Key Points
The complete response rate for first-line bendamustine/rituximab was statistically noninferior to R-CHOP or R-CVP in indolent NHL or MCL.
The safety profile of bendamustine/rituximab is distinct from that of R-CHOP/R-CVP.
Abstract
This randomized, noninferiority (NI), global, phase 3 study evaluated the efficacy and safety of bendamustine plus rituximab (BR) vs a standard rituximab-chemotherapy regimen (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP] or rituximab plus cyclophosphamide, vincristine, and prednisone [R-CVP]) for treatment-naive patients with indolent non-Hodgkin’s lymphoma or mantle cell lymphoma. Investigators preassigned the standard treatment regimen they considered most appropriate for each patient; patients were randomized to receive BR (n = 224) or standard therapy (R-CHOP/R-CVP, n = 223) for 6 cycles; 2 additional cycles were permitted at investigator discretion. Response was assessed by a blinded independent review committee. BR was noninferior to R-CHOP/R-CVP, as assessed by the primary end point of complete response rate (31% vs 25%, respectively; P = .0225 for NI [0.88 margin]). The overall response rates for BR and R-CHOP/R-CVP were 97% and 91%, respectively (P = .0102). Incidences of vomiting and drug-hypersensitivity reactions were significantly higher in patients treated with BR (P < .05), and incidences of peripheral neuropathy/paresthesia and alopecia were significantly higher in patients treated with standard-therapy regimens (P < .05). These data indicate BR is noninferior to standard therapy with regard to clinical response with an acceptable safety profile. This trial was registered at www.clinicaltrials.gov as #NCT00877006.
Introduction
Rituximab-based immunochemotherapy regimens have become the standard initial treatment of patients with symptomatic advanced indolent non-Hodgkin’s lymphoma (NHL) and mantle cell lymphoma (MCL). In patients with advanced indolent NHL, adding rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy has been shown to improve progression-free survival (PFS),1 and adding rituximab to cyclophosphamide, vincristine, and prednisone (R-CVP) chemotherapy has been shown to improve overall survival (OS).2
Bendamustine is an alkylating agent that contains a bifunctional mechlorethamine derivative and a unique benzimidazole heterocyclic ring structure. Bendamustine has demonstrated clinical activity in patients with chronic lymphocytic leukemia3 and in patients with indolent NHL that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.4 The combination of bendamustine plus rituximab (BR) was also shown to be active in patients with advanced indolent NHL.5,6
The BRIGHT study was initiated to evaluate the efficacy and safety of BR compared with the standard rituximab-chemotherapy regimens (R-CHOP and R-CVP) for patients with treatment-naive indolent NHL or MCL. The primary objective of this study was to determine whether the complete response (CR) rate with BR was noninferior to standard treatment, as assessed by a blinded independent review committee (IRC).
Patients and methods
Accrual to this multicenter, phase 3, open-label, active-controlled, randomized study began in April 27, 2009, and data collection for this report continued until March 31, 2012. The clinical centers were located in Canada, the United States, Brazil, Peru, Mexico, Australia, and New Zealand. An independent external data safety monitoring board was implemented at the beginning of the study for review of all available safety data on an ongoing basis as per the data safety monitoring board charter. Response assessments were performed by the investigators and an IRC (CoreLab Partners, Princeton, NJ) according to the International Working Group (IWG) criteria.7
Patients
Eligible adult patients (≥18 years of age) had CD20-positive indolent NHL with one of the following histologies: follicular lymphoma (grade 1 or 2), lymphoplasmacytic lymphoma, splenic marginal zone B-cell lymphoma, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type, nodal marginal zone B-cell lymphoma, or MCL. Additional criteria included bidimensional measurable disease; Eastern Cooperative Oncology Group performance status score of 0, 1, or 2; estimated life expectancy of ≥6 months; and adequate hematologic, renal (serum creatinine of ≤2.0 mg/dL or creatinine clearance ≥50 mL/min via the Cockcroft-Gault method), and hepatic function (≤2.5 times the upper limit of normal for alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase and total bilirubin within normal limits). Patients were required to be treatment-naive with a need for treatment as indicated by the presence of ≥1 of the following: B symptoms, large tumor mass (characterized by lymphomas with a diameter >3 cm in 3 or more regions or by a lymphoma with a diameter >7 cm in 1 region), presence of lymphoma-related complications, or hyperviscosity syndrome attributed to monoclonal gammopathy. Patients preassigned to the standard R-CHOP treatment group were further required to have a left ventricular ejection fraction ≥50%. Patients who had chronic lymphocytic leukemia, small lymphocytic lymphoma, or follicular lymphoma (grade 3) were excluded from the study. Patients were excluded from the study if they received prior treatment of NHL with the exception of locally delimited radiation therapy (in which the radiation field did not exceed 2 adjacent lymph node regions), had Ann Arbor stage I disease, or had a history of central nervous system or leptomeningeal lymphoma. Additional ineligibility criteria included the following: transformed disease; a malignancy other than NHL within the previous 3 years with the exception of localized prostate cancer treated with hormone therapy, cervical carcinoma in situ, breast cancer in situ, or nonmelanoma skin cancer that was definitively treated; cardiac disorder, such as New York Heart Association Class III or IV heart failure, or evidence of ischemia or myocardial infarction within the previous 6 months; evidence of HIV or active hepatitis B or C infection; or received corticosteroids for treatment of lymphoma within 28 days of study entry. Pregnant or lactating women were also excluded from the study.
Study design
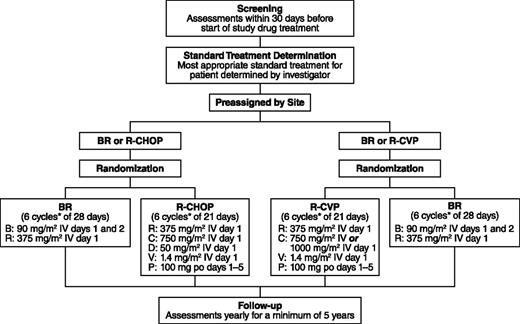
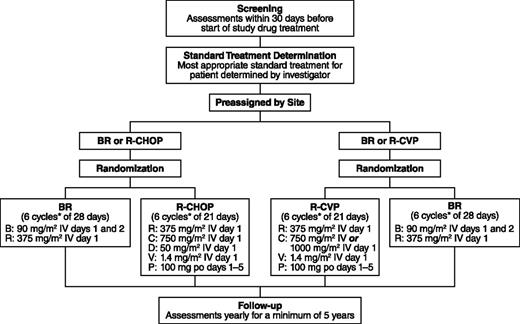
During screening, the investigators preassigned patients to the most appropriate standard treatment (R-CHOP/R-CVP) based on their performance status, comorbidities, and general health. After confirmation that patients met the eligibility criteria for the study, the preassigned patients were then randomized to open-label treatment with either BR or the standard therapy at a 1:1 ratio (Figure 1). Patients were considered to have met the eligibility criteria upon a central review of the inclusion/exclusion criteria and pathology-report review by a central medical monitor. Randomization was also stratified by the investigator’s predetermined standard treatment (R-CHOP or R-CVP) and by lymphoma type (indolent NHL or MCL).
Study design. *Up to 8 cycles at investigator discretion; B, bendamustine; C, cyclophosphamide; D, doxorubicin; P, prednisone; R, rituximab; V, vincristine.
Study design. *Up to 8 cycles at investigator discretion; B, bendamustine; C, cyclophosphamide; D, doxorubicin; P, prednisone; R, rituximab; V, vincristine.
The primary objective of this study was to determine whether BR was noninferior to standard treatment, as assessed by the CR rate among treatment-naive patients with postbaseline data during the randomized-treatment period; CR was defined by the IWG criteria7 and evaluated by an IRC in a blinded manner. Computed tomography, magnetic resonance imaging, and/or fluorodeoxyglucose positron emission tomography was conducted according to the following schedule: screening, which had to be completed within 6 weeks prior to the use of study drug; the last week of treatment cycles 3, 6, and (if applicable) 8; or the end-of-treatment visit. All images were sent to the IRC for review by 2 blinded readers, with adjudication if needed. An end-of-treatment visit was scheduled if the last treatment received was not at cycle 3, 6, or 8. Safety and tolerability were assessed. The final sample size was determined to be 218 patients per group based on the noninferiority (NI) ratio and an assumed 5% superiority (Sup) of BR compared with R-CHOP/R-CVP treatment based on available literature. Consistency of treatment effect for the primary efficacy end point was also checked with a subgroup analysis by preassigned standard-therapy stratum (BR vs R-CHOP or BR vs R-CVP).
Secondary objectives were to compare the following between the BR and standard-therapy treatment group: overall response rate, which was defined as CR plus partial response; PFS, or the time from randomization to disease progression or relapse, or death from any cause; event-free survival, or time from randomization to treatment failure; median duration of response; OS; quality of life; and safety and tolerability. The CR rate and CR-rate ratio were calculated for the subpopulations, and P values for NI and Sup were calculated. Quality of life was measured by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire at screening and after cycles 1, 3, 6, and 8. Time-to-event (ie, PFS, event-free survival, duration of response, and OS) data are being collected, but these data and analyses are not yet mature, because patients are still in the 5-year follow-up period. Time-to-event and quality of life findings will be discussed in future publications.
Safety of the treatment regimens was evaluated among patients who received ≥1 dose of study drug. Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities version 15.0, and AEs were recorded and graded according to the Common Terminology Criteria for AEs version 3.0. Patients were queried as to whether they experienced AEs during each scheduled visit to receive a cycle of therapy and, if applicable, an end-of-treatment visit. Safety data are presented for the individual treatment strata (BR vs R-CHOP and BR vs R-CVP).
The protocol was submitted to the appropriate local independent ethics committee/institutional review board for each site. All patients provided written and dated informed consent in accordance with local policies, federal regulations, and the Declaration of Helsinki.
Treatment
Six cycles were planned for all treatment arms, with a maximum of 8 cycles at the discretion of the investigator. In the BR arm, rituximab was administered intravenously at 375 mg/m2 on day 1 according to standard procedures at each center. After the administration of rituximab, bendamustine was administered intravenously over 30 minutes at a dosage of 90 mg/m2/d on days 1 and 2. Cycles of BR were repeated every 28 days. In the standard-therapy arm, rituximab was administered intravenously at 375 mg/m2 on day 1, cyclophosphamide intravenously at 750 mg/m2 (with the option of 1000 mg/m2 for patients assigned to R-CVP) on day 1, vincristine intravenously at 1.4 mg/m2 (2-mg maximum) on day 1, and prednisone orally at 100 mg/d on days 1 to 5; patients assigned to R-CHOP also received doxorubicin intravenously at 50 mg/m2 on day 1. Cycles of R-CHOP or R-CVP were repeated every 21 days. During the assessment period for response to treatment with BR or standard therapy (ie, prior to the follow-up period), no patient received maintenance rituximab.
Supportive therapy (eg, antiemetics, antipyretics, and antibiotics) was given according to the standard of care at the study center. Cytokines could be prophylactically administered or used in response to severe myelosuppression according to American Society of Clinical Oncology guidelines.
Statistical methods
The safety analysis included all patients who received ≥1 dose of any study drug. The set of efficacy-evaluable patients included all treated patients who had a baseline and ≥1 postbaseline response evaluation based on computed tomography/magnetic resonance imaging or fluorodeoxyglucose positron emission tomography and clinical data by the IRC, or who discontinued treatment due to progressive disease and did not have major protocol violations. The set of efficacy-evaluable patients is considered of primary interest for the efficacy analysis of NI.
The primary efficacy end point of NI was assessed by stratified z statistics. To be sufficiently powered for the primary end point, planned study enrollment was increased to 218 patients per treatment group (BR or R-CHOP/R-CVP standard therapy) due to recalculation of the NI margin as 0.88 for the CR-rate ratio (BR vs R-CHOP/R-CVP), based on a meta-analysis following the “FDA Draft Guidance for Industry: Non-Inferiority Clinical Trials”8 and reserve 50% active control effect. If NI was established, then Sup was assessed using the Cochran–Mantel–Haenszel test stratified by the predetermined standard-therapy assignment and lymphoma type. Statistical tests were 2-sided at the α level of 0.05.
Results
Patient disposition and baseline characteristics
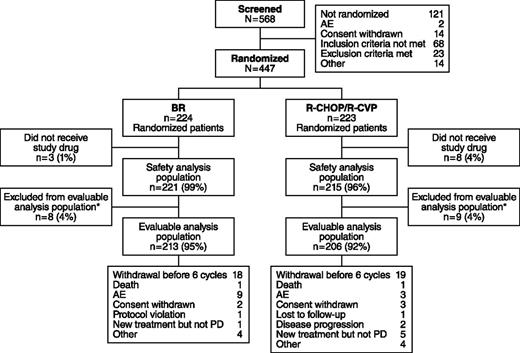
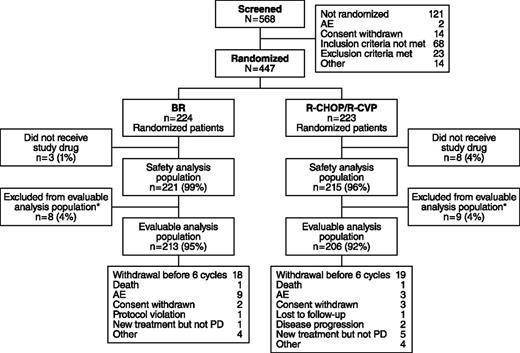
Of 447 patients who met the eligibility criteria and were enrolled, 224 were randomized to receive BR and 223 to standard therapy (Figure 2). Among patients receiving standard therapy, 104 were treated with R-CHOP and 119 with R-CVP. Nine patients in the BR treatment group and 3 patients in the standard-therapy treatment group withdrew because of AEs. The distribution of demographics and baseline characteristics was balanced across treatment arms (Table 1). The histologic subtypes were lymphoplasmacytic (n = 11), marginal zone (n = 46), MCL (n = 74), and follicular lymphoma (n = 314); histologic subtypes were not recorded for 2 patients.
Patient disposition in the BRIGHT study. *Efficacy-evaluable population of all treated patients who have a baseline and ≥1 postbaseline efficacy evaluation, or who discontinued due to progressive disease (PD) and did not have major protocol violations.
Patient disposition in the BRIGHT study. *Efficacy-evaluable population of all treated patients who have a baseline and ≥1 postbaseline efficacy evaluation, or who discontinued due to progressive disease (PD) and did not have major protocol violations.
Efficacy
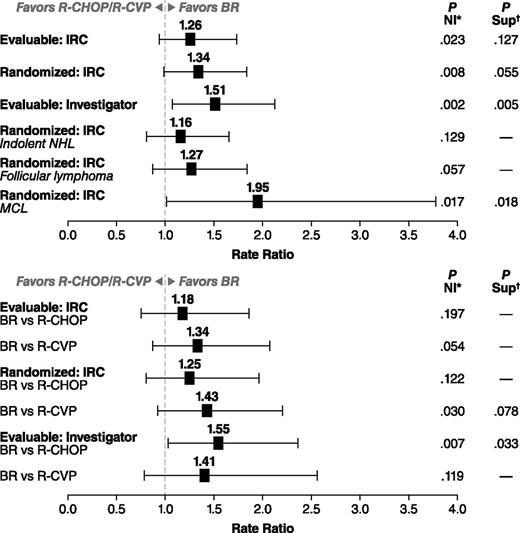
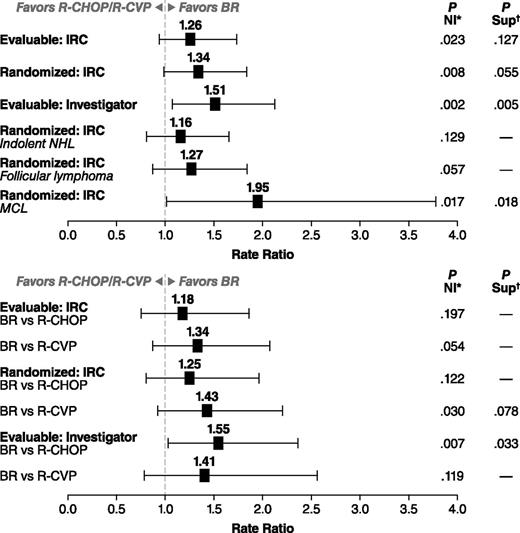
In the evaluable patient population, there were 213 patients in the BR treatment group and 206 patients in the standard-therapy treatment group. BR therapy was noninferior to the standard therapy by IRC-assessed CR rate: 31% in the BR treatment group and 25% in the standard-therapy treatment group (CR-rate ratio 1.26; P = .0225 for NI); the CR rate for BR was greater than the 22% threshold for NI (ie, >88% of the CR rate for standard therapy). The higher CR rate with BR treatment was not statistically superior to standard therapy (P = .1269) (Table 2). Overall response rates were 97% for the BR treatment group and 91% for the standard-therapy treatment group, which was statistically superior for the BR treatment group (CR-rate ratio: 1.04; 95% confidence interval [CI]: 0.99-1.09; P = .0102). Analyses by treatment stratum among evaluable patients demonstrated numerically higher CR rates for BR compared with R-CHOP (P = .197 for NI) and R-CVP (P = .054 for NI), and overall response rates of 96% (95% CI: 89.8-98.9) for BR compared with 96% (95% CI: 89.8-98.9) for R-CHOP among patients preassigned to R-CHOP and 97% (95% CI: 92.6-99.5) for BR compared with 86% (95% CI: 78.3-92.1) for R-CVP among patients preassigned to R-CVP.
IRC-evaluated CR rates in the randomized population results were generally similar to the primary analysis of the evaluable population (Figure 3). The investigator-assessed analysis of the evaluable population found that BR was significantly superior to standard therapy (CR-rate ratio: 1.51; 95% CI: 1.07-2.12; P = .005). Subanalyses were also conducted by histology (Table 3). NI was not reached when comparing treatment arms in patients with indolent NHL, as determined by IRC analysis (CR-rate ratio: 1.16; 95% CI: 0.81-1.65; P = .1289) (Figure 3). NI approached significance in the follicular-lymphoma subset (CR-rate ratio: 1.27; 95% CI: 0.87-1.84; P = .0569) (Figure 3). In contrast, Sup was demonstrated when comparing BR with standard therapy in patients with MCL (CR-rate ratio: 1.95; 95% CI: 1.01-3.77; P = .018; 22 patients received R-CHOP and 11 R-CVP) (Figure 3). Analyses by treatment stratum demonstrated a greater difference in response rate between BR and R-CVP than between BR and R-CHOP when assessed by the IRC, whereas the reverse was true for the assessment by the investigators (Figure 3). The agreement between IRC and investigator assessment of response to BR or standard therapy was relatively high (70% for BR and 71% for R-CHOP/R-CVP), with only 7% of patients determined to be a responder by one assessment and not the other. The main difference between the assessments was in the degree of response (partial response vs CR). In R-CHOP/R-CVP–treated patients, a lower CR rate was assessed by the investigators (19%) compared with the IRC (25%). In contrast, IRC and investigator assessments of the CR rate for BR patients were equivalent (31% for both).
CR-rate ratios with 95% CIs. CR-rate ratio and P value for a Sup test are calculated using the Cochran–Mantel–Haenszel test stratified by predetermined standard treatment and lymphoma type (mantle cell vs other types). P value is calculated based on weighted z statistics for an NI test of CR-rate ratio (BR vs R-CHOP/R-CVP) of 0.88. (Top) BR compared with combined R-CHOP/R-CVP group. (Bottom) Analysis by preassigned treatment group. *CR-rate ratio and P value for a Sup test are calculated using the Cochran–Mantel–Haenszel test stratified by predetermined standard treatment and lymphoma type (mantle cell vs other types). †P value is calculated based on weighted z statistics for an NI test of CR-rate ratio (BR vs R-CHOP/R-CVP) of 0.88.
CR-rate ratios with 95% CIs. CR-rate ratio and P value for a Sup test are calculated using the Cochran–Mantel–Haenszel test stratified by predetermined standard treatment and lymphoma type (mantle cell vs other types). P value is calculated based on weighted z statistics for an NI test of CR-rate ratio (BR vs R-CHOP/R-CVP) of 0.88. (Top) BR compared with combined R-CHOP/R-CVP group. (Bottom) Analysis by preassigned treatment group. *CR-rate ratio and P value for a Sup test are calculated using the Cochran–Mantel–Haenszel test stratified by predetermined standard treatment and lymphoma type (mantle cell vs other types). †P value is calculated based on weighted z statistics for an NI test of CR-rate ratio (BR vs R-CHOP/R-CVP) of 0.88.
Safety profile
The safety population consisted of 103 and 98 patients in the BR and R-CHOP treatment strata, respectively, and 118 and 116 patients in the BR and R-CVP treatment strata, respectively. Analysis of treatment exposure showed that 92% of the BR treatment groups, 95% of the R-CHOP treatment group, and 88% of the R-CVP treatment group received ≥6 treatment cycles.
Mean relative dose intensities were ≥96% for individual drugs except prednisone (93% to 94%) and vincristine (70% to 73%). Vincristine as per protocol was given up to a maximum dose of 2 mg. Treatment delays and dose reductions are shown for the 4 treatment strata (supplemental Table 1, available on the Blood Web site). Neutropenia was the most common specified reason for dose delay across the groups. Dose reductions for AEs were most common for bendamustine (8% of patients) and vincristine (12% of patients in the R-CHOP treatment stratum and 19% of patients in the R-CVP treatment stratum [reductions from cycle 1 dose, not related to 2-mg maximum]). The most common reasons for dose reductions were neutropenia, thrombocytopenia, and rash for bendamustine; infusion-related reactions for rituximab; neutropenia for cyclophosphamide and doxorubicin; and peripheral neuropathy and sensory neuropathy for vincristine.
Several differences were apparent in the safety profile of the different treatment regimens. Among the most common nonhematologic AEs of any grade, the standard rituximab-chemotherapy regimens (ie, R-CHOP/R-CVP) were associated with a significantly higher incidence (P < .05) of peripheral neuropathy/paresthesia and alopecia vs BR (Table 4). The R-CVP treatment regimen was also associated with a significantly higher incidence (P < .05) of constipation. The BR regimen was associated with a significantly higher incidence (P < .05) of drug hypersensitivity, vomiting, and nausea when compared with the R-CVP regimen. The use of 5-hydroxytryptamine3 (5-HT3) receptor antagonists was similar across treatments. However, aprepitant was used by a higher proportion of patients in the R-CHOP arm (23% overall, 19% cycle 1) than with the other treatment regimens (BR 9% overall, 2% cycle 1; and R-CVP 3% overall, 2% cycle 1). In most cases in this study, aprepitant was used in addition to 5-HT3 receptor antagonists. Prednisone was given as a component of the standard-therapy regimens; additional low-dose corticosteroids were also given as concomitant therapy (eg, to prevent infusion-like reactions in subsequent cycles for patients who previously experienced infusion reactions) to 86% of patients receiving BR and 75% of patients receiving R-CHOP/R-CVP. The incidence of infections was not statistically different across treatment arms. The incidence of opportunistic infections was slightly higher in patients receiving BR (10%) vs R-CHOP (7%) and in patients receiving BR (12%) vs R-CVP (9%). The most common nonhematologic AEs of grade ≥3 are shown in Table 4.
Twenty-one patients in the study had died at the point of data cutoff in March 2012; 12 deaths (5%) were in the BR treatment arm and 9 (4%) in the standard-therapy arm. Three deaths, 2 in the group preassigned to R-CVP and treated with BR (cardiac arrest; respiratory failure and septic shock secondary to pneumonia) and 1 in the R-CVP treatment group (septic shock), occurred during treatment or within 30 days after the last dose of study drug. The deaths of 3 patients in the BR treatment group were possibly related to treatment (pneumonia, chronic obstructive pulmonary disease, and sepsis). Grade 3/4 reductions in lymphocyte count were more common in patients receiving BR, and grade 3/4 reductions in neutrophils were more common in patients receiving the standard chemotherapy regimens, most notably R-CHOP (Table 4). Hematologic supportive care is summarized by cycle in Table 5. In the preassigned treatment strata, a greater number of patients receiving R-CHOP (61%) used growth colony-stimulating factors than in the corresponding patients receiving BR (27%). Use in patients receiving R-CVP (27%) was similar to that in the corresponding patients receiving BR (30%).
Discussion
The primary objective of this randomized, multicenter, global, phase 3 study in patients with treatment-naive indolent NHL and MCL was met; the BR regimen was noninferior to standard therapy (R-CHOP/R-CVP) with regard to CR rate as assessed by the IRC among efficacy-evaluable patients (31% vs 25%, respectively; P = .0225 for NI). The overall response rates were 97% for the BR treatment group and 91% for the standard-therapy treatment group (P = .0102). The difference in CR rate between BR and the standard chemotherapy regimens was greatest in patients preassigned to R-CVP, although the difference was not statistically significant. The last patient was enrolled on July 11, 2011, so time-to-event results are not sufficiently mature, as per the 5-year minimum follow-up specified in the protocol, to evaluate the secondary endpoints of PFS and OS; follow-up is ongoing. Overall, the proportion of patients preassigned to R-CVP was higher than expected based on the literature,9 especially in Canada. The ratio of R-CHOP to R-CVP preassignment was similar for all regions (53% to 58% of patients being preassigned to R-CHOP), with the exception of Canada, where only 9% of patients were preassigned R-CHOP. The rate of use in Canada may reflect the favorable toxicity profile of R-CVP and that OS has not been shown to be inferior to R-CHOP therapy.10,11
Response rates in the BRIGHT study can be compared with the phase 3 trial of BR vs R-CHOP in patients with indolent NHL and MCL performed by the Study Group for Indolent Lymphomas (StiL) trial in Germany.6 In the cooperative StiL trial, in which response was determined by the investigators with the World Health Organization criteria,12 the CR rate in the BR treatment group was significantly higher than in the R-CHOP treatment group (40% vs 30%, respectively; P = .021). These results are very similar to the investigator-determined CR rate (by IWG criteria) in the BRIGHT patients preassigned to R-CHOP (40% for BR vs 26% for R-CHOP; P = .033, evaluable population). The R-CVP regimen was not studied in the StiL trial, but CR with BR was numerically higher than R-CVP (P = .119 for NI) in BRIGHT.
The analysis of AEs and other safety parameters showed that there were some statistically significant differences between the treatment regimens. The finding that the BR treatment regimens resulted in a higher incidence of nausea and vomiting was not expected. A review of antiemetic treatment revealed that although the use of 5-HT3 antagonists was similar between regimens, the use of aprepitant in addition to 5-HT3 antagonists in the BRIGHT study was much lower in patients receiving BR, particularly in the first cycle, suggesting that long-term experience with R-CHOP and R-CVP led to better anticipation of the degree of nausea and vomiting associated with those regimens. Of note, low-dose corticosteroids were used by approximately similar proportions of patients in the BR and standard-therapy arms (eg, to prevent severe reactions in subsequent cycles in patients who had previously experienced infusion reactions); however, the R-CHOP and R-CVP regimens also incorporate 5 days of prednisone 100 mg/day, which the BR regimen does not. Skin reactions and drug hypersensitivity were also more commonly reported for the BR regimen than for R-CHOP or R-CVP. Severe reactions were infrequent (<3%); however, these require careful management, as a fatal event of toxic epidermal necrolysis in a patient receiving BR has been reported.5 Also, the nausea and vomiting for the BR treatment group were observed at rates similar to those seen in prior clinical development studies, as were drug hypersensitivity and skin reactions.4,13,14
The incidence of fatigue was similar between BR and the standard chemotherapy regimens but was higher in patients preassigned to R-CVP. This is likely attributable to the factors that led investigators to preassign these patients to the R-CVP arm. The increase relative to the BR treatment arm in both peripheral neuropathy and alopecia was expected with the standard treatment regimens. Together with the long-term consequences of doxorubicin with regard to cardiac safety and myelosuppression, these toxicities are the main concerns for physicians and patients in the standard treatment of indolent NHL and MCL. In the current study, the use of colony-stimulating factors in patients receiving R-CHOP was approximately double that in patients receiving BR or R-CVP. Even so, the incidence of grade 3/4 neutropenia for R-CHOP was still greater than for BR or R-CVP.
Comparison of the AE profiles with those reported in the StiL trial6 shows differences and similarities, probably due to differences in methods. For example, the World Health Organization toxicity scale used in the StiL trial is designed to be expanded by investigators,12 whereas Common Terminology Criteria for AEs (version 3.0), which was used in the BRIGHT study due to regulatory requirements, includes a comprehensive list of AEs. The increases in peripheral neuropathy/paresthesia and alopecia in the R-CHOP treatment groups are observed in both studies, as are higher rates of skin reactions and drug hypersensitivity in the BR treatment groups. Alopecia was reported at 100% in the StiL trial for the R-CHOP treatment group, which is broadly consistent with experience. It is not known why a lower rate of 51% was reported in the current study. The higher rate of infection reported for the R-CHOP group in the StiL trial was not observed in the BRIGHT study, in which the rates of infection were similar in the R-CHOP and BR treatment groups.
There are many options for the frontline treatment of patients with low-grade NHL and MCL. In the FOLL05 study, treatment-naive patients with advanced follicular lymphoma were randomized into an R-CHOP, R-CVP, or fludarabine and mitoxantrone (FM) arm. Of note, the R-CHOP and FM regimens were superior to R-CVP, as assessed by time-to-treatment failure and PFS. FM had a worse AE profile than R-CHOP, because FM was associated with a higher rate of grade 3/4 neutropenia and more secondary cancers.15,16 Moreover, the R-CHOP and R-CVP overall response rates from the FOLL05 study were comparable with findings from the BRIGHT study; whereas the overall response rates from the FOLL05 study were 93% for the R-CHOP arm and 88% for the R-CVP arm,16 the overall response rates were 96% for patients receiving R-CHOP and 86% for patients receiving R-CVP in the BRIGHT study.
In conclusion, the BRIGHT study has demonstrated that BR has a unique safety profile distinct from that of the standard chemotherapy regimens studied and one that in several important aspects is favorable. The efficacy of the BR regimen as measured by CR and overall response rates was equivalent to the standard regimens. Follow-up is continuing for PFS and OS, and new studies should provide further data on long-term toxicities. The combination of these results and the long-term safety data from other studies suggests that BR may be an important alternative treatment option for the initial therapy of patients with low-grade NHL and MCL.5,6,13,17
Presented as 2 oral sessions at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8 to 11, 2012.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peter Brown (Teva Branded Pharmaceutical Products R&D, Inc.) for his gracious review of this manuscript. A complete list of the BRIGHT Study Investigators appears in the “Appendix.” A special thank you goes to all investigators, study and clinical research staff, and patients and their families for participating or being involved in this study.
This research was sponsored and conducted by Teva Branded Pharmaceutical Products R&D, Inc., Frazer, PA. Funding for editorial, design, and production support was provided by Teva Branded Pharmaceutical Products R&D, Inc., to The Curry Rockefeller Group, LLC, Tarrytown, NY (Susan Kralian, John Norwood).
Authorship
Contribution: I.W.F., R.v.d.J., B.S.K., P.W., T.E.H., D.M., M.H., Y.-L.K., D.S., M.C., K.K., S.I., R.C., D.M.H., M.M., L.C., and J.M.B. designed the study; I.W.F., R.v.d.J., B.S.K., P.W., T.E.H., D.M., M.H., Y.-L.K., D.S., M.C., K.K., S.I., and J.M.B. performed research; I.W.F., R.v.d.J., B.S.K., P.W., T.E.H., D.M., M.H., Y.-L.K., D.S., M.C., K.K., S.I., J.M.B., L.C., and M.M. analyzed and interpreted data; L.C. performed statistical analysis; I.W.F., R.v.d.J., B.S.K., P.W., T.E.H., D.M., M.H., Y.-L.K., D.S., M.C., K.K., S.I., R.C., D.M.H., M.M., L.C., and J.M.B. wrote and revised the manuscript; and I.W.F.,R.v.d.J., B.S.K., P.W., T.E.H., D.M., M.H., Y.-L.K., D.S., M.C., K.K., S.I., R.C., D.M.H., M.M., L.C., and J.M.B. approved the final draft.
Conflict-of-interest disclosure: I.W.F. has conducted clinical research projects funded, in whole or in part, by Teva. R.v.d.J. has served as a consultant or on the scientific advisory board of Lundbeck and Teva; received honoraria from Roche, Celgene, and Novartis; and conducted clinical research projects funded, in whole or in part, by Lundbeck, Roche, Teva, Celgene, and CTI. B.S.K. has served as a consultant or on the scientific advisory board of Genentech and Teva; and conducted clinical research projects funded, in whole or in part, by Genentech. D.M. has served as a consultant or on the scientific advisory board of Lundbeck Canada and Roche. K.K. has conducted clinical research projects funded, in whole or in part, by Pharmacyclics. R.C., M.M., and L.C. are employees of and have stock ownership in Teva. D.M.H. is an employee of Teva. J.M.B. has served as a consultant or on the scientific advisory board of Spectrum Pharmaceuticals, Genomic Health, Dendreon, Alexion Pharmaceuticals, and Seattle Genetics; and conducted clinical research projects funded, in whole or in part, by Genentech. The remaining authors declare no competing financial interests.
Correspondence: Ian W. Flinn, Sarah Cannon Research Institute, 250 25th Ave North, Suite 412, Nashville, TN 37203; e-mail: iflinn@tnonc.com.