, defining HAC is challenging because morphologic and/or clinical criteria have not been established within the hematology community. This study shows that certain atypical morphologic features, such as those included in our definition of HAC, appear to have a clinical correlation and relevance. Second, SUVmax may have been influenced by interobserver variability in evaluating the FDG/PET images. Moreover, PET imaging technique evolved over the period covered by this study and SUVmax vary with different imaging techniques.27 Such variability was minimized because the majority of patients were imaged and reported at our institution following published guidelines.28,29 However, cutoff SUVmax may not be generalizable if the PET technique substantially differs from that used in our study.28,29 In addition, specificity and NPV of FDG/PET in detecting RS may have been influenced in part by those patients (90/764, 12%) who underwent FDG/PET to rule out RS, but were not subsequently biopsied in view of a low SUVmax. This limitation can be overcome by prospective studies that systematically correlate FDG/PET and histologic findings. Third, the SUVmax cutoff of 10 derived from our population needs to be validated in an independent cohort of patients with transformed CLL. Because of the rarity of RS, a multi-institutional effort to collect sufficiently large numbers of patients will be needed to confirm this finding. Fourth, we recognize that treatments given after the FDG/PET in our study were not uniform; however, similar proportions of patients with RS or HAC received intensive chemoimmunotherapy as a first or subsequent line of treatment after the index FDG/PET. Prospective studies with specifically designed treatment approaches are warranted in these patients. Finally, because a proportion of patients with HAC or RS died before or shortly after treatment initiation, we cannot exclude that the patient population evaluable for response may have been positively selected, with potential overestimation of the response rates.
In conclusion, our data show that FDG/PET is a useful diagnostic tool in patients with CLL and suspected transformation. Biopsy is the preferred approach to obtaining histologic confirmation of RS because FNA resulted in a high rate of false negatives. Patients with HAC show higher SUVmax, worse clinical characteristics, and worse prognosis compared with those with HIC. SUVmax likely reflects tumor aggressiveness and patients with different CLL phases, but similar SUVmax have a similar outcome. Studies designed to prospectively validate these findings are warranted.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.F. designed research, performed research, collected data, analyzed and interpreted data, and wrote the manuscript; M.J.K. designed research, treated patients, analyzed and interpreted data, and approved the manuscript; E.M.M., M.T.T., E.J.S., and R.L.S. collected and interpreted data, and wrote the manuscript; L.T. and S.C.S. collected data, analyzed and interpreted data, and approved the manuscript; X.W. analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; Z.E., S.O., and W.G.W. treated patients, analyzed and interpreted data, and approved the manuscript; N.J. analyzed and interpreted data, and approved the manuscript; S.L. collected data, contributed analytical tools, and approved the manuscript; and A.F. designed research, performed research, treated patients, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandra Ferrajoli, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: aferrajo@mdanderson.org.