Key Points
NK cells and their expression of FcRγIII (CD16) are decreased in MDS and inversely correlate with a substantial increase in MDSCs.
CD16xCD33 BiKE potently activates blood and marrow MDS-NK cells at all diseases stages to lyse CD33+ MDS and CD33+ MDSC targets.
Abstract
Myelodysplastic syndromes (MDS) are stem cell disorders that can progress to acute myeloid leukemia. Although hematopoietic cell transplantation can be curative, additional therapies are needed for a disease that disproportionally afflicts the elderly. We tested the ability of a CD16xCD33 BiKE to induce natural killer (NK) cell function in 67 MDS patients. Compared with age-matched normal controls, CD7+ lymphocytes, NK cells, and CD16 expression were markedly decreased in MDS patients. Despite this, reverse antibody-dependent cell-mediated cytotoxicity assays showed potent degranulation and cytokine production when resting MDS-NK cells were triggered with an agonistic CD16 monoclonal antibody. Blood and marrow MDS-NK cells treated with bispecific killer cell engager (BiKE) significantly enhanced degranulation and tumor necrosis factor-α and interferon-γ production against HL-60 and endogenous CD33+ MDS targets. MDS patients had a significantly increased proportion of immunosuppressive CD33+ myeloid-derived suppressor cells (MDSCs) that negatively correlated with MDS lymphocyte populations and CD16 loss on NK cells. Treatment with the CD16xCD33 BiKE successfully reversed MDSC immunosuppression of NK cells and induced MDSC target cell lysis. Lastly, the BiKE induced optimal MDS-NK cell function irrespective of disease stage. Our data suggest that the CD16xCD33 BiKE functions against both CD33+ MDS and MDSC targets and may be therapeutically beneficial for MDS patients.
Introduction
Myelodysplastic syndromes (MDS) are clonal heterogeneous stem cell disorders characterized by normal or hypercellular bone marrow (BM) with peripheral blood (PB) cytopenias and an increased risk of progressing to frank acute myeloid leukemia (AML).1 The only curative treatment of MDS is hematopoietic cell transplantation, but many patients are ineligible due to advanced age (median age at diagnosis is 70-75 years),1 performance status, and comorbidities. Accordingly, alternative therapies are offered, but due to the heterogeneous nature of MDS, overall responses and duration of responses are suboptimal.2 As a result, new therapeutic strategies are urgently needed to reduce MDS burden and improve overall survival.
The ability of natural killer (NK) cells to control human hematologic malignancies has been increasingly recognized. Consequently, NK cells are acknowledged to play an important role in tumor immunosurveillance.3-5 NK cell function is regulated by a repertoire of inhibitory and activating surface receptors.6 NK cell killing can occur by distinct mechanisms that involve NKG2D and natural cytotoxicity receptors, which mediate natural cytotoxicity, or through the potent activating receptor CD16 (FcγRIII), which mediates antibody-dependent cell-mediated cytotoxicity (ADCC).6-8 NK cells from MDS patients show impairments in both natural cytotoxicity and cytokine production.9-11 However, the ability of MDS-NK cells to function through CD16 to induce an ADCC response has not been investigated.
The therapeutic potential of manipulating NK cell function via CD16 for the treatment of cancer has been demonstrated through the use of monoclonal antibody (mAb) therapies.12,13 Currently, novel single-chain variable fragment (scFv) recombinant reagents termed bispecific and trispecific killer cell engagers (BiKE and TriKE), which specifically target CD16 expressed on effector NK cells and antigens of interest on tumor cells, are being developed and tested for clinical use.14-18 We recently developed a novel BiKE that targets CD16 along with the myeloid differentiation antigen CD33 (CD16xCD33) and demonstrated its ability to facilitate effective NK cell elimination of primary CD33+ AML targets.16 Here, we tested the CD16xCD33 BiKE using primary MDS patient samples. We show that CD16 function is intact in MDS patients and can induce BiKE-mediated NK cell killing of CD33+ MDS targets and CD33+ immunosuppressive MDSC targets. Our data demonstrate the therapeutic potential of the CD16xCD33 BiKE and suggest that this reagent may be efficacious in patients with MDS.
Methods
Patient and clinical data collection
Patient samples, demographics, and MDS pathology details were supplied by the National Marrow Donor Program (NMDP) and Center for International Blood and Marrow Transplant Research. Deidentified samples and healthy controls were approved by the University of Minnesota institutional review board in accordance with the Declaration of Helsinki. Pathologic classification data were available in the French-American-British (FAB) format (refractory anemia [RA], RA with ringed sideroblasts, RA with excess blasts, RA with excess blasts in transformation, and chronic myelomonocytic leukemia), which shows the overlap between MDS and AML. Raw cytogenetic data were available in the majority of patients (n = 48). We further subclassified the raw cytogenetic data into the defined International Prognosis Scoring System (IPSS) classification categories of favorable (−Y, 20q, 5q−), poor (complex [≥3 abnormalities] or chromosome 7 abnormalities), or intermediate (all others). Blast percentage at the time of transplant was also available and categorized by IPSS classification.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) from MDS patients were collected pretransplant, prior to treatment, and cryopreserved by the NMDP Research Repository. PBMCs from age-matched normal donors (median age, 59 years; range, 22-80 years; 44% female, 56% male) were isolated from adult blood obtained from Memorial Blood Center (Minneapolis, MN) by centrifugation using a Histopaque gradient (Sigma-Aldrich, St. Louis, MO) and cryopreserved. Paired blood and marrow samples were obtained from the Leukemia Tissue Bank, a shared resource at the University of Minnesota. All samples were obtained after informed consent using guidelines approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota and the NMDP in accordance with the Declaration of Helsinki.
Cell lines
The CD33+ human acute promyelocytic leukemia cell line HL-60 (ATCC, Manassas, VA) was cultured at 37°C with 5% CO2 in Iscove’s medium (Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum (Gibco-Invitrogen) and 100 U/mL penicillin and 100 U/mL streptomycin (Invitrogen). The murine mastocytoma cell line P815 (ATCC) was cultured at 37°C with 5% CO2 in Dulbecco’s modified Eagle medium (DMEM), high glucose (Invitrogen) supplemented with 10% fetal bovine serum and 100 U/mL penicillin and 100 U/mL streptomycin.
Cytokine-derived MDSC targets
In vitro generation and characterization of cytokine-derived human MDSC targets from normal PBMCs were performed as previously described.19 The phenotype of in vitro cytokine-derived MDSCs was evaluated by flow cytometry for the expression of CD45, CD33, CD11b, CD14, and HLA-DR. Quantitative reverse-transcription polymerase chain reaction was used for the evaluation of specific, differentiation-associated gene expression as previously described.19 The ability to suppress the proliferation of carboxyfluorescein diacetate succinimidyl ester–labeled (Invitrogen) allogeneic T and NK cells in 5-day MDSC cocultures (effector:target [E:T] ratio = 1:2) was evaluated by flow cytometry as previously described.19
Flow cytometry
Cells were immunophenotyped with the following fluorescent-labeled mAbs against: phycoerythrin (PE)-Cy7–conjugated CD56 (HCD56; BioLegend, San Diego, CA), energy-coupled dye–conjugated CD3 (UCHT1; Beckman Coulter, Brea, CA), allophycocyanin (APC)-Cy7-conjugated CD16 (3G8; BioLegend), PE-conjugated CD7 (M-T701; BD Biosciences, San Jose, CA), Pacific Blue–conjugated CD45 (HI30; BioLegend), fluorescein isothiocyanate (FITC)-conjugated CD2 (S5.2; BD Biosciences), PerCP-Cy5.5–conjugated anti-human CD107a (LAMP-1) (H4A3; BioLegend), Pacific Blue–conjugated anti-human interferon (IFN)-γ (4S.B3; BioLegend), FITC-conjugated tumor necrosis factor-α (TNF-α) (MAb11; BioLegend), FITC-conjugated CD33 (P67.6; BD Biosciences), PE-conjugated CD11b/Mac-1 (ICRF44; BD Biosciences), APC-Cy7–conjugated CD14 (M5E2; BioLegend), PE-Cy5–conjugated HLA-DR (L243; BioLegend), and APC-conjugated CD45 (HI30; BioLegend). Phenotypic acquisition of cells was performed on the LSRII (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Construction, expression, and purification of the bispecific-scFv CD16xCD33 BiKE
The synthesis and assembly of hybrid genes encoding the CD16x33 BiKE reagent were accomplished by using DNA shuffling and DNA ligation techniques as previously described.16,20,21 DNA-sequencing analysis (Biomedical Genomics Center, University of Minnesota) was used to verify that the gene was correct in sequence and cloned in frame. For bacterial protein expression and purification by ion exchange and size exclusion chromatography, methods were used as previously described.20
Cytokine/chemokine production and degranulation (CD107a) assay
Thawed PBMCs from MDS patients and normal donors were incubated overnight at 37°C, 5% CO2 in basal medium or basal medium supplemented with interleukin (IL)-12 (10 ng/mL) and IL-18 (100 ng/mL). Cells were then treated with the CD16xCD33 BiKE (10 μg/mL), scFvCD16 control reagent (10 μg/mL), or no reagent and cocultured with (E:T ratio = 10:1) or without HL-60 or cytokine-derived MDSC targets, and CD107a expression and intracellular IFN-γ and TNF-α production were evaluated as previously described.16
51Cr release cytotoxicity assay
Cytotoxicity was evaluated by 4-hour 51-chromium (51Cr)-release assays. Briefly, resting PBMCs from normal donors treated with the CD16xCD33 BiKE (10 μg/mL), scFvCD16 control reagent (10 μg/mL), or no reagent were cocultured for 4-hours with 51Cr-labeled cytokine-derived MDSCs or HL-60 targets at varying E:T ratios. 51Cr release was measured by a γ scintillation counter (Perkin Elmer, Waltham, MA), and specific target lysis was determined.16
Reverse antibody-dependent cell-mediated cytotoxicity (R-ADCC) assay
Resting PBMCs from MDS patients and normal donors were coated with 10 μg/mL of the following agonistic mAb in various combinations for 30 minutes at room temperature: anti-CD16 (3G8; BioLegend), anti-DNAM-1 (11A8; BioLegend), anti-CD2 (TS1/8; BioLegend), anti-2B4 (C1.7; BioLegend), and mouse immunoglobulin G (MOPC-21; BioLegend). Coated PBMCs were then cocultured with P815 targets in basal medium for 4 hours at 37°C with 5% CO2, and CD107a expression and intracellular IFN-γ and TNF-α production were evaluated as previously described.16
Statistical analysis
Grouped data were expressed as mean ± standard error of the mean. Differences between 2 groups were analyzed by Student t test or the Mann-Whitney U test. Differences between more than 2 groups were analyzed by 2-way analysis of variance followed by Bonferroni or Tukey-Kramer posttests. Spearman ρ tests were used to evaluate correlative relationships (GraphPad Prism Statistical Software, La Jolla, CA).
Results
MDS patient characteristics
MDS patient samples were collected pretransplant, prior to treatment, from a cohort undergoing adult unrelated-donor allogeneic transplantation. Among the 67 MDS patients, 60% were male and 40% were female, with a median age of 47 years (range, 19-74 years). According to FAB classification, 13% had RA, 12% RA with ringed sideroblasts, 36% RA with excess blasts, 15% RA with excess blasts in transformation, and 24% were classified as other (see supplemental Table 1 available at the Blood Web site). Based on data available from the era of sample collection (1990-2005), 48 patients had cytogenetic data available and could be stratified by an IPSS cytogenetic classification (36% of patients had a favorable karyotype, 15% intermediate, and 22% poor) and 57 patients had blast percentage at date of transplant data available and could be stratified by IPSS categories (52% of patients had <5% blasts, 21% had 5%-10% blasts, 4% had 11%-20% blasts and 8% had ≥ 21% blasts).
NK cells and their CD16 expression are significantly decreased in MDS patients
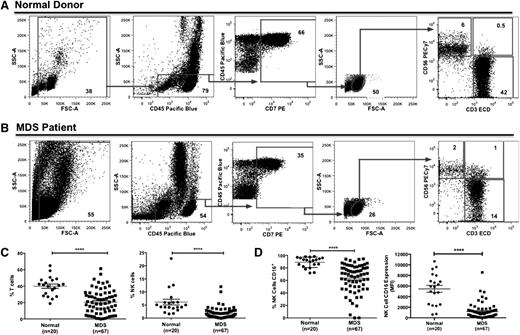
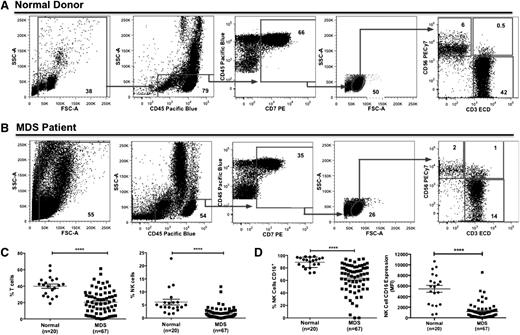
PBMC populations from normal donors and MDS patients were analyzed by flow cytometry to determine the frequencies of CD7+ lymphocytes, CD56−/CD3+ T cells, CD56+/CD3+ NKT cells, and CD56+/CD3− NK cells (Figure 1A-B). Compared with normal donors, MDS patients had a significant decrease in CD7+ lymphocytes and T and NK cells, whereas no significant difference was observed for the NKT cell population (Figure 1C, supplemental Figure 1A, and Table 1). Further evaluation of the NK cell CD56bright and CD56dim subpopulations did not reveal any significant differences between normal donors and MDS patients (Table 1). CD16 expression was significantly decreased on MDS NK cells compared with normal donor PB-NK cells (percent: 63% ± 3% vs 89% ± 2%; mean fluorescence intensity: 1169 ± 177 vs 5470 ± 656; P < .0001; Figure 1D and supplemental Figure 1B).
Aberrant NK cell frequency and NK cell CD16 expression in MDS PBMCs. (A-C) PBMCs from normal donors and MDS patients were stained with anti-CD56, anti-CD3, and anti-CD16 mAbs. (A-B) Gating strategy for the evaluation of the lymphocyte populations in normal donors (A) and MDS patients (B). Plots are representative from 1 normal donor (#12 of 20) and 1 higher-risk MDS patient (#49 of 67), and gate frequency indicates population percent normalized to the all-cell fraction based on the forward scatter (FSC)/side scatter (SSC) gate excluding debris. (C) Percent of CD56−/CD3+ T cells and CD56+/CD3− NK cells (normalized to all-cell fraction based on the FSC/SSC gate excluding debris) and (D) percent of CD16+ NK cells (calculated as the percent of CD56+/CD3− NK cells) and mean fluorescence intensity of CD16 expression on NK cells were determined by fluorescence-activated cell sorter (FACS) analysis (****P < .0001). ECD, energy-coupled dye.
Aberrant NK cell frequency and NK cell CD16 expression in MDS PBMCs. (A-C) PBMCs from normal donors and MDS patients were stained with anti-CD56, anti-CD3, and anti-CD16 mAbs. (A-B) Gating strategy for the evaluation of the lymphocyte populations in normal donors (A) and MDS patients (B). Plots are representative from 1 normal donor (#12 of 20) and 1 higher-risk MDS patient (#49 of 67), and gate frequency indicates population percent normalized to the all-cell fraction based on the forward scatter (FSC)/side scatter (SSC) gate excluding debris. (C) Percent of CD56−/CD3+ T cells and CD56+/CD3− NK cells (normalized to all-cell fraction based on the FSC/SSC gate excluding debris) and (D) percent of CD16+ NK cells (calculated as the percent of CD56+/CD3− NK cells) and mean fluorescence intensity of CD16 expression on NK cells were determined by fluorescence-activated cell sorter (FACS) analysis (****P < .0001). ECD, energy-coupled dye.
Activation of NK cells through CD16 is intact in MDS patients
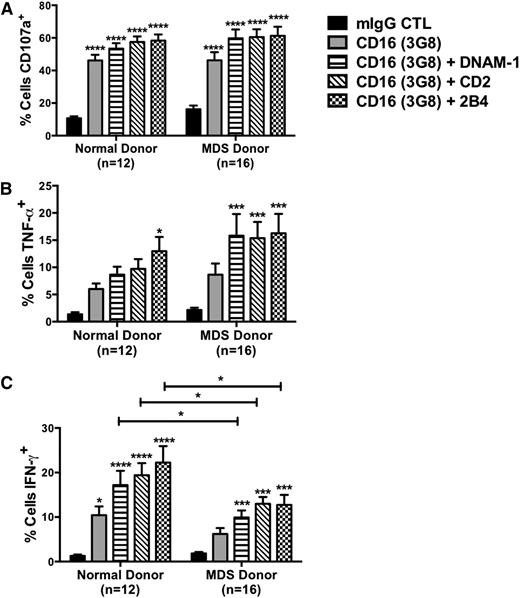
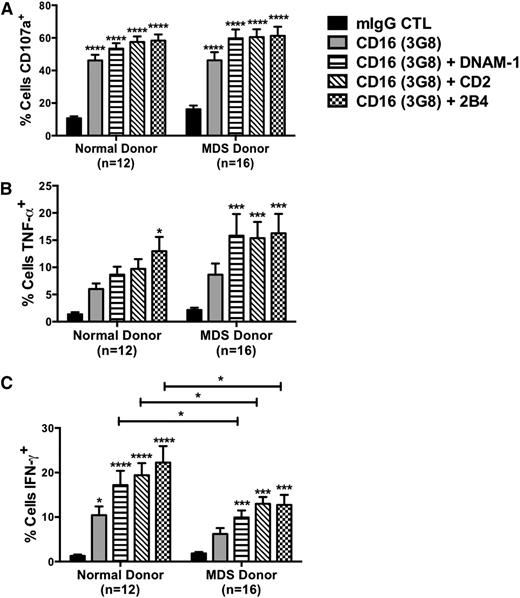
An agonistic mAb against CD16 was used to induce NK cell function in an R-ADCC assay. Compared with the immunoglobulin G control, CD16 crosslinking of resting MDS-NK cells led to a significant increase in degranulation (16% ± 2% vs 46% ± 5%, P < .0001, n = 16), which was similarly observed in normal donors (11% ± 1% vs 46% ± 3%, P < .0001, n = 12; Figure 2A). Intracellular cytokine production induced via CD16 triggering was evaluated, and a comparison of MDS patients to normal donors did not show any significant differences in TNF-α (MDS: 9% ± 2% vs normal: 6% ± 1%, P = .331; Figure 2B) or IFN-γ (6% ± 1% vs 10% ± 2%, P = .113; Figure 2C) production, further demonstrating the ability of MDS-NK cells to function through CD16 despite the lower expression levels. Activation of resting NK cells occurs via synergistic interactions between CD16 and the coactivating receptors DNAM-1, CD2, and 2B4.22 To further evaluate the function of CD16 on MDS-NK cells, CD16 was cocrosslinked in pairwise combinations and function was measured. Compared with the immunoglobulin G control, all receptor combinations with CD16 induced significant increases in CD107a expression (Figure 2A), TNF-α (Figure 2B), and IFN-γ (Figure 2C) production in MDS-NK cells. No significant differences in degranulation and TNF-α production induced by cocrosslinking receptor pairs were observed between normal donors and MDS patients. However, MDS-NK cells displayed a significant decrease in IFN-γ production compared with normal donor NK cells for CD16 crosslinking combinations with DNAM-1, CD2, and 2B4. No differences in DNAM-1, CD2, and 2B4 receptor expression were observed between normal donor and MDS-NK cells (supplemental Figure 2). To evaluate whether the diminished IFN-γ production by MDS-NK cells was specific to CD16 synergistic activation, resting MDS-NK cells were stimulated overnight with saturating concentrations of IL-12 and IL-18. Compared with normal donors, MDS-NK cells displayed a significant reduction in IFN-γ production in response to cytokine stimulation (supplemental Figure 3), revealing a broader defect in the activation threshold for the IFN-γ pathway.
NK cell activation induced through CD16 crosslinking is intact in MDS-NK cells. (A-C) Resting PBMCs from normal donors and MDS patients were coated with 10 μg/mL of the indicated mAbs and cocultured with P815 targets, and an R-ADCC assay was performed. NK cell CD107a expression (A) and intracellular TNF-α (B) and IFN-γ (C) production were evaluated by FACS analysis (*P < .05, ***P < .001, ****P < .0001). mIgG, mouse immunoglobulin G.
NK cell activation induced through CD16 crosslinking is intact in MDS-NK cells. (A-C) Resting PBMCs from normal donors and MDS patients were coated with 10 μg/mL of the indicated mAbs and cocultured with P815 targets, and an R-ADCC assay was performed. NK cell CD107a expression (A) and intracellular TNF-α (B) and IFN-γ (C) production were evaluated by FACS analysis (*P < .05, ***P < .001, ****P < .0001). mIgG, mouse immunoglobulin G.
CD16xCD33 BiKE enhances NK cell activity against primary CD33+ MDS targets
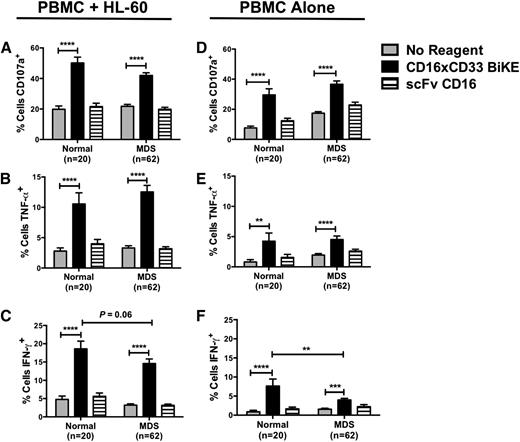
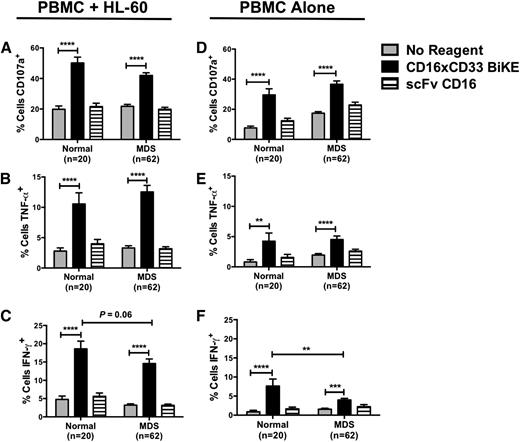
To evaluate the therapeutic potential of targeting CD16 in MDS patients, PBMCs from normal donors and MDS patients were treated with the CD16xCD33 BiKE, scFv CD16 control, or no reagent and cocultured with the CD33+ HL-60 cell line. NK cell degranulation and TNF-α and IFN-γ production were then evaluated by flow cytometry. Compared with the no reagent and scFv CD16 controls, MDS-NK cell degranulation (Figure 3A), TNF-α (Figure 3B), and IFN-γ (Figure 3C) production were significantly increased. Moreover, in the absence of HL-60 targets, MDS-NK cells treated with the CD16xCD33 BiKE targeted endogenous CD33+ cells, significantly enhancing degranulation (Figure 3D) and TNF-α (Figure 3E) and IFN-γ (Figure 3F) production. As with CD16 synergistic crosslinking and IL-12 and IL-18 stimulation, CD16xCD33 BiKE stimulation of MDS-NK cells resulted in lower levels of IFN-γ production in the presence (Figure 3C) and absence (Figure 3F) of HL-60 targets compared with normal donors.
CD16xCD33 BiKE enhances MDS-NK cell function against CD33+ targets. Resting PBMCs from normal donors and MDS patients were coated with 10 μg/mL of the CD16xCD33 BiKE or scFv CD16 control and cocultured with (A-C) or without (D-F) CD33+ HL-60 targets. NK cell CD107a expression and intracellular TNF-α and IFN-γ production were evaluated by FACS analysis (*P < .05, **P < .01, ***P < .001, ****P < .0001).
CD16xCD33 BiKE enhances MDS-NK cell function against CD33+ targets. Resting PBMCs from normal donors and MDS patients were coated with 10 μg/mL of the CD16xCD33 BiKE or scFv CD16 control and cocultured with (A-C) or without (D-F) CD33+ HL-60 targets. NK cell CD107a expression and intracellular TNF-α and IFN-γ production were evaluated by FACS analysis (*P < .05, **P < .01, ***P < .001, ****P < .0001).
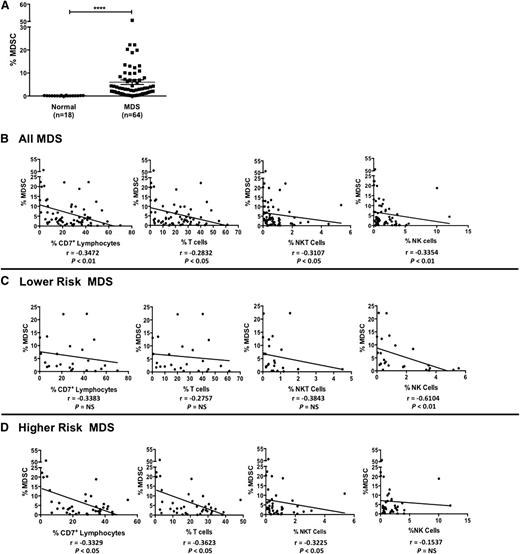
The CD33+ MDSC population is significantly increased in MDS patients and negatively correlates with MDS lymphocyte populations
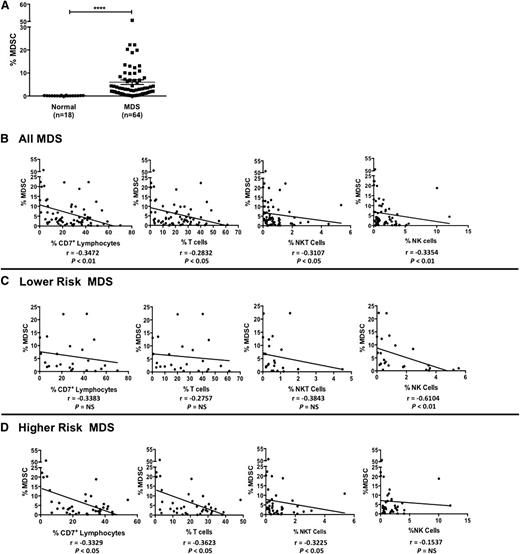
Suppressive MDSCs are phenotypically defined as CD33+/CD11b+/CD14−/HLA-DRlo/− and are increased during oncogenesis and in the presence of inflammatory cytokines.23 We therefore evaluated the presence of MDSCs in MDS. Within the all-cell fraction, the total CD33+ cell population frequency was significantly higher in MDS patients compared with normal donors (29% ± 2% vs 16% ± 2%, P = .002; Table 1). CD7+ lymphocytes and T and NK cells from normal donors and MDS patients lacked expression of CD33 (supplemental Figure 4A-E). Additional subgating within the CD33+ fraction showed a distinct MDSC population in MDS patients that was not observed in normal donors (supplemental Figure 5), where MDS-MDSCs comprised 21% ± 2% of the total CD33+ cell fraction (vs normal: 0.7% ± 0.1%, P < .0001) and 6% ± 1% of the all-cell fraction (vs 0.1% ± 0.02%, P < .0001; Figure 4A). The relationships between MDSC and lymphocyte populations were next evaluated in MDS patients. Among all MDS patients, there was a statistically significant negative correlation between percent MDSCs and percent CD7+ lymphocytes, T cells, NKT, and NK cells (Figure 4B). Interestingly, when stratifying the MDS patients by disease status, a statistically significant stronger negative correlation was observed between MDSCs and NK cells (r = −0.6104, P < .01) for lower-risk MDS patients, whereas no significant correlations were observed for CD7+ lymphocytes and T and NKT cells (Figures 4C). Among higher-risk MDS patients, there were significant negative correlations between MDSCs and CD7+ lymphocytes and T and NKT cell populations (Figure 4D). Moreover, a statistically significant negative correlation between MDS-MDSC and MDS-NK cell CD16 expression was also observed (supplemental Figure 6), suggestive of a MDSC immunosuppressive mechanism against NK cells. Taken together, these findings support a negative relationship between the MDSC population and lymphocyte populations in MDS patients and further support the evidence that immunosuppressive MDSCs help facilitate ineffective hematopoiesis in MDS.24
MDS patients have a significant increase in MDSCs that negatively correlates with lymphocyte populations. (A) PBMCs from normal donors and MDS patients were stained with anti-CD45, anti-CD33, anti-CD11b, anti-CD14, and anti-HLA-DR mAbs and the percent of MDSCs (phenotypically defined as CD33+/CD11b+/CD14−/HLA-DRlo/−) of the all-cell fraction was evaluated (****P < .0001). (B-D) Based on the all-cell fraction, the correlation between percent MDSCs and percent CD7+ lymphocytes, percent T cells, percent NKT cells, and percent NK cells among all MDS patients (B), lower-risk MDS patients (C), and higher-risk MDS patients (D) is shown. Correlation coefficients (r) and statistical significance are indicated in the figure.
MDS patients have a significant increase in MDSCs that negatively correlates with lymphocyte populations. (A) PBMCs from normal donors and MDS patients were stained with anti-CD45, anti-CD33, anti-CD11b, anti-CD14, and anti-HLA-DR mAbs and the percent of MDSCs (phenotypically defined as CD33+/CD11b+/CD14−/HLA-DRlo/−) of the all-cell fraction was evaluated (****P < .0001). (B-D) Based on the all-cell fraction, the correlation between percent MDSCs and percent CD7+ lymphocytes, percent T cells, percent NKT cells, and percent NK cells among all MDS patients (B), lower-risk MDS patients (C), and higher-risk MDS patients (D) is shown. Correlation coefficients (r) and statistical significance are indicated in the figure.
Immunosuppressive MDSCs are targeted by the CD16xCD33 BiKE
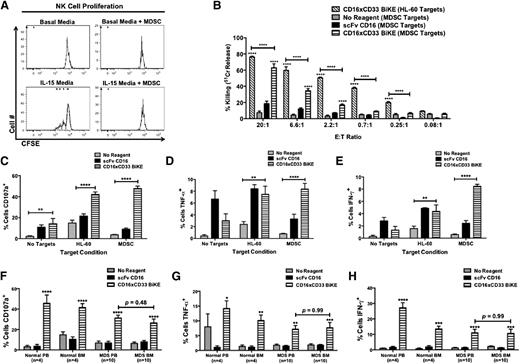
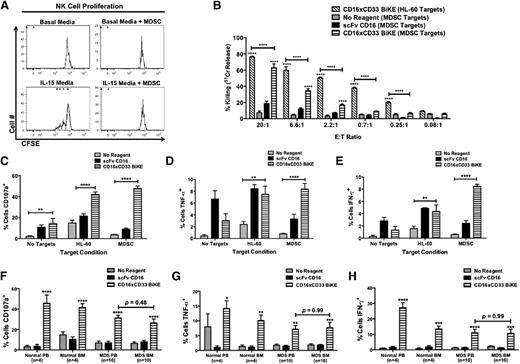
Because MDSCs have been shown to suppress NK cell activity,25,26 we next evaluated the ability of the CD16xCD33 BiKE to activate NK cells in the presence of immunosuppressive MDSCs. Cytokine-derived MDSCs were generated from PBMCs isolated from normal donors, and postinduction characterization analysis verified the MDSC phenotype and gene expression signature (data not shown). Cytokine-derived MDSCs functioned to suppress T-cell (supplemental Figure 7A) and NK cell proliferation (Figure 5A). The MDSC targets were then labeled with 51Cr and cocultured with normal donor resting NK cells in the absence or presence of the CD16xCD33 BiKE or scFv CD16 control. Without reagent treatment or when treated with the scFv CD16 control, NK cell cytotoxicity was suppressed in the presence of MDSC targets. However, when treated with the CD16xCD33 BiKE, there was a significant increase in MDSC killing (CD16xCD33 BiKE vs no reagent: [20:1] 63% ± 5% vs 7% ± 2%, P < .0001; Figure 5B). Evaluation of NK cell degranulation and intracellular cytokine production revealed significant increases in CD107a expression (Figure 5C), TNF-α (Figure 5D), and IFN-γ (Figure 5E) production as well in the presence of MDSC targets when resting NK cells were treated with the CD16xCD33 BiKE. We next evaluated the ability of the CD16xCD33 BiKE to enhance PB and BM MDS-NK cell function against MDSC targets. Significant increases in degranulation (Figure 5F), TNF-α (Figure 5G), and IFN-γ (Figure 5H) production were observed in the presence of the BiKE. Similar results were found using HL-60 targets (supplemental Figure 7B-D). Notably, no significant differences were observed between PB and BM MDS-NK cell BiKE-mediated function. Overall, these data demonstrate the ability of the CD16xCD33 BiKE to induce blood and marrow MDS-NK cell activation to overcome MDSC immunosuppression.
CD16xCD33 BiKE enhances NK cell cytotoxicity and cytokine production against MDSC targets. (A) Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled NK cells were cultured for 5 days in basal medium or medium supplemented with 10 ng/mL IL-15 in the presence or absence (E:T ratio = 1:2) of cytokine-derived MDSCs from normal PBMCs, and proliferation was evaluated via FACS analysis. Histogram plots represent 1 of 6 normal donors. (B) PBMCs from normal donors were coated with 10 μg/mL of CD16xCD33 BiKE or scFv CD16 control and cocultured with cytokine-derived MDSC or HL-60 targets, and NK cell-mediated cytotoxicity was evaluated via a 51Cr-release assay (****P < .0001; n = 4). (C-E) PBMCs from normal donors were coated with 10 μg/mL of CD16xCD33 BiKE or scFv CD16 control and cocultured with cytokine-derived MDSC or HL-60 targets, and NK cell CD107a expression and intracellular TNF-α and IFN-γ production were evaluated via FACS analysis (**P < .01, ****P < .0001; n = 6). (F-H) Mononuclear cells from paired PB and BM samples were isolated from normal donors (n = 4) and MDS patients (n = 10), coated with 10 μg/mL of CD16xCD33 BiKE or scFv CD16 control, and cocultured with cytokine-derived MDSC, and NK cell (F) CD107a expression and intracellular (G) TNF-α and (H) IFN-γ production were evaluated via FACS analysis (*P < .05, **P < .01, ***P < .01, ****P < .0001).
CD16xCD33 BiKE enhances NK cell cytotoxicity and cytokine production against MDSC targets. (A) Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled NK cells were cultured for 5 days in basal medium or medium supplemented with 10 ng/mL IL-15 in the presence or absence (E:T ratio = 1:2) of cytokine-derived MDSCs from normal PBMCs, and proliferation was evaluated via FACS analysis. Histogram plots represent 1 of 6 normal donors. (B) PBMCs from normal donors were coated with 10 μg/mL of CD16xCD33 BiKE or scFv CD16 control and cocultured with cytokine-derived MDSC or HL-60 targets, and NK cell-mediated cytotoxicity was evaluated via a 51Cr-release assay (****P < .0001; n = 4). (C-E) PBMCs from normal donors were coated with 10 μg/mL of CD16xCD33 BiKE or scFv CD16 control and cocultured with cytokine-derived MDSC or HL-60 targets, and NK cell CD107a expression and intracellular TNF-α and IFN-γ production were evaluated via FACS analysis (**P < .01, ****P < .0001; n = 6). (F-H) Mononuclear cells from paired PB and BM samples were isolated from normal donors (n = 4) and MDS patients (n = 10), coated with 10 μg/mL of CD16xCD33 BiKE or scFv CD16 control, and cocultured with cytokine-derived MDSC, and NK cell (F) CD107a expression and intracellular (G) TNF-α and (H) IFN-γ production were evaluated via FACS analysis (*P < .05, **P < .01, ***P < .01, ****P < .0001).
CD16xCD33 BiKE enhanced NK cell activity functions regardless of MDS disease stage
MDS is a heterogeneous disease, and treatment options depend on several clinical factors specific to each patient. Therefore, the ability of the CD16xCD33 BiKE to enhance NK cell function among the various MDS groups stratified by clinical features was evaluated. No significant differences in NK cell (Figure 6A), total CD33+ (Figure 6B), and MDSC (Figure 6C) population frequencies were observed among FAB classification and IPSS karyotype groups in MDS patients. Upon evaluation of CD16xCD33 BiKE-induced MDS-NK cell function (degranulation, TNF-α, and IFN-γ production) in the absence of HL-60 targets (Figure 6D) and in the presence of HL-60 targets (Figure 6E), no significant differences between MDS groups were observed. Stratifying by blast percentage also showed no significant differences in the study variables (supplemental Table 2). Because the median age of our patient cohort indicated a younger population, respectively, the study variables were also stratified by age to evaluate a potential age effect. No significant differences were observed in percent NK cells, total CD33+ cells, MDSCs, or CD16xCD33 BiKE-induced NK cell function among age strata in MDS patients (supplemental Figure 8). Altogether, these data indicate that despite disease heterogeneity, the CD16xCD33 BiKE can consistently function to enhance MDS-NK cell activity against the CD33+ target cell population, which includes immunosuppressive MDSCs, regardless of disease stage.
CD16xCD33 BiKE consistently enhances NK cell function at all disease stages. The percent of CD56+/CD3− MDS-NK cells (A), total CD33+ cells (B), and MDS-MDSC (C) of the all-cell fraction was stratified according to their respective FAB classification and IPSS karyotype groups. (D-E) CD16xCD33 BiKE-induced MDS-NK cell functions (degranulation [CD107a], intracellular TNF-α and IFN-γ production) in the absence of HL-60 targets (D) and in the presence of HL-60 targets (E) were stratified according to their respective FAB classification and IPSS karyotype groups. The y-axes of graphs in panels D and E represent the percent of NK cells positive for each function listed in the graph legend.
CD16xCD33 BiKE consistently enhances NK cell function at all disease stages. The percent of CD56+/CD3− MDS-NK cells (A), total CD33+ cells (B), and MDS-MDSC (C) of the all-cell fraction was stratified according to their respective FAB classification and IPSS karyotype groups. (D-E) CD16xCD33 BiKE-induced MDS-NK cell functions (degranulation [CD107a], intracellular TNF-α and IFN-γ production) in the absence of HL-60 targets (D) and in the presence of HL-60 targets (E) were stratified according to their respective FAB classification and IPSS karyotype groups. The y-axes of graphs in panels D and E represent the percent of NK cells positive for each function listed in the graph legend.
Discussion
We examined the expression and function of CD16 on MDS-NK cells and the ability of a CD16xCD33 BiKE to mediate MDS-NK cell targeting of the CD33+ cell population that contains both the premalignant clone and immunosuppressive MDSCs. Previous studies of MDS-NK cells from PB and BM have evaluated NK cell function in the context of natural cytotoxicity mediated by NKp46, NKp30, NKG2D, 2B4, and DNAM-1.9-11 Kiladjian et al described decreased cytolytic function of PB NK cells from all MDS subtypes against K562 targets despite normal expression of natural cytotoxicity receptors, as well as decreased IFN-γ and TNF-α secretion in response to IL-2 stimulation.9 In contrast, Epling-Burnette et al observed downregulation of NKp30 and NKG2D and correlated PB MDS-NK cell dysfunction with decreased NKG2D expression.10 In another study, Carlsten et al found significant decreases in DNAM-1 and NKG2D expression in BM MDS-NK cells that were not observed in PB MDS-NK cells; despite this difference, both sources of MDS-NK cells displayed impaired degranulation in the presence of K562 cells and in an R-ADCC assay triggering through NKG2D, 2B4, and DNAM-1.11 Our study demonstrated that despite reduced CD16 expression on MDS-NK cells, degranulation and TNF-α and IFN-γ production could be induced through CD16 stimulation by an agonistic mAb and the CD16xCD33 BiKE, indicating a functional ADCC response in MDS patients. This agrees with early evidence that showed an intact lymphocyte ADCC response in patients with primary preleukemic syndrome while abnormalities in other lymphocyte functions were observed.27 Notably, when we cocrosslinked MDS-NK cells with pairwise combinations of CD16 and coactivating receptors, synergistic activation was significantly diminished for IFN-γ production, but not degranulation or TNF-α production. Decreases in IFN-γ production were also found when MDS-NK cells were stimulated with IL-12 and IL-18 and the CD16xCD33 BiKE. Our data agree with previous work that showed decreased NK cell IFN-γ production despite stimulation with IL-12 and IL-18 in the context of a murine hepatic tumor model containing immunosuppressive MDSCs.25 Importantly, however, we demonstrate cytotoxicity was not impaired in MDS patients and could be driven by CD16xCD33 BiKE stimulation. Fauriat et al have demonstrated that NK cell IFN-γ production has a greater threshold of activation than that of degranulation and TNF-α production.28 Our data, therefore, suggest NK cell functions that have a lower threshold of activation are intact in MDS patients, whereas the higher activation threshold required for stimulation of the IFN-γ pathway may be impaired.
Increases in the frequency of circulating MDSCs have been well described in solid tumors, and studies have shown MDSCs are an important factor in facilitating immune evasion and tumor progression.23,29-32 Although the roles of MDSCs in hematologic malignancies are still evolving, a recent study demonstrated an increase in MDSC frequency in the PB of multiple myeloma patients that increased with disease progression and promoted tumor growth via suppression of CD4+ T, CD8+ T, and NKT-cell lymphocyte populations.33 We demonstrated here that the percent of MDSCs is significantly increased in the PB of MDS patients and negatively correlates with CD7+ lymphocytes and T, NKT, and NK cell populations. Interestingly, when MDS patients were stratified by disease status, this negative relationship was only significant for NK cells in patients with lower risk. By contrast, the negative correlations between MDSCs and total lymphocytes and T and NKT cells were significant for patients with higher risk. MDSCs are generated from immature myeloid cells, which includes immature dendritic cells (DCs).23 The crosstalk between NK cells and DCs has been well studied, and it has been shown that NK cells play a role in DC maturation via the secretion of TNF-α and IFN-γ as well as cell-to-cell contact mechanisms that involve NKp30.34-37 Expansion and activation of MDSCs require several different factors that include TNF-α and IFN-γ.38-41 Because our data show a statistically significant negative correlation between MDSCs and NK cells for lower-risk disease, it is possible the altered BM microenvironment in MDS primes the induction and expansion of immature DCs to MDSCs and interaction with NK cells promotes MDSC functional activation via NK cell secretion of TNF-α and IFN-γ, which in turn ultimately leads to suppression of the enabling NK cells. Moreover, studies have shown activated MDSCs promote the development and expansion of regulatory T cells,42-44 which is a characteristic of MDS patients with advanced disease.45-47 This supports our data showing a statistically significant negative correlation between MDSC and T cells in MDS patients with higher risk. Taken together, these data suggest a potential model whereby interactions early in disease between MDSC-primed immature myeloid cells and NK cells promote the functional activation of MDSCs, leading to suppression of this innate lymphocyte population and promotion of regulatory T cell development, which ultimately function in concert with MDSCs to suppress adaptive immunity and facilitate disease progression. Further studies are underway to investigate this hypothesis.
MDSCs use multiple mechanisms to suppress antitumor immunity. Downregulation of l-selectin (CD62L) on T cells has been shown to impair T-cell homing to lymph nodes and activation.23 Hanson et al demonstrated MDSCs constitutively express the sheddase ADAM 17 (a disintegrin and metalloproteinase domain 17) on their plasma membrane and suggest ADAM 17 is the enzyme responsible for cleaving the ectodomain of l-selectin.48 We recently demonstrated that NK cell CD16 surface expression is regulated by ADAM 17, which functions to promote attenuation of NK cell function through cleavage of CD16 from the cell surface.49 We show here a statistically significant negative correlation between MDSC and MDS-NK cell expression of CD16 suggesting a novel role for MDSC suppression of NK cell function, namely the induction of NK cell CD16 shedding. Accordingly, further testing of an ADAM 17 inhibitor in combination with the CD16xCD33 BiKE in MDS is warranted and may further serve to inhibit MDSC immunosuppression and enhance NK cell effector function against CD33+ targets.
Immune suppression has a crucial role in promoting tumor progression, and the supporting data clearly indicate that elimination of suppressing factors is required for cancer immunotherapies to be successful.50 Our data and others suggest that MDSCs contribute to ineffective hematopoiesis in MDS.24 Thus, the ability to target this immunosuppressive population through the CD16xCD33 BiKE may be therapeutically beneficial for patients with MDS and other diseases characterized by increased MDSCs. Acknowledging that the CD16xCD33 BiKE can also target normal CD33+ cells (monocytes and committed myeloid progenitors), transient myelosuppression may be expected with this therapy, not unlike most cytotoxic therapies. Clinical trials show CD3xCD19 bispecific T-cell engager therapy is efficacious for acute lymphoblastic leukemia while targeting of the normal cell compartment is well tolerated.51-53 This is in part due to the ease with which drug exposure can be managed, an important feature that helps control on-target/off-tumor effects allowing regeneration of the normal cell compartment after drug elimination. Moreover, for NK cells, other receptor-ligand interactions, facilitated by the BiKE-mediated effector-target cell synapse, may play an important role in target cell elimination. In conclusion, we propose that CD16xCD33 BiKE is a promising therapy for MDS and other myeloid malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health Center Core grant (P30CA77598) utilizing the following Masonic Cancer Center, University of Minnesota shared resources: sample procurement and cell processing services from the Translational Therapy Core and Minnesota Masonic Charities through the Cancer Experimental Therapeutics Initiative. These studies were also supported in part by the National Institutes of Health Research Program grants (P01CA111412, P0165493, P30CA77598), National Cancer Institute Research Training grant (T32CA099936), Senior Scientist Research and Mentorship Award (K05CA157439), and Research Project grant (R01CA142714), and R01CA142714), the Minnesota Masonic Charities, the US Public Health Service (grant R01CA36725), the Randy Shaver Foundation, the Lion’s Children’s Cancer Fund, the William Lawrence and Blanche Hughes Fund, and a grant from the University of Minnesota-Mayo Clinic Partnership. The Center for International Blood and Marrow Transplant Research is supported by Public Health Service grant/cooperative agreement U24CA76518 from the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; grant/cooperative agreement 5U01HL069294 from the National Heart, Lung and Blood Institute and National Cancer Institute; contract HHSH234200637015C with Health Resources and Services Administration; and two grants from the Office of Naval Research (N00014-12-1-0142 and N00014-13-1-0039).
Authorship
Contribution: M.K.G. designed the research plan, performed experiments, analyzed and interpreted data, and wrote the manuscript; J.A.R., P.K.E.-B., B.R.B., and D.A.V. designed the research plan, performed data analysis and interpretation, and prepared the paper; E.D.W., T.C.L., M.R.V., A.W., S.S., M.D.H., M.R.L., and D.J.W., performed data analysis and interpretation and prepared the paper; A.J.L. performed experiments and data analysis and interpretation; L.M.W. provided concept development, experimental platform design, and the scFv anti-CD16 reagent; and J.S.M. designed the research plan, performed data analysis and interpretation, prepared the paper, and was responsible for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Correspondence: Jeffrey S. Miller, Professor of Medicine, University of Minnesota Masonic Cancer Center, MMC 806, Harvard St at East River Rd, Minneapolis, MN 55455, e-mail: mille011@umn.edu.

![Figure 6. CD16xCD33 BiKE consistently enhances NK cell function at all disease stages. The percent of CD56+/CD3− MDS-NK cells (A), total CD33+ cells (B), and MDS-MDSC (C) of the all-cell fraction was stratified according to their respective FAB classification and IPSS karyotype groups. (D-E) CD16xCD33 BiKE-induced MDS-NK cell functions (degranulation [CD107a], intracellular TNF-α and IFN-γ production) in the absence of HL-60 targets (D) and in the presence of HL-60 targets (E) were stratified according to their respective FAB classification and IPSS karyotype groups. The y-axes of graphs in panels D and E represent the percent of NK cells positive for each function listed in the graph legend.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/19/10.1182_blood-2013-10-533398/4/m_3016f6.jpeg?Expires=1765075042&Signature=EiAYzK1u4ffreCbQJJDcaOLVn3xJVdYZT4tNkbWwedgzTg6xxL6kCqi3cQq0Y9POrNhdhLpBx-KYHxoG-vQO2hpM9qB1i1rEizq2rS6xCAsGFAk197ImExGusBXFE91x6R4nmApmTQbB2-UxsJIn8iKQwEPNHeyDKegZyHs6gNhWBoGOpRwyrzlHa~T-arlu9-ScdrT6RryTOfFdIlmqUUG~bs9gvjEZyGT076kM7oPj3nRYXa~kZ1RCvZ6~tEico34Wxc4Abxz97eFB8kQteTDxbb69BGo1Tvp12lFDzpspgBRwTSA-ZMsMTSxxw63zpcbBJq90kywlhEX7SjAiAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. CD16xCD33 BiKE consistently enhances NK cell function at all disease stages. The percent of CD56+/CD3− MDS-NK cells (A), total CD33+ cells (B), and MDS-MDSC (C) of the all-cell fraction was stratified according to their respective FAB classification and IPSS karyotype groups. (D-E) CD16xCD33 BiKE-induced MDS-NK cell functions (degranulation [CD107a], intracellular TNF-α and IFN-γ production) in the absence of HL-60 targets (D) and in the presence of HL-60 targets (E) were stratified according to their respective FAB classification and IPSS karyotype groups. The y-axes of graphs in panels D and E represent the percent of NK cells positive for each function listed in the graph legend.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/19/10.1182_blood-2013-10-533398/4/m_3016f6.jpeg?Expires=1765575512&Signature=1eKLvMsTJ9O-QGN9GTrtEcomSKmbG4hP-6zjITfgUqgRXUjcoWgXokk-f7mAKltC~uncTbJT5EISsv834~Rqv6zAVYJo0kz2-ZeaDNvAbFCkKlgX5FvdrM0mvLHfmst7IJsephPmmXkKSUW~7Jxz~BuHnabEaIjZC8bypxLZMiRNQZ~Ls4-Hp1~5CC1BbLMhLsdQ48xIH~A8qUAt4oGfvvTKtoV6625mrVNAjTGmRjw0kjKFUWAif9tzNVJIkp7uwY6G2dH9ghotRAAerQKN5vSrWK4wco4mLe0BgWXD~BPs9Zy3XbNnnTC7wxPPhlN5eLc4kdc2bBSFi6bKFL~J6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)