Key Points
Loss of the tumor suppressor, PTEN, results in enhanced blood stem cell proliferation and arrested differentiation, hallmarks of leukemia.
Pten mutant zebrafish embryos display defective hematopoiesis and constitute an excellent tool to assess drug treatment.
Abstract
Self-renewing hematopoietic stem/progenitor cells (HSPCs) produce blood cells of all lineages throughout life. Phosphatase and tensin homolog (PTEN), a tumor suppressor that antagonizes phosphatidylinositol 3-kinase (PI3K) signaling, is frequently mutated in hematologic malignancies such as bone marrow failure and leukemia. We set out to investigate whether Pten is required for hematopoiesis. Analysis of zebrafish mutants lacking functional Pten revealed that HSPCs colonized the caudal hematopoietic tissue normally. There, HSPCs hyperproliferated and engaged in all blood lineages. However, they failed to differentiate into mature blood cells. Hence, Pten mutant zebrafish embryos displayed hallmarks of leukemia in humans. Inhibition of PI3K signaling in mutants lacking functional Pten suppressed hyperproliferation and released the differentiation arrest. We conclude that Pten has an essential role in the balance between proliferation and differentiation of blood cells.
Introduction
Understanding the development of blood stem cells has been a challenge for decades and still little is known about the ontogeny of hematopoietic stem/progenitor cells (HSPCs) that give rise to all blood lineages. All vertebrates possess 2 waves of hematopoiesis: the primitive wave and the definitive wave.1-3 The primitive wave gives rise to primitive erythrocytes and macrophages, as well as megakaryocytes in mice4 and neutrophils in zebrafish,5 whereas the definitive wave or adult hematopoiesis generates all hematopoietic cell lineages. In mammals and zebrafish, HSPCs emerge from the ventral wall of the dorsal aorta in a conserved region known as the aorta-gonad-mesonephros (AGM).6-8 After leaving the AGM, mammalian HSPCs transiently colonize the fetal liver before seeding the bone marrow. In zebrafish, HSPCs colonize the caudal hematopoietic tissue (CHT)9 before seeding the definitive hematopoietic organs, the thymus, and head kidney. In the hematopoietic organs, HSPCs commit to progenitors before maturing and giving rise to all blood cell populations.
HSPCs are tightly regulated in terms of dormancy and self-renewal, proliferation, and differentiation. Disrupting this balance leads to pathological consequences such as bone marrow failure or hematologic malignancy. For example, T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematologic tumor arising from the malignant transformation of hematopoietic progenitors.10 To date, several genes and underlying pathways have been linked to T-ALL. One of these genes is the tumor suppressor, phosphatase and tensin homolog (PTEN). Deletion mutations in PTEN appear in 5% to 10% of T-ALL cases, and about 17% of patients lack PTEN expression.10,11 Recent work using a conditional knockout mouse model demonstrated that loss of Pten in bone marrow HSPCs causes expansion of the short-term population and declining of the long-term population of HSPCs. Mice with Pten-deficient bone marrow HSPCs develop myeloproliferative disorder (MPD) eventually.12-15
PTEN is a phosphatase specific for the D3 position of the second messenger, phosphatidylinositol (3,4,5)-triphosphate, which is formed by phosphatidylinositol 3-kinase (PI3K). Thus, PTEN is one of the few known lipid phosphatases counteracting the PI3K-Akt (also known as protein kinase B [PKB]) pathway.15 Loss of Pten function is embryonic lethal in many organisms, including the mouse, Caenorhabditis elegans, and Drosophila.16-18 The zebrafish genome encodes 2 pten genes with redundant function, designated ptena and ptenb.19 Single mutants display no morphologic phenotype and are viable and fertile, but mutants that retain only a single wild-type copy of pten develop hemangiosarcomas during adulthood.20 Ptena−/−ptenb−/− mutants, hereafter referred to as Pten mutants, lack functional Ptena and Ptenb, are embryonic lethal at 5 to 6 days postfertilization (dpf), and display hyperplasia and dysplasia.19
We used zebrafish mutants lacking functional Pten to address hematopoiesis. Zebrafish embryos survive without circulating erythrocytes21 and hence provide an excellent system to investigate the early stages of hematopoiesis, which have not been addressed in other systems before. In particular, we investigated whether homing and proliferation of definitive HSPCs in the CHT, and their maturation into differentiated blood cells are Pten dependent. Here, we report that progenitors of all blood lineages are formed in the CHT in Pten mutants, but their differentiation into mature blood cells is arrested. Finally, we show that inhibition of PI3K signaling once the HSPCs have colonized the CHT allowed their differentiation into definitive blood cells.
Methods
Zebrafish husbandry
Confocal, fluorescence, brightfield microscopy
Fluorescence images of transgenic embryos were acquired using a TCS-SPE confocal microscope (Leica) and processed with Image J (http://rsb.info.nih.gov/ij/). Embryos were anesthetized with tricaine23 and mounted on a glass cover dish with 0.7% low-melting agarose and covered with standard E3 medium. Whole-mount images from in situ hybridizations and Sudan Black (SB)-stained embryos were taken using a Zeiss Axioplan microscope connected to a Leica DFC480 camera.
In situ hybridization
Whole-mount immunohistochemistry
Immunohistochemistry was performed using anti–phosphorylated Histone 3 (pHis3) (Abcam)19 with slight modifications. Briefly, 4 dpf embryos were fixed overnight in 2% paraformaldehyde and subsequently washed with phosphate-buffered saline (PBS) including 0.3% Triton X-100. Permeabilization was performed by incubating embryos for 5 minutes on ice in 2.5 mg/mL trypsin solution (Worthington Biochemical Corporation). Subsequently, embryos were rinsed 3 times for 5 minutes with PBS including 0.3% Triton X-100. Embryos were blocked for several hours with 0.1% bovine serum albumin (BSA), 2% lamb serum, 0.1% dimethylsulfoxide (DMSO) and 1% Triton-X100 in PBS and incubated with polyclonal anti-pHis3 antibody2 (1:1000 in blocking buffer) overnight. Embryos were washed with 0.3% Triton X-100 in PBS several times and incubated in blocking buffer for several hours. Secondary antibody (Alexa 568, 1:1000) was applied overnight and embryos were washed 10 × 10 minutes with 0.3% Triton X-100 in PBS and processed for imaging.
Quantification of GFPlow and GFPhigh cells using Tg(cd41:eGFP)
Ptena+/−ptenb−/− with Tg(cd41:eGFP) were crossed, offspring were mounted at 4 dpf, and the entire CHT was imaged by confocal microscopy. Green fluorescent protein (GFP)low- and GFPhigh-expressing cells were quantified using Volocity software.
SB staining
Five-dpf-old embryos were fixed and stained using SB as described.5 Images of SB-stained embryos were taken and cells were counted using Image J software.
LY294002 treatment
Embryos were incubated with 4μM LY 294002 from 82 hpf onwards, fixed at 5 dpf, and processed for in situ hybridization or SB staining. SB+ cells were counted in the head, yolk sac, trunk, tail, and CHT using Image J software.
Results
Primitive wave of hematopoiesis is independent of Pten
To assess hematopoiesis systematically, we first analyzed the primitive wave of hematopoiesis. In situ hybridization was performed at 32 hpf using key markers that are associated with early erythrocytes (gata-1) and cells of the primitive myeloid lineage (pu.1, l-plastin, and c-fms).2,5,9,27-29 Expression pattern analysis for gata1, pu.1, l-plastin, and c-fms revealed no differences between siblings and Pten mutant embryos (supplemental Figure 1, available on the Blood website), indicating that initiation of the primitive wave was not affected in Pten mutants.
Homing and hyperproliferation of HSPCs in Pten mutant embryos
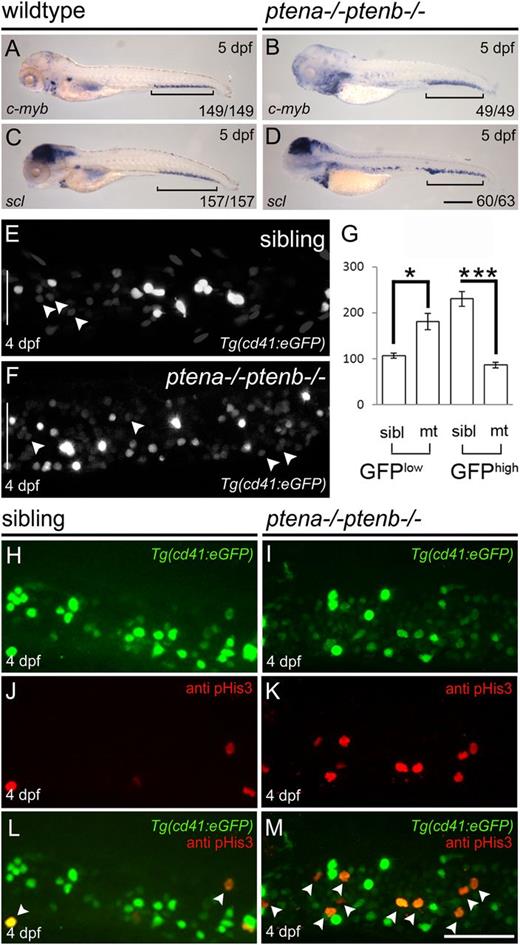
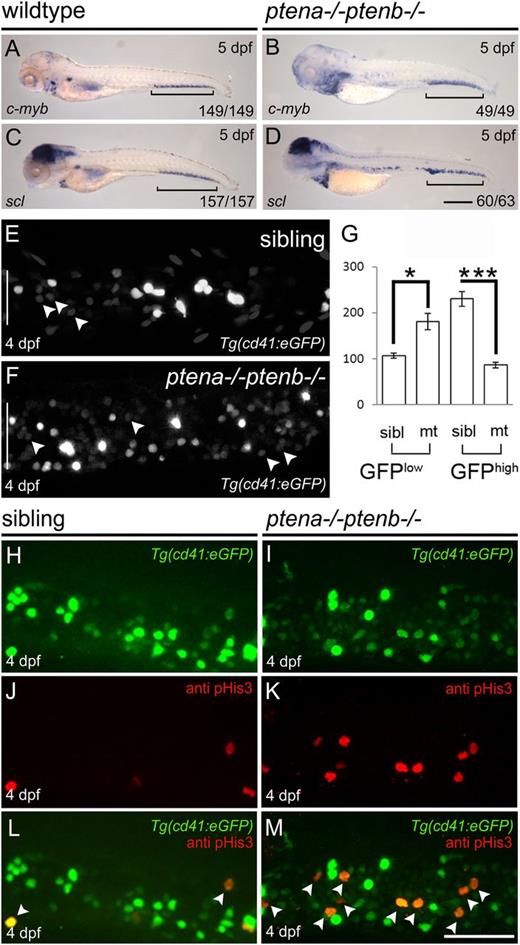
To investigate definitive hematopoiesis in Pten mutants, we first determined expression of c-myb and scl that mark HSPCs9,30 at 5 dpf. We detected expression of both genes in the CHT (Figure 1A-D brackets), indicating that homing of HSPCs was not dependent on Pten function. However, Pten mutants displayed more c-myb+ and scl+ cells, compared with wild-type embryos at 5 dpf (Figure 1A-D). We wondered whether the loss of function of Pten was associated with an elevated number of HSPCs in the CHT. To investigate this, we used the Tg(cd41:eGFP) line in which HSPCs express a low level of GFP (GFPlow) and thrombocytes a high level of GFP (GFPhigh).31 The number of GFPlow+ HSPCs in the CHT was significantly increased in Pten mutants at 4 dpf, compared with siblings (Figure 1E-G arrowheads). Next, we investigated whether the elevated number of HSPCs was due to enhanced proliferation. Immunohistochemistry using pHis3-specific antibodies in the Tg(cd41:eGFP) line revealed an increased number of mitotic GFPlow+ HSPCs in Pten mutant embryos compared with siblings at 4 dpf (Figure 1H-M). Predominantly GFPlow+ HSPCs stained positive for pHis3 in Pten mutant embryos (Figure 1I,M). Our data demonstrate that Pten is not required for HSPCs to colonize the CHT, but loss of Pten led to enhanced proliferation of HSPCs.
Enhanced proliferation of HSPCs in the CHT of Pten mutants.C-myb (A-B) and scl expression (C-D) were examined by in situ hybridization at 5 dpf in wild-type and Pten mutant (ptena−/−ptenb−/−) embryos as indicated. Representative embryos are depicted with anterior to the left. Brackets indicate the CHT; scale bar (200 µm) in panel D is representative for panels A-D. (E-G) The CHTs of sibling and Pten mutant embryos in the Tg(cd41:eGFP) background were imaged by confocal microscopy with a ×40 objective. Maximum projections of z-planes (step size, 2 µm) are shown (E-F). The number of HSPCs (GFPlow) (indicated by arrowheads) and thrombocytes (GFPhigh) were counted in the entire CHT (height of CHT is depicted by a white bar in panels E and F) at 4 dpf using Volocity (siblings, n = 14; ptena−/−ptenb−/−, n = 10). (G) Results are expressed as average number of cells per CHT and error bars indicate SEM. Normal distribution of data points was assessed with the Shapiro-Wilk test. Statistical comparisons of groups were performed by the 2-tailed t test, respectively, with the Mann-Whitney U test. *P < .05, **P < .01, ***P < .001 vs control. (H-M) Cell proliferation was assessed in the CHT of 4-dpf-old Pten mutant Tg(cd41:eGFP) embryos and siblings by immunohistochemistry using pHis3-specific antibodies. Representative Tg(cd41:eGFP) (H-I), pHis3 (J-K), and merged images are shown (L-M). GFP and pHis3 double-positive cells are indicated with arrowheads in panels L and M. Scale bar, 50 μm.
Enhanced proliferation of HSPCs in the CHT of Pten mutants.C-myb (A-B) and scl expression (C-D) were examined by in situ hybridization at 5 dpf in wild-type and Pten mutant (ptena−/−ptenb−/−) embryos as indicated. Representative embryos are depicted with anterior to the left. Brackets indicate the CHT; scale bar (200 µm) in panel D is representative for panels A-D. (E-G) The CHTs of sibling and Pten mutant embryos in the Tg(cd41:eGFP) background were imaged by confocal microscopy with a ×40 objective. Maximum projections of z-planes (step size, 2 µm) are shown (E-F). The number of HSPCs (GFPlow) (indicated by arrowheads) and thrombocytes (GFPhigh) were counted in the entire CHT (height of CHT is depicted by a white bar in panels E and F) at 4 dpf using Volocity (siblings, n = 14; ptena−/−ptenb−/−, n = 10). (G) Results are expressed as average number of cells per CHT and error bars indicate SEM. Normal distribution of data points was assessed with the Shapiro-Wilk test. Statistical comparisons of groups were performed by the 2-tailed t test, respectively, with the Mann-Whitney U test. *P < .05, **P < .01, ***P < .001 vs control. (H-M) Cell proliferation was assessed in the CHT of 4-dpf-old Pten mutant Tg(cd41:eGFP) embryos and siblings by immunohistochemistry using pHis3-specific antibodies. Representative Tg(cd41:eGFP) (H-I), pHis3 (J-K), and merged images are shown (L-M). GFP and pHis3 double-positive cells are indicated with arrowheads in panels L and M. Scale bar, 50 μm.
Arrest of differentiation in all blood cell lineages in Pten mutants
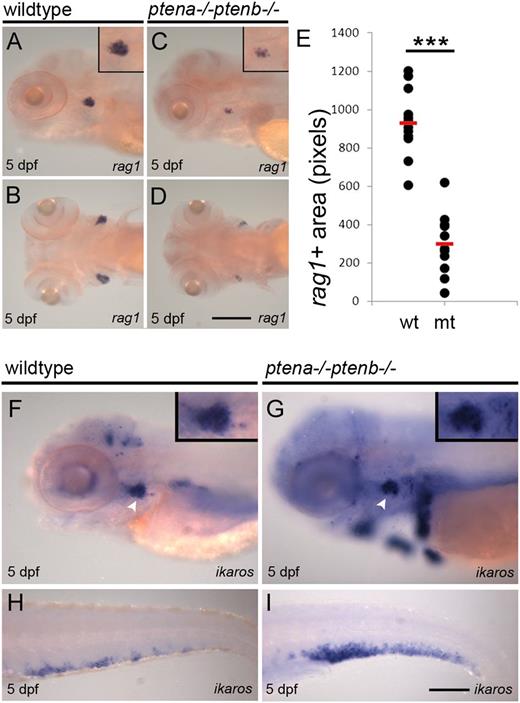
What are the consequences of lack of Pten function for the various definitive blood lineages generated from the HSPCs? We first investigated the lymphoid lineage. Pten mutants contained less rag1+ cells in the nascent thymus, compared with siblings (Figure 2A-D). Quantification of the rag1+ area in wild-type and Pten mutant embryos indicated a significant reduction in the rag1+ area in Pten mutants (Figure 2E). To assess whether reduced expression of rag1 was a consequence of reduced numbers of lymphoid progenitors, ikaros expression was evaluated. Surprisingly, ikaros expression was detected in the mutant thymus at the same level as in wild-type (Figure 2F-G inset). In addition, ectopic ikaros expression was detected in Pten mutants (Figure 2G) and ikaros expression was strongly enhanced in the CHT of Pten mutants (Figure 2H-I). The reduced number of rag1+ thymocytes and elevated number of ikaros+ lymphoid progenitors indicate that in Pten mutants, most lymphoid progenitors are arrested at a specific stage between ikaros and rag1 expression.
Differentiation arrest of lymphoid progenitors in Pten mutants.Rag1 is expressed in lymphocytes and highlights the thymus. Representative lateral and dorsal views of wild-type (A-B) and Pten mutant embryos (C-D) are shown; close-ups of the thymus in the insets. (E) Rag1+ area in wild-type and Pten mutant embryos at 5 dpf was quantified and is depicted in scatter plot; wild-type, n = 14; Pten mutant (mt), n = 11. The average rag1+ area is highlighted with a red bar and is significantly reduced in Pten mutants (P < .001, 2-tailed t test). (F-I) Ikaros was used as a marker for lymphoid progenitors in 5-dpf wild-type (F,H) and Pten mutant embryos (G,I) in the thymus and CHT, respectively; thymus is indicated with arrowhead. Representative embryos are depicted with anterior to the left. Scale bars in panels D and I represent 200 µm. wt, wild type.
Differentiation arrest of lymphoid progenitors in Pten mutants.Rag1 is expressed in lymphocytes and highlights the thymus. Representative lateral and dorsal views of wild-type (A-B) and Pten mutant embryos (C-D) are shown; close-ups of the thymus in the insets. (E) Rag1+ area in wild-type and Pten mutant embryos at 5 dpf was quantified and is depicted in scatter plot; wild-type, n = 14; Pten mutant (mt), n = 11. The average rag1+ area is highlighted with a red bar and is significantly reduced in Pten mutants (P < .001, 2-tailed t test). (F-I) Ikaros was used as a marker for lymphoid progenitors in 5-dpf wild-type (F,H) and Pten mutant embryos (G,I) in the thymus and CHT, respectively; thymus is indicated with arrowhead. Representative embryos are depicted with anterior to the left. Scale bars in panels D and I represent 200 µm. wt, wild type.
The Tg(cd41:eGFP) line revealed that the number of GFPhigh thrombocytes in the CHT was significantly reduced by 4 dpf in the CHT (Figure 1E-F), suggesting that thrombocytic differentiation was impaired in Pten mutants.
To assess erythropoiesis, we used in situ hybridization and found elevated numbers of globin+ and gata1+ early erythroid progenitors in the CHT of Pten mutants (Figure 3A-D). Further differentiation of these early erythroid progenitors could not be addressed, for in fish, erythroid differentiation normally occurs very gradually over several days within the circulation.21,32 The death of PTEN mutants by 5 to 6 dpf19 only allows us to detect the definitive committed (gata1+, globin+) early erythroid progenitors, that are mostly not yet circulating by that time and hence are located in the CHT.33
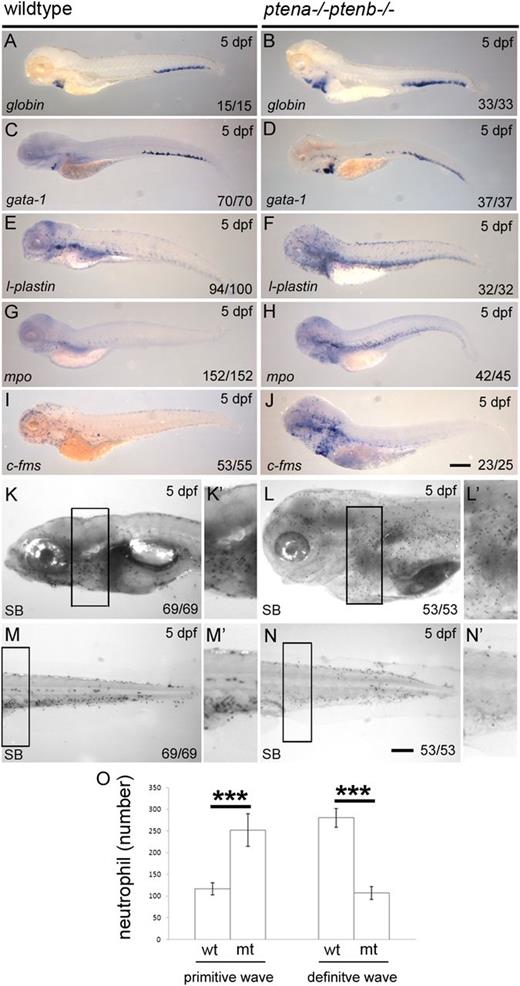
Enhanced numbers of progenitors and arrest of neutrophil differentiation in Pten mutants. A panel of in situ hybridization markers for blood lineages was used on 5 dpf wild-type and Pten mutant (ptena−/−ptenb−/−) embryos: (A-B) Globin and (C-D) gata1, both indicative of erythroblasts; (E-F) l-plastin, marking lymphocytes, macrophages, and neutrophils; (G-H) mpo, indicating neutrophils; (I-J) c-fms, indicative of macrophages. (K-N) At 5 dpf, SB staining was used to assess the number of mature neutrophils in various parts of the embryo. Panels K′-N′ represent magnifications of the boxed areas in the corresponding panels. (O) SB+ cells were counted in the head and yolk sac (mainly the primitive wave) and in the trunk, tail, and CHT region (mainly the definitive wave) as indicated in supplemental Figure 2. The results are expressed as average number of cells per embryo with the error bars indicating SEM; wild type, n = 6, ptena−/−ptenb−/− (n = 7). Normal distribution of data points was assessed with the Shapiro-Wilk test. Statistical comparison of groups was performed by the 2-tailed t test. *P < .05, **P < .01, ***P < .001 as indicated. Representative embryos are depicted; scale bars in panel J (200 µm) and N (100 µm) are representative for panels A-J, respectively, panels K-N. wt, wild type; mt, Pten mutant.
Enhanced numbers of progenitors and arrest of neutrophil differentiation in Pten mutants. A panel of in situ hybridization markers for blood lineages was used on 5 dpf wild-type and Pten mutant (ptena−/−ptenb−/−) embryos: (A-B) Globin and (C-D) gata1, both indicative of erythroblasts; (E-F) l-plastin, marking lymphocytes, macrophages, and neutrophils; (G-H) mpo, indicating neutrophils; (I-J) c-fms, indicative of macrophages. (K-N) At 5 dpf, SB staining was used to assess the number of mature neutrophils in various parts of the embryo. Panels K′-N′ represent magnifications of the boxed areas in the corresponding panels. (O) SB+ cells were counted in the head and yolk sac (mainly the primitive wave) and in the trunk, tail, and CHT region (mainly the definitive wave) as indicated in supplemental Figure 2. The results are expressed as average number of cells per embryo with the error bars indicating SEM; wild type, n = 6, ptena−/−ptenb−/− (n = 7). Normal distribution of data points was assessed with the Shapiro-Wilk test. Statistical comparison of groups was performed by the 2-tailed t test. *P < .05, **P < .01, ***P < .001 as indicated. Representative embryos are depicted; scale bars in panel J (200 µm) and N (100 µm) are representative for panels A-J, respectively, panels K-N. wt, wild type; mt, Pten mutant.
Next, expression of myeloid cell markers was assessed. Clearly more l-plastin+, mpo+ (neutrophil lineage), and c-fms+ (macrophage lineage) cells dispersed in peripheral tissues were detected in Pten mutant than in wild-type embryos at 5 dpf (Figure 3E-J). SB stains the granules of neutrophils5 and was used to quantify mature neutrophils in Pten mutant embryos and siblings. SB+ cells in the head and yolk sac region are predominantly derived from the primitive wave, whereas SB+ cells in the trunk, tail, and CHT are mainly definitive wave-derived.5 The number of SB+ neutrophils was counted in the different areas of 6 wild-type and 7 Pten mutant embryos as illustrated in supplemental Figure 2. The number of SB+ cells from the primitive wave was enhanced more than twofold in Pten mutants (Figure 3K,L,O), whereas the number of SB+ cells from the definitive wave was reduced more than twofold (Figure 3M-O). The elevated number of mpo+ cells in the CHT, combined with reduced number of SB+ cells in the trunk, tail, and CHT, suggests an arrest in differentiation of neutrophils in the absence of Pten function. To verify whether cell death might cause the lack of differentiated cells, we performed acridine orange staining. Pten mutant embryos did not exhibit enhanced apoptosis in the CHT (supplemental Figure 3), suggesting that the absence of differentiated cells is rather due to perturbed maturation. These results demonstrate that in the absence of Pten all blood lineages are specified, yet differentiation is arrested, suggesting a requirement for Pten in terminal blood cell differentiation.
Arrested differentiation in Pten mutants is reversed by inhibition of PI3K signaling
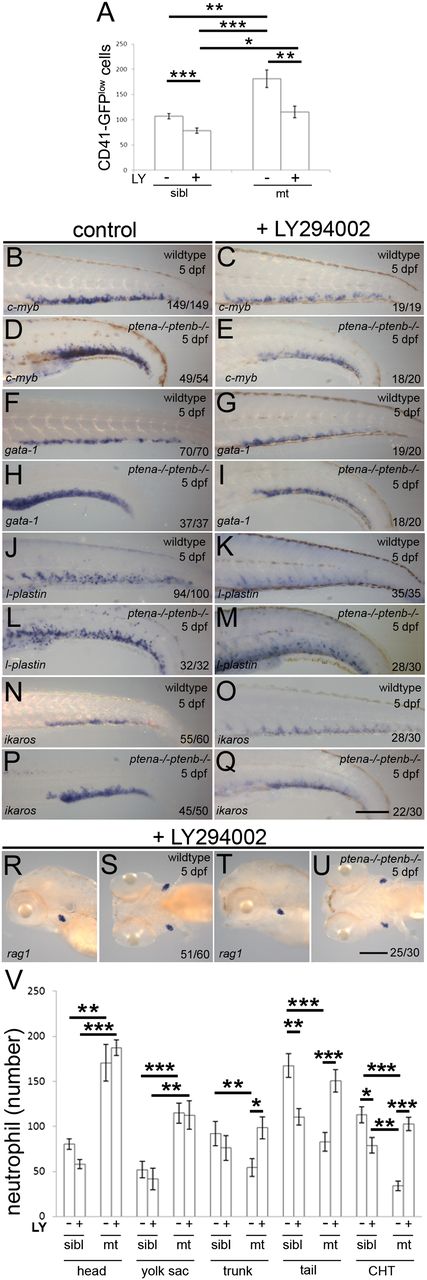
Our data suggest that proliferation of definitive blood cell progenitors was enhanced, while concomitantly differentiation was arrested in Pten mutant embryos. To address directly whether the lack of Pten function was causative, we treated Pten mutant embryos with the PI3K inhibitor, LY294002, from 82 hpf onwards, that is, after the HSPCs had colonized the CHT. Pten mutant embryos displayed enhanced numbers of HSPCs at 5 dpf, compared with siblings (Figure 4A), consistent with our data at 4 dpf (Figure 1E-G). Treatment with 4µM LY294002 from 82 hpf onwards significantly decreased the number of CD41-GFPlow cells in Pten mutants and siblings as well (Figure 4A). Expression of the progenitor marker, c-myb, which was enhanced in the Pten mutants at 5 dpf, was also diminished by treatment with LY294002 from 82 hpf (Figure 4B-E) in both mutant and wild-type embryos, consistent with the CD41-GFPlow data. These data suggest that inhibition of PI3K signaling after colonization of the CHT suppressed proliferation of HSPCs.
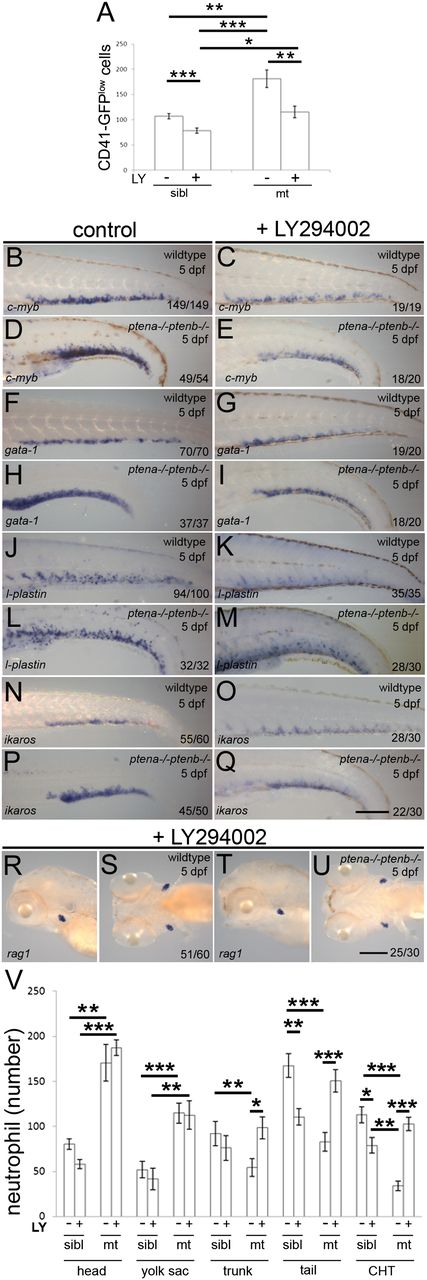
Inhibition of PI3K signaling suppressed enhanced proliferation and released differentiation arrest in Pten mutants. Embryos were treated with 4μM LY294002 or DMSO (control) from 82 hpf onward. (A) The CHTs of sibling (sibl) and Pten mutant (mt) embryos in Tg(cd41:eGFP) background were imaged. The number of HSPCs (GFPlow) were counted in the whole CHT at 4 dpf (sibling, n = 14; sibling LY294002 treated, n = 10; ptena−/−ptenb−/−, n = 10; ptena−/−ptenb−/− LY294002 treated, n = 9). Results are expressed as the average number of cells per CHT and error bars indicate SEM. Normal distribution of data points was assessed with the Shapiro-Wilk test. Statistical comparisons of groups were performed by the 2-tailed t test, respectively, with the Mann-Whitney U test. *P < .05, **P < .01, ***P < .001 vs control. (B-U) In situ hybridization markers for blood lineages were used on 5-dpf wild-type and Pten mutant (ptena−/−ptenb−/−) embryos: (B-E) c-myb is expressed in HSPCs; (F-I) gata-1, indicative of erythroblasts; (J-M) l-plastin, marking lymphocytes, macrophages, and neutrophils; (N-Q) ikaros, indicating lymphoblasts and (R-U) rag1, expressed in lymphocytes. Representative embryos are depicted; scale bars in panels Q and U (200 µm) are representative for panels B-U. (V) At 5 dpf, SB staining was used to assess the number of neutrophils in various parts of the embryo. SB+ cells in control (−, n = 10) and LY294002-treated (+, n = 16) siblings (sibl) and control (−, n = 11) and LY294002-treated (+, n = 17) Pten mutant (mt) embryos were counted in various parts of the embryo as indicated in supplemental Figure 2 and the results are expressed as average number of cells per embryo with the error bars indicating SEM. Normal distribution of data points was assessed with the Shapiro-Wilk test. Statistical comparison of groups was performed by the 2-tailed t test. *P < .05, **P < .01, ***P < .001 as indicated.
Inhibition of PI3K signaling suppressed enhanced proliferation and released differentiation arrest in Pten mutants. Embryos were treated with 4μM LY294002 or DMSO (control) from 82 hpf onward. (A) The CHTs of sibling (sibl) and Pten mutant (mt) embryos in Tg(cd41:eGFP) background were imaged. The number of HSPCs (GFPlow) were counted in the whole CHT at 4 dpf (sibling, n = 14; sibling LY294002 treated, n = 10; ptena−/−ptenb−/−, n = 10; ptena−/−ptenb−/− LY294002 treated, n = 9). Results are expressed as the average number of cells per CHT and error bars indicate SEM. Normal distribution of data points was assessed with the Shapiro-Wilk test. Statistical comparisons of groups were performed by the 2-tailed t test, respectively, with the Mann-Whitney U test. *P < .05, **P < .01, ***P < .001 vs control. (B-U) In situ hybridization markers for blood lineages were used on 5-dpf wild-type and Pten mutant (ptena−/−ptenb−/−) embryos: (B-E) c-myb is expressed in HSPCs; (F-I) gata-1, indicative of erythroblasts; (J-M) l-plastin, marking lymphocytes, macrophages, and neutrophils; (N-Q) ikaros, indicating lymphoblasts and (R-U) rag1, expressed in lymphocytes. Representative embryos are depicted; scale bars in panels Q and U (200 µm) are representative for panels B-U. (V) At 5 dpf, SB staining was used to assess the number of neutrophils in various parts of the embryo. SB+ cells in control (−, n = 10) and LY294002-treated (+, n = 16) siblings (sibl) and control (−, n = 11) and LY294002-treated (+, n = 17) Pten mutant (mt) embryos were counted in various parts of the embryo as indicated in supplemental Figure 2 and the results are expressed as average number of cells per embryo with the error bars indicating SEM. Normal distribution of data points was assessed with the Shapiro-Wilk test. Statistical comparison of groups was performed by the 2-tailed t test. *P < .05, **P < .01, ***P < .001 as indicated.
Next, we addressed whether the blood cell lineage commitment was affected by treatment with LY294002 from 82 hpf onward. Expression of gata-1, l-plastin, and ikaros were determined for the erythroid, myeloid, and lymphoid lineages, respectively. While 5 dpf Pten mutant embryos exhibited elevated expression of gata-1, l-plastin, and ikaros in the CHT compared with wild-type embryos (Figure 4F-Q), treatment with LY294002 from 82 hpf onward suppressed this upregulation of gata-1, l-plastin, and ikaros in Pten mutant embryos to levels that were comparable to wild type (Figure 4F-Q). These results indicate that LY294002-induced inhibition of elevated PI3K activity in Pten mutants leads to suppression of the enhanced numbers of blood cell progenitors committed to the erythroid, myeloid, and lymphoid lineages.
Finally, we addressed whether arrested differentiation in Pten mutant embryos is caused by elevated PI3K signaling. Rag1 expression that is greatly reduced in Pten mutant embryos at 5 dpf (Figure 2A-D) was fully restored after treatment with LY294002 (Figure 4R-U). Furthermore, quantification of SB+ mature neutrophils at 5 dpf following late LY294002 treatment demonstrated a dramatic increase in the number of definitive wave-derived mature neutrophils in the CHT, trunk, and tail of Pten mutants, but not siblings (Figure 4V). In contrast, LY294002 treatment did not significantly affect the number of primitive wave-derived SB+ cells in the head and yolk sac of Pten mutants and siblings (Figure 4V). Taken together, these data indicate that inhibition of PI3K signaling in Pten mutant embryos suppressed the enhanced proliferation of HSPCs in the CHT and at the same time released the blood cell differentiation arrest.
Discussion
PTEN is a lipid phosphatase that counteracts PI3K activity and is one of the most frequently mutated tumor suppressor genes in a wide range of cancer types, including leukemia.12-15,34,35 PI3K signaling regulates hematopoietic stem cell (HSC) function. For instance, deletion of the downstream factors, Akt1 and Akt2, increases HSC quiescence,36 whereas activation of PI3K signaling promotes HSC proliferation and depletion.12,13 In the mouse embryo, HSPCs emerge from the dorsal aorta at embryonic day 10.5 (E10.5) and transiently colonize the fetal liver before seeding the organs of adult hematopoiesis.8,37-39 Similarly, zebrafish HSPCs emerge from the ventral wall of the dorsal aorta and transiently colonize the CHT, which is homologous to the fetal liver in mouse, before colonization of the adult organs of hematopoiesis.9 We used zebrafish mutant embryos lacking functional Pten to investigate whether loss of Pten affects homing of HSPCs to the CHT and further we addressed whether Pten is required for blood lineage specification and differentiation.
Our data revealed that Pten is not required for colonization of the CHT by HSPCs. Surprisingly, quantification of CD41low-positive HSPCs and analysis of c-myb and scl expression patterns revealed an increased number of HSPCs in the CHT during the course of development. PTEN is a tumor suppressor and loss of PTEN function is associated with enhanced cell proliferation. Reminiscent of studies in the mouse,12,13 we found increased numbers of mitotic cells in the CHT and conclude that in the absence of Pten, HSPCs colonize the niche and hyperproliferate.
PTEN is frequently mutated in leukemia resulting in replacement of normal bone marrow cells with leukemic cells. Acute myeloid leukemia patients suffer from a drop in erythrocytes, platelets, and normal white blood cells. Our investigation revealed that HSPCs lacking functional Pten engage in all blood lineages, indicating that lineage specification is a Pten-independent process. However, definitive differentiation of the thromboid, myeloid, lymphoid, and possibly other blood lineages is arrested in Pten mutants. We observed elevated numbers of mitotic HSPCs in the CHT of Pten mutant embryos, but no obvious differences in apoptosis between wild-type and Pten mutant embryos, indicating that enhanced proliferation caused the observed elevated number of HSPCs in mutant embryos. It is well established that proliferation and differentiation of stem cells, including HSCs, are inversely correlated.40 Our results are consistent with this notion. Yet, the potential of HSPCs to commit to distinct blood lineages appears not to be affected in Pten mutants, in that we observed progenitors of all the main lineages. Moreover, inhibition of PI3K after the HSPCs had colonized the CHT, released the arrest of differentiation, and resulted in the production of definitively differentiated blood cells, including SB+ neutrophils and rag1+ thymocytes, indicating that these cells can produce mature blood cells.
The role of Pten in mouse HSCs has been addressed by conditional knockout of Pten using the Mx-1 promoter driving Cre recombinase in adult bone marrow cells, respectively, the VE-cadherin promoter in fetal liver.12,13,41 HSCs are driven into the cell cycle in the absence of Pten, resulting in mobilization of HSCs from the bone marrow or fetal liver and transient expansion of the spleen, leading to depletion of HSCs. These conditional Pten-deficient mice die of a MPD that resembles acute myeloid/lymphoid leukemia.12,13,41 Our observations that HSPCs hyperproliferate in the CHT of Pten mutant zebrafish embryos are consistent with the expansion of bone marrow HSCs in conditional mouse models.
Here, we report that zebrafish mutant embryos lacking functional Pten develop multiple hematopoietic abnormalities during development. In particular, we show that whereas Pten is not required for HSPCs to colonize the CHT/niche, loss of Pten results in enhanced proliferation of HSPCs. Additionally, enhanced PI3K signaling in response to loss of Pten does not favor a certain blood lineage in that all progenitors are defined. However, differentiation into mature blood cells is impaired in Pten mutants, which is reversed by antagonizing PI3K signaling by treatment with LY294002. We conclude that the zebrafish Pten mutants are a powerful tool to further study hematopoietic abnormalities/hematologic malignancies and demonstrate that zebrafish mutants are a suitable model for drug screening.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mark Reijnen and the animal caretakers for excellent management of the fish facility. Microscopy was done at the Hubrecht Imaging Centre. The authors also thank Jeroen Paardekooper Overman for technical support.
This work was supported in part by a European Union (EU) (FP7) grant, ZF-CANCER (HEALTH-F2-2008-201439).
Authorship
Contribution: S.C. and J.d.H. designed experiments with input from K.K. and P.H.; S.C. and R.K. performed experiments; and S.C. and J.d.H. wrote the manuscript with input from P.H. and K.K.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.C. and K.K. is Institut National de la Santé et de la Recherche Médicale, Unités Mixtes de Recherche 5235, Dynamique des Interactions Membranaires Normales et Pathologiques, Université Montpellier 2, Montpellier, France.
Correspondence: Jeroen den Hertog, Hubrecht Institute, Uppsalalaan 8, 3584 CT, Utrecht, The Netherlands; e-mail: j.denhertog@hubrecht.eu.