Key Points
Haploinsufficiency of Egr1, Apc, and Tp53 in mice cooperate to model the pathogenesis of the early stages of t-MN with a del(5q).
Exposure of an Apc haploinsufficient BM microenvironment to radiation and/or an alkylating agent accelerates disease development.
Abstract
Therapy-related myeloid neoplasms (t-MN) are a late complication of the successful use of cytotoxic therapy for patients with cancer. A heterozygous deletions of the long arm of chromosome 5 [del(5q)], observed in 40% of patients, is associated with prior exposure to alkylating agents, and a high frequency of TP53 loss or mutation. In previous studies, we demonstrated that haploinsufficiency of 2 del(5q) genes, Egr1, and Apc, individually play a role in the pathogenesis of hematologic disease in mice. We now show that loss of one copy of Egr1 or Tp53 in an Apc haploinsufficient background (Apcdel/+) accelerated the development of a macrocytic anemia with monocytosis, early features of t-MN. The development of anemia was significantly accelerated by treatment of mice with the alkylating agent, N-ethyl-N-nitrosourea (ENU), regardless of the levels of expression of Egr1 and Tp53. Transplantation of either wild type; Egr1+/−; Tp53+/−; Apcdel/+; or Egr1+/−, Apcdel/+ bone marrow cells into lethally irradiated Apcdel/+ recipients resulted in rapid development of anemia that was further accelerated by administration of ENU to recipients, demonstrating that the Apcdel/+-induced anemia was cell extrinsic and potentiated by ENU mutagenesis. These data emphasize the synergistic role of cell intrinsic and cell extrinsic (microenvironment) factors in the pathogenesis of t-MN, and raise awareness of the deleterious effects of cytotoxic therapy on the stromal microenvironment.
Introduction
Therapy-related myeloid neoplasms (t-MN) are a late complication of cytotoxic treatment of primary malignant diseases, and include myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML).1 The incidence of t-MN is rising commensurate with improvements in cancer treatments and increased survival. The prognosis for these patients has not improved substantially in several decades (median survival, 8 months); thus, t-MN is an increasingly challenging clinical problem. Recurring chromosomal abnormalities correlate with prior treatment and clinical features of the disease.2,3 For example, heterozygous deletions of the long arm of chromosome 5 [del(5q)] are among the most common genetic abnormalities (∼40%) in alkylator-induced t-MN, with a long latency period of 5 to 7 years, trilineage dysplasia, and a poor outcome. A del(5q) also occurs in 10% to 15% of patients with primary MDS, a subset of whom have MDS with an isolated del(5q),4 as well as in 15% of patients with AML de novo.
Using molecular mapping approaches, 2 minimally deleted regions have been identified: 5q31.2 in t-MN and MDS/AML, and 5q33.1 in MDS with an isolated del(5q).1,5,6 Current studies support a haploinsufficiency model, in which loss of a single allele of more than one gene on 5q cooperate in the development of myeloid neoplasms. In fact, a number of genes and several micro RNA sequences located on 5q, including APC, EGR1, CTNNA1, DIAPH1, HNRNPA0, HSPA9, RPS14, and miR-145/146a, have been implicated in the development of myeloid disorders due to a gene dosage effect.1,7,8 For example, RPS14 and miR-145/146a cooperate in the pathogenesis of MDS with an isolated del(5q).6 We previously demonstrated that EGR1 (5q31.2), which is deleted in all t-MN patients with a del(5q) and APC (5q22) and is deleted in >95% of patients, are both expressed at haploinsufficient levels, and inactivating mutations have not been identified in the remaining alleles,9,10 confirming that these genes are not acting as typical tumor suppressor genes. Moreover, using mouse models, we showed that haploinsufficiency of Egr1 or Apc individually recapitulates some features of human MDS,8,10 further supporting their role in the pathogeneis of t-MN with a del(5q).
The early growth response 1 gene (EGR1) encodes a transcription factor that is a direct transcriptional regulator of many known tumor suppressor genes, including TP53, CDKN1A, (p21), and PTEN,11 and it has been shown to play a role in maintaining hematopoietic stem cell (HSC) quiescence and retention in the bone marrow (BM) niche.12 The adenomatous polyposis coli (APC) gene encodes a tumor suppressor protein best known for its involvement in the WNT signaling cascade via the regulation of β-catenin stability13 ; loss of APC acutely activates WNT signaling.14,15 Activation of WNT signaling in the BM stromal niche plays a role in maintaining the HSC pool throughout life, and WNT signaling in leukemia stem cells is critical for their self-renewal.16-18 Of note, there is emerging evidence that a large percentage of MDS/AML patients with abnormalities of chromosomes 5 and/or 7 show constitutive activation of canonical WNT signaling in osteoblast stromal precursors.19 Additional roles for APC include the regulation of mitosis, via control of spindle orientation and chromosome segregation, as well as cell migration.20 In addition to the loss of 5q genes, the results of recent high-throughput sequencing studies have confirmed that loss of TP53 activity, through mutation or loss, is significantly associated with t-MN with a del(5q).21,22 The well-characterized tumor suppressor gene, TP53, has been shown to play a role in cell cycle arrest, DNA repair, and apoptosis after cellular stress,23 as well as maintenance of stem cell self-renewal.24,25
We and others have previously established a role for reduced expression of EGR1, APC, or TP53 in the pathogenesis of myeloid diseases.8,10,25 In this report, we modeled the simultaneous reduction in expression of all three genes in mice. We observed an accelerated development of macrocytic anemia in double and triple heterozygous (Apcdel/+) mice that was reminiscent of the early stages of t-MN, suggesting that haploinsufficiency of APC cooperates with loss of EGR1 or TP53. We next examined whether this effect occurred entirely within the hematopoietic compartment (where deletion of 5q genes occur), and demonstrated that alterations within the stromal cell compartment due, in part, to deregulation of the Apc/WNT pathway, were largely responsible for the phenotype. Interestingly, we also demonstrated that exposure of the BM microenvironment to N-ethyl-N-nitrosourea (ENU) accelerates disease development in an Apc haploinsufficient background, raising awareness of the effects that alkylating agent therapy may have on the stromal microenvironment in patients.
Methods
Mouse strains and transplantation studies
All studies were approved by the University of Chicago Institutional Animal Care & Use Commitee. Mice were housed in a fully-Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. Mx1-Cre−Apcfl/+ (Apcfl/+) and Mx1-Cre+Apcfl/+ (Apcdel/+) mice were generated by crossing Apcfl/fl mice26 with Mx1-Cre transgenic mice.27 The efficiency of deletion in hematopoietic cells, after 3 intraperitoneal injections with 10 mg/kg polyinosinic-polycytidylic acid (pI-pC) (GE Healthcare, Pittsburgh, PA) when mice were 2 months old, was verified by polymerase chain reaction (PCR), as previously described.10 Conditional Apc mice, Egr1 knockout mice (provided by Dr Jeffrey Milbrandt), and Tp53 knockout mice (strain Trp53tm1Tyi/J, developed in Tyler Jacks’ Laboratory) were all backcrossed onto the C57BL/6 (CD45.2) background. Egr1+/−, Apcdel/+; Tp53+/−, Apcdel/+; and Egr1+/−, Tp53+/−, Apcdel/+ mice and corresponding Apcfl/+ controls were generated by crossing Mx1-Cre+Apcfl/fl mice with Egr1+/− and Tp53+/− mice. CD45.1/CD45.2 wild-type (WT) mice were generated by crossing C57BL/6 (CD45.2) and B6.SJL (CD45.1). Total BM cells (∼5 × 106 cells, of varying genotype indicated in individual figure legends) were transplanted, via retro-orbital injection, into lethally irradiated (8.6 Gy) Apcfl/+ or Apcdel/+ mice that had been pretreated, 3 to 4 weeks prior to irradiation, with pIpC to induce deletion. When Apcdel/+ mice were used as donors, BM cells were harvested 3 to 4 weeks after pIpC. Mice were monitored daily and sacrificed either at ×3 months post-pIpC, or when they were moribund and/or severely anemic. ENU was administered at 100 mg/kg either 1 month post-pIpC or 2 weeks post-pIpC in Apc recipient mice used for transplants. Stromal cells were isolated from Apcfl/+ or Apcdel/+ mice 4 weeks post-pIpC, as described by Soleimani and Nadri.28 For Apcdel/del mice, cells were isolated 1 week post-pIpC.
Peripheral blood analyses and histology
A complete blood count (CBC) from heart blood was determined with a Hemavet 950 counter (CDC Technologies, Oxford, CT). All organs were recovered, fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 4 to 5 µm, and stained with hematoxylin and eosin for histologic examination by a pathologist (J.A.). Peripheral blood, BM aspirates, and spleen touch preparations were stained with Wright–Giemsa. Images were obtained using an Olympus microscope (Model BX51; Tokyo, Japan), equipped with an Optronics 3CCD 1080p digital camera (Goleta, CA), and processed with Adobe Photoshop (San Jose, CA).
Flow cytometric analysis
Single-cell suspensions of BM and spleen were stained with fluorochrome-conjugated antibodies specific for CD71, Ter119, Gr-1, Mac-1 (CD11b), CD19, IgM, CD4, CD8, and Annexin V (BD Biosciences, San Jose, CA). Flow cytometry was performed on a FACSCanto or LSRFortessa (BD Biosciences), and data were analyzed with the FlowJo software (Tree Star, Inc., Ashland, OR).
Statistical analysis
Survival times (time to sacrifice) were estimated by the Kaplan-Meier method and compared between groups via log rank tests. Blood counts were compared using pairwise two-sample Student t tests (Apcfl/+ vs Apcdel/+; Egr1+/−, Apcfl/+ vs Egr1+/−, Apcdel/+; etc).
Results
Loss of one allele of Egr1 and/or Tp53 accelerates Apcdel/+-induced fatal macrocytic anemia
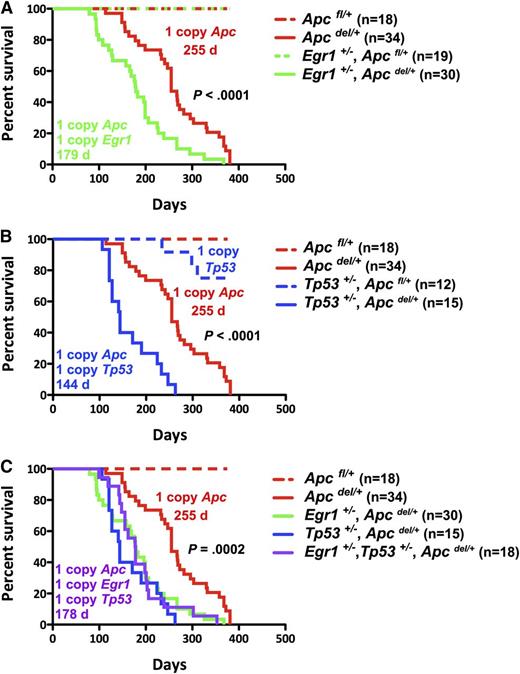
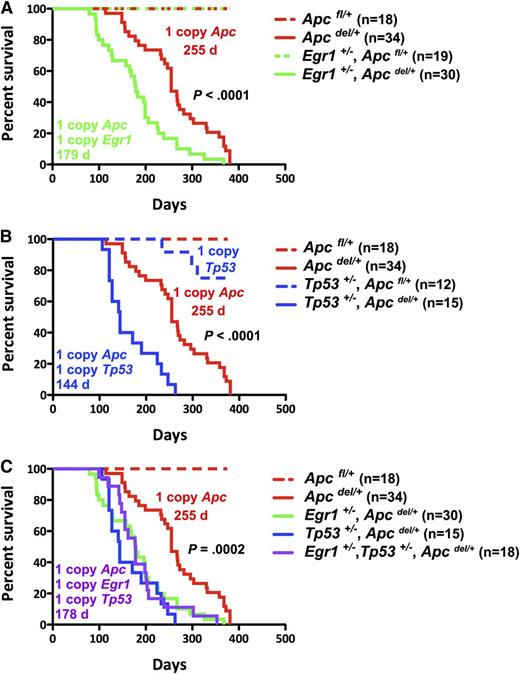
We hypothesized that loss of 1 copy of Egr1 may cooperate with Apc haploinsufficiency in the pathogenesis of anemia or other myeloid disorders, and that loss of Tp53 function may further cooperate in disease progression. To test this hypothesis, we first generated mice expressing a single allele of Egr1 and Apc: Egr1+/−, Apcdel/+, or a single allele of Tp53 and Apc: Tp53+/−, Apcdel/+, and the corresponding controls in the Apcfl/+ (Apc WT) background (Figure 1). Loss of Apc is under the control of an interferon-inducible Mx1-Cre promoter, and we induced deletion of a single allele of Apc by the injection of 3 doses of the interferon-inducer pI-pC, when the mice were 2–months-old. Consistent with our previous studies, Apcdel/+ mice developed a fatal macrocytic anemia at 4 to 12 months after deletion of Apc. Interestingly, Egr1+/−, Apcdel/+ mice developed disease significantly faster with a median survival of 179 vs 255 days (P < .0001); the control Egr1+/−, Apcfl/+ mice remain healthy (Figure 1A). In addition, Tp53+/−, Apcdel/+ mice had a significantly reduced median survival of 144 days vs 255 days (P < .0001) (Figure 1B). To examine whether haploinsufficiency of Egr1 and Apc cooperate with reduced Tp53 function, we generated triple heterozygous mice (Figure 1C). Egr1+/−, Tp53+/−, and Apcdel/+ mice died at a significantly faster rate than Apcdel/+ mice, with a median survival of 178 days vs 255 days (P = .0002). However, there was no significant difference in the survival of the double or triple heterozygous mice (P = .66). In the absence of Apc haploinsufficiency, Egr1+/−, Apcfl/+; Tp53+/−, Apcfl/+; and Egr1+/−, Tp53+/−, Apcfl/+ control mice did not develop a macrocytic anemia, but some died of T lymphomas or soft tissue sarcomas at much later time points (>300 days). In summary, loss of 1 allele of Egr1, or Tp53, or both, equally accelerated the Apcdel/+-induced fatal anemia.
Loss of 1 Allele of Egr1, Tp53, or both genes equally accelerates Apcdel/+-induced fatal anemia. Kaplan-Meier survival curve of Apcfl/+ control mice and Apcdel/+mice, crossed with Egr1+/− (A), Tp53+/− (B), or Egr1+/− and Tp53+/− (C). Percent survival (time to sacrifice of moribund animals) is plotted vs time in days, post-pIpC treatment (to induce Apc deletion). The number of mice in each cohort is shown. Egr1+/−, Apcdel/+; Tp53+/−, Apcdel/+; and Egr1+/−, Tp53+/−, Apcdel/+ mice all had significantly decreased survival compared with Apcdel/+ mice (P < .0001). The deletion of a single allele of Apc was induced in Mx1-Cre+Apcfl/+ mice treated with 3 doses of pI-pC, referred to as Apcdel/+mice. Control Mx1-Cre−Apcfl/+ mice are referred as Apcfl/+mice.
Loss of 1 Allele of Egr1, Tp53, or both genes equally accelerates Apcdel/+-induced fatal anemia. Kaplan-Meier survival curve of Apcfl/+ control mice and Apcdel/+mice, crossed with Egr1+/− (A), Tp53+/− (B), or Egr1+/− and Tp53+/− (C). Percent survival (time to sacrifice of moribund animals) is plotted vs time in days, post-pIpC treatment (to induce Apc deletion). The number of mice in each cohort is shown. Egr1+/−, Apcdel/+; Tp53+/−, Apcdel/+; and Egr1+/−, Tp53+/−, Apcdel/+ mice all had significantly decreased survival compared with Apcdel/+ mice (P < .0001). The deletion of a single allele of Apc was induced in Mx1-Cre+Apcfl/+ mice treated with 3 doses of pI-pC, referred to as Apcdel/+mice. Control Mx1-Cre−Apcfl/+ mice are referred as Apcfl/+mice.
All mice with Apc haploinsufficiency developed macrocytic anemia due to block at early erythroblast stage
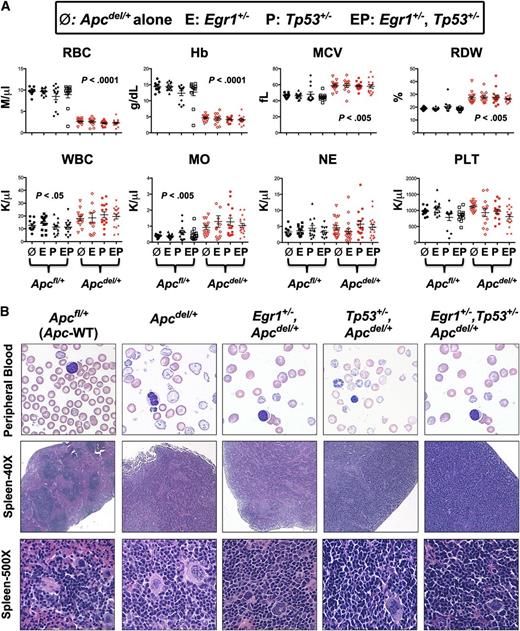
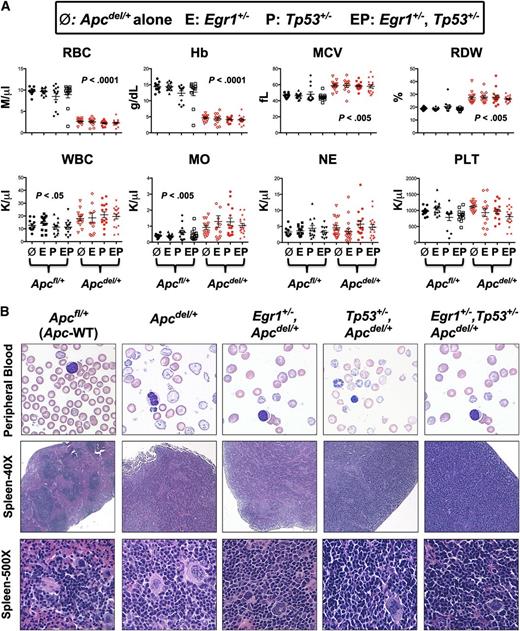
All mice with Apc haploinsufficiency succumbed to a fatal anemia. To determine if there were any distinct characteristics among the 4 different genotypic cohorts, hematologic parameters for Apcdel/+ mice or Apcdel/+ mice crossed with Egr1+/− or Tp53+/−, or Egr1+/− and Tp53+/− were measured and compared with Apcfl/+ (WT-Apc) corresponding controls (Figure 2A). Compared with Apcfl/+ control mice, the red blood cell (RBC) and hemoglobin (Hb) count were significantly decreased, and the mean cell volume (MCV) and red cell distribution width (RDW) were significantly increased in all mouse cohorts with Apc haploinsufficiency. There was also a significantly increased monocyte count in all Apcdel/+ cohorts, consistent with the development of monocytosis. The peripheral blood smears from all Apc haploinsufficient mice showed varying degrees of macrocytosis, anisocytosis, and poikilocytosis (Figure 2B), a common feature in t-MN patients.
All mice with Apc haploinsufficiency develop a macrocytic anemia with monocytosis. (A) CBC of Apcdel/+and Apcfl/+mice alone or crossed with Egr1+/−(E), or Tp53+/− (P), or Egr1+/−, Tp53+/− (EP) are shown. CBCs of Apcdel/+ mice were taken at the time of sacrifice, when the mice were severely anemic and moribund. Blood counts of Apcfl/+ control mice were taken during a similar timeframe, but mice were not moribund. (B) Representative peripheral blood smears (×1000) and spleen sections at ×40 and ×500 from anemic Apcdel/+mice alone or crossed and of Apcfl/+ control mice. Images were obtained using an Olympus microscope (Model BX51), equipped with an Optronics 3CCD 1080p digital camera, and processed with Adobe Photoshop. MO, monocytes; NE, neutrophils; PLT, platelets; WBC, white blood cells.
All mice with Apc haploinsufficiency develop a macrocytic anemia with monocytosis. (A) CBC of Apcdel/+and Apcfl/+mice alone or crossed with Egr1+/−(E), or Tp53+/− (P), or Egr1+/−, Tp53+/− (EP) are shown. CBCs of Apcdel/+ mice were taken at the time of sacrifice, when the mice were severely anemic and moribund. Blood counts of Apcfl/+ control mice were taken during a similar timeframe, but mice were not moribund. (B) Representative peripheral blood smears (×1000) and spleen sections at ×40 and ×500 from anemic Apcdel/+mice alone or crossed and of Apcfl/+ control mice. Images were obtained using an Olympus microscope (Model BX51), equipped with an Optronics 3CCD 1080p digital camera, and processed with Adobe Photoshop. MO, monocytes; NE, neutrophils; PLT, platelets; WBC, white blood cells.
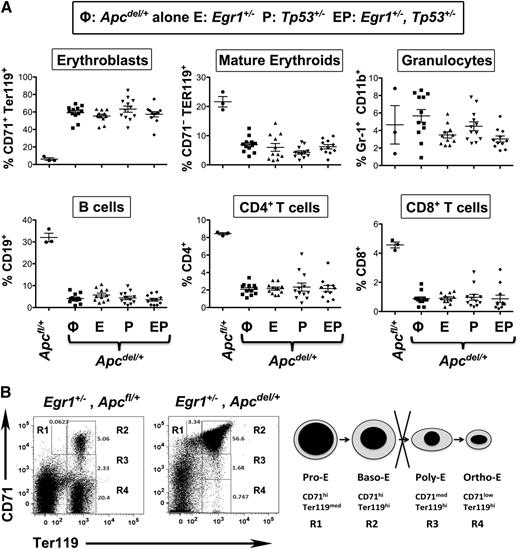
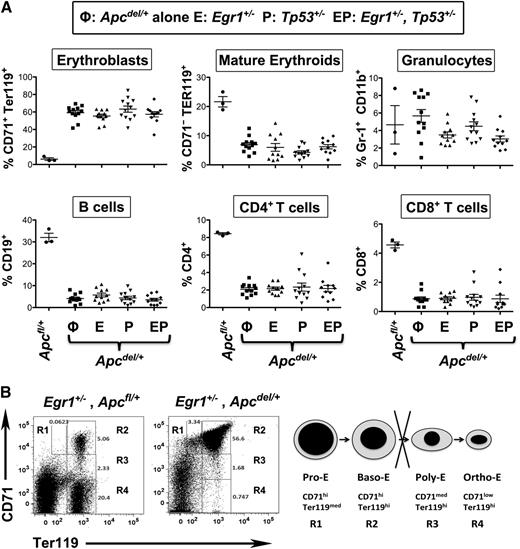
In all of the anemic mice, the spleen was enlarged threefold to 10-fold suggestive of extramedullary erythropoiesis. The stages of erythroid development can be defined by the expression of CD71 and Ter119. In all anemic mice with Apc haploinsufficiency, there was an increased proportion of CD71+Ter119+ erythroblasts and a decreased percentage of CD71−Ter119+ mature erythroid cells (Figure 3A). Morphologic and fluorescence-activated cell sorter analysis confirmed that this phenotype corresponded to a block at the basophilic erythroblast, or R2 (CD71+Ter119+) stage (Figures 2B and 3B). Histologic analysis also revealed that there was an effacement of the normal architecture in the spleen, with an expansion of the red pulp (Figure 2B). Effacement of splenic white pulp was reflected by a decrease in the proportion of B and T lymphocytes. Thus, loss of 1 copy of Egr1 and/or Tp53 accelerates the development of the macrocytic anemia in Apcdel/+ mice; however, the phenotype of the mice appears identical at time of sacrifice, with a block at the early erythroblast stage leading to a fatal macrocytic anemia.
Anemia in all Apc haploinsufficient mice is caused by a block at an early erythroblast stage. (A) Splenocytes were isolated from Apcfl/+ control mice, Apcdel/+ mice, or Apcdel/+mice crossed with Egr1+/−(E), Tp53+/− (P), or Egr1+/−, Tp53+/− (EP), and flow cytometric analysis of immature erythroblasts (CD71+Ter119+), mature erythroid cells (CD71−Ter119+), granulocytes (Gr1+Mac1+), B cells (CD19+), and T cells (CD4+ or CD8+) was performed when mice displayed severe anemia. (B) Flow cytometric analysis of spleen cells from a representative, anemic Apc haploinsufficient mouse. The 4r erythroid cell populations from least to most differentiated are indicated as R1 to R4. R1, pro-erythroblasts; R2, basophilic erythroblasts; R3, polychromatophilic erythroblasts; R4, orthochromatophilic erythroblasts.
Anemia in all Apc haploinsufficient mice is caused by a block at an early erythroblast stage. (A) Splenocytes were isolated from Apcfl/+ control mice, Apcdel/+ mice, or Apcdel/+mice crossed with Egr1+/−(E), Tp53+/− (P), or Egr1+/−, Tp53+/− (EP), and flow cytometric analysis of immature erythroblasts (CD71+Ter119+), mature erythroid cells (CD71−Ter119+), granulocytes (Gr1+Mac1+), B cells (CD19+), and T cells (CD4+ or CD8+) was performed when mice displayed severe anemia. (B) Flow cytometric analysis of spleen cells from a representative, anemic Apc haploinsufficient mouse. The 4r erythroid cell populations from least to most differentiated are indicated as R1 to R4. R1, pro-erythroblasts; R2, basophilic erythroblasts; R3, polychromatophilic erythroblasts; R4, orthochromatophilic erythroblasts.
Erythropoietic block occurs more rapidly in double heterozygous mice and is not due to increased apoptosis
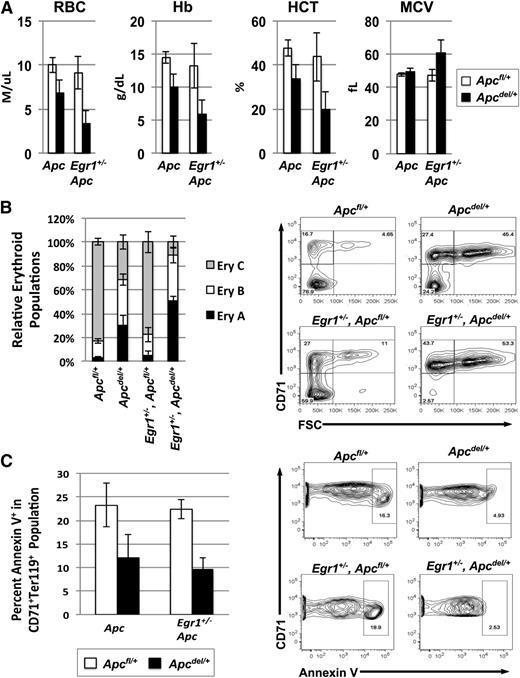
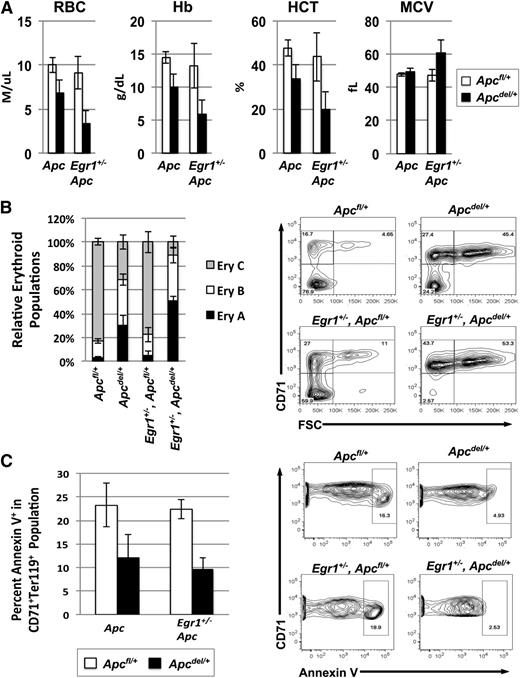
To determine why the Egr1+/−, Apcdel/+ double heterozygous mice developed the fatal anemia at a significantly faster rate, we examined the kinetics of disease development by comparing the phenotype of mice at 3 months post-pIpC treatment (Apc deletion). A comparison of the CBC values shows that early signs of a macrocytic anemia were more pronounced in double heterozygous mice, as the RBC, Hb, and hemocrit counts were lower, and MCV and RDW were slightly increased (Figure 4A). We next compared the stages of erythropoiesis. When Ter119high cells are analyzed using both the CD71 and forward scatter (FSC) parameters, they consistently resolve into 3 principal populations: Ery.A cells (Ter119highCD71highFSChigh) are basophilic erythroblasts, and Ery.B cells (Ter119highCD71highFSClow) are late basophilic and polychromatic erythroblasts, whereas Ery.C cells (Ter119highCD71lowFSClow) are orthochromatic erythroblasts and reticulocytes. We found that at 3 months postApc deletion, the ratio of the Ery.A vs Ery.C population was significantly higher in the double heterozygous mice (Egr1+/−, Apcdel/+) than in Apcdel/+ and control mice, suggesting that ineffective erythropoiesis develops more rapidly in the double heterozygous mice (Figure 4B). To compensate for the loss of mature erythroid cells, the number of erythroid colony-forming units in the spleen is increased in both the Apcdel/+and Egr1+/−, Apcdel/+ mice (supplemental Figure 1). In other mouse models (eg, Stat5a/b−/−), decreased survival of early erythroblasts accounts for the block in differentiation.29,30 We measured the percentage of Annexin V+ cells in the CD71+Ter119+ (R2) population in the spleen, and consistently observed decreased apoptosis in both the Apcdel/+ and Egr1+/−, Apcdel/+ mice, suggesting that the block in differentiation was not due to decreased survival of erythroblasts (Figure 4C). A similar phenotype was observed in Tp53+/−, Apcdel/+ mice (supplemental Figure 2). Although Egr1 and Tp53 expression induces apoptosis in tumor cells,31 a reduction in the dosage of Egr1 or Tp53 did not significantly decrease apoptosis in the Apc haploinsufficient background. Thus, the kinetics of disease development appear to be due to a block in early erythroid differentiation that occurs more rapidly in double heterozygous mice, but is not associated with an increase in apoptosis.
Anemia and block in erythropoiesis is detected earlier in Egr1+/−, Apcdel/+double heterozygous mice compared with Apcdel/+mice. (A) Peripheral blood counts of Apcfl/+, Apcdel/+, and Apcdel/+, Egr1+/− mice 3 months after induction. (B) Flow cytometric analysis of spleen cells isolated from mice, 3 months postinduction. Ter119high cells were analyzed using the CD71 and FSC parameters. Ery.A (Ter119highCD71highFSChigh) are basophilic, and Ery.B (Ter119highCD71highFSClow) are late basophilic and polychromatic, whereas Ery.C (Ter119highCD71lowFSClow) are orthochromatic erythroblasts and reticulocytes. A representative plot is shown on the right. Data represents the relative erythroid populations and standard error of the mean for 3 mice for each genotype. (C) The frequency of apoptosis in the R2 (CD71+Ter119+) population was measured by Annexin V staining. A representative contour plot is shown on the right. Apcdel/+ and Egr1+/−, Apcdel/+ mice consistently displayed a trend toward less apoptosis in the CD71+Ter119+ population compared with controls. For all experiments, the average and standard error of the mean of 3 mice per genotype is shown. HCT, hemocrit.
Anemia and block in erythropoiesis is detected earlier in Egr1+/−, Apcdel/+double heterozygous mice compared with Apcdel/+mice. (A) Peripheral blood counts of Apcfl/+, Apcdel/+, and Apcdel/+, Egr1+/− mice 3 months after induction. (B) Flow cytometric analysis of spleen cells isolated from mice, 3 months postinduction. Ter119high cells were analyzed using the CD71 and FSC parameters. Ery.A (Ter119highCD71highFSChigh) are basophilic, and Ery.B (Ter119highCD71highFSClow) are late basophilic and polychromatic, whereas Ery.C (Ter119highCD71lowFSClow) are orthochromatic erythroblasts and reticulocytes. A representative plot is shown on the right. Data represents the relative erythroid populations and standard error of the mean for 3 mice for each genotype. (C) The frequency of apoptosis in the R2 (CD71+Ter119+) population was measured by Annexin V staining. A representative contour plot is shown on the right. Apcdel/+ and Egr1+/−, Apcdel/+ mice consistently displayed a trend toward less apoptosis in the CD71+Ter119+ population compared with controls. For all experiments, the average and standard error of the mean of 3 mice per genotype is shown. HCT, hemocrit.
ENU accelerates Apcdel/+-induced macrocytic anemia
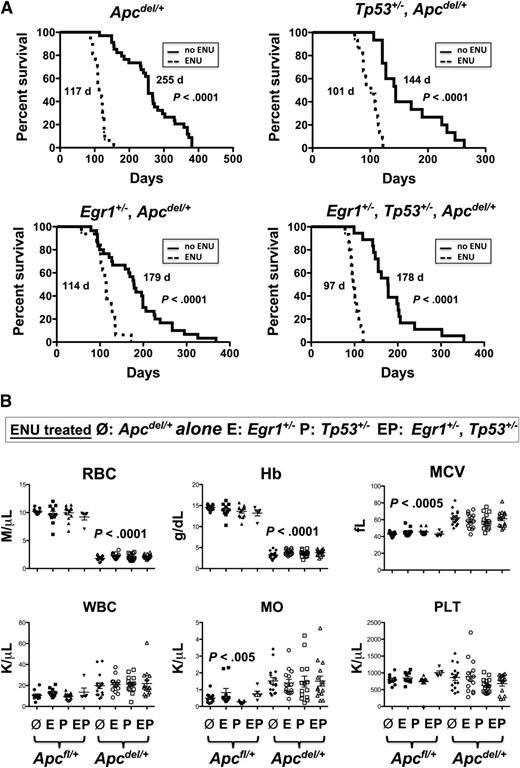
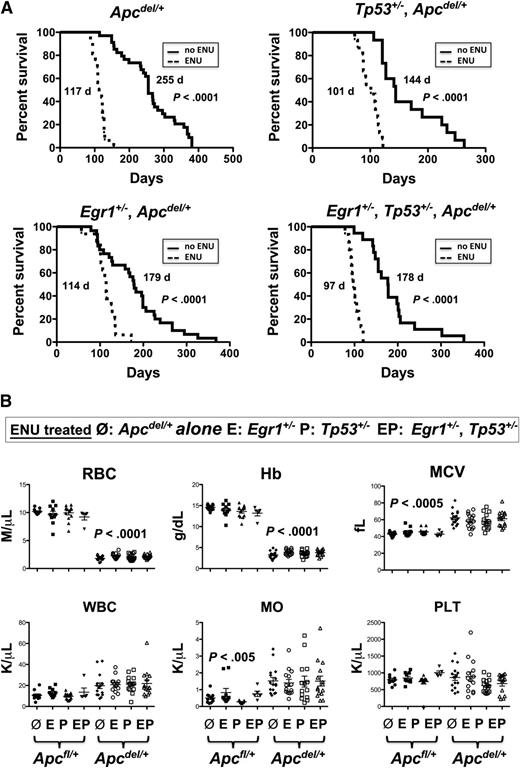
Therapy-related myeloid neoplasms with a del(5q) is associated with prior exposure to alkylating agents and mutations of Tp53.3,21 We previously showed that the loss of 1 copy of the del(5q) gene, Egr1, cooperates with secondary mutations, induced by the alkylating agent ENU, resulting in a myeloproliferative disease with ineffective erythropoiesis in mice.8 To examine the interaction of Apc, Egr1, and Tp53, and the impact of cooperating mutations, we treated mice with haploinsufficient levels of Apc, Egr1, and Tp53 with ENU. The ENU equally accelerated the Apcdel/+-induced fatal anemia, regardless of the Egr1 or Tp53 gene dosage (Figure 5A). The median survival in ENU-treated Apcdel/+ mice was 117 days vs 255 days for no ENU treatment (P < .0001). Similarly, the median survival in ENU-treated Egr1+/−, Apcdel/+ mice was 114 days vs 179 days (P < .0001), and 101 days vs 144 days (P < .0001) in ENU-treated Tp53+/−, Apcdel/+ mice as compared with mice with no ENU treatment. In addition, ENU treatment of triple heterozygous mice (Egr1+/−, Tp53+/−, Apc del/+) also accelerated anemia development (median survival was 97 days vs 178 days; P < .0001), and did not appear to alter the phenotype.
ENU-treated mice with Apc haploinsufficiency develop a fatal macrocytic anemia at an accelerated pace, regardless of Egr1 or Tp53 expression. (A) Kaplan-Meier survival curve of Apcdel/+mice, or mice crossed with Egr1+/−; Tp53+/−; or Egr1+/− and Tp53+/−. Mice were treated with 3 doses of pI-pC at 2 months of age, and ENU, where indicated, at 3 months. All mice treated with ENU died at a significantly faster rate (P < .0001). (B) CBC are shown for ENU-treated Apcfl/+and Apcdel/+mice or Apcdel/+mice crossed with Egr1+/−(E), Tp53+/− (P), or Egr1+/−, Tp53+/− (EP). CBCs of Apcdel/+ mice were taken at the time of sacrifice, when the mice were severely anemic and moribund. Blood counts of ENU-treated Apcfl/+ control mice were taken during a similar timeframe, but mice were not moribund. MO, monocytes; PLT, platelets; WBC, white blood cells.
ENU-treated mice with Apc haploinsufficiency develop a fatal macrocytic anemia at an accelerated pace, regardless of Egr1 or Tp53 expression. (A) Kaplan-Meier survival curve of Apcdel/+mice, or mice crossed with Egr1+/−; Tp53+/−; or Egr1+/− and Tp53+/−. Mice were treated with 3 doses of pI-pC at 2 months of age, and ENU, where indicated, at 3 months. All mice treated with ENU died at a significantly faster rate (P < .0001). (B) CBC are shown for ENU-treated Apcfl/+and Apcdel/+mice or Apcdel/+mice crossed with Egr1+/−(E), Tp53+/− (P), or Egr1+/−, Tp53+/− (EP). CBCs of Apcdel/+ mice were taken at the time of sacrifice, when the mice were severely anemic and moribund. Blood counts of ENU-treated Apcfl/+ control mice were taken during a similar timeframe, but mice were not moribund. MO, monocytes; PLT, platelets; WBC, white blood cells.
To examine why ENU-treated mouse cohorts with Apc haploinsufficiency died of a rapid and fatal anemia, we initially evaluated the hematologic parameters. The RBC and Hb count were significantly decreased and the MCV and RDW were significantly increased in all ENU-treated mouse cohorts with Apc haploinsufficiency compared with Apcfl/+ (Apc-WT) controls bled at a similar time point (Figure 5B). There was also a significantly increased monocyte count, consistent with the development of monocytosis. There were 2/15 Egr1+/−, Tp53+/−, Apc del/+, and 1/16 Egr1+/−, Apc del/+ mice that developed T lymphoma in addition to the anemia. All ENU-treated Apcdel/+ cohorts displayed splenomegaly with a decreased percentage of CD71−Ter119+ mature erythroid cells, and an increased proportion of CD71+Ter119+ erythroblasts, corresponding to a block at the basophilic erythroblast stage (supplemental Figure 3). These data suggest that all ENU-treated, Apcdel/+ cohorts develop a macrocytic anemia with monocytosis that is similar to non-ENU treated, Apcdel/+ cohorts; however, they develop this phenotype at an accelerated pace, regardless of the loss of Egr1 or Tp53 expression.
Apcdel/+-induced fatal anemia is cell extrinsic
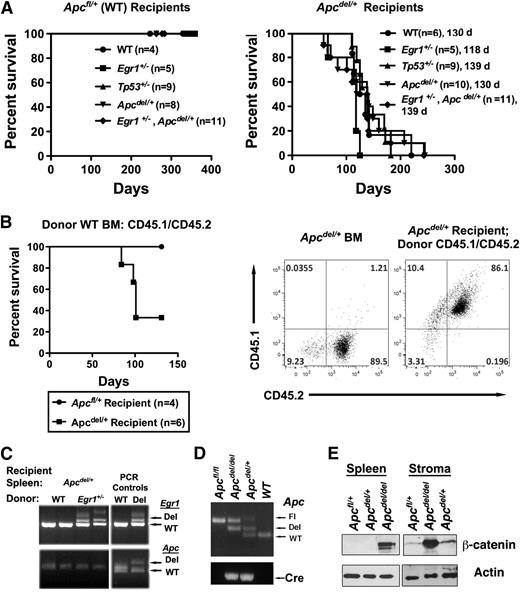
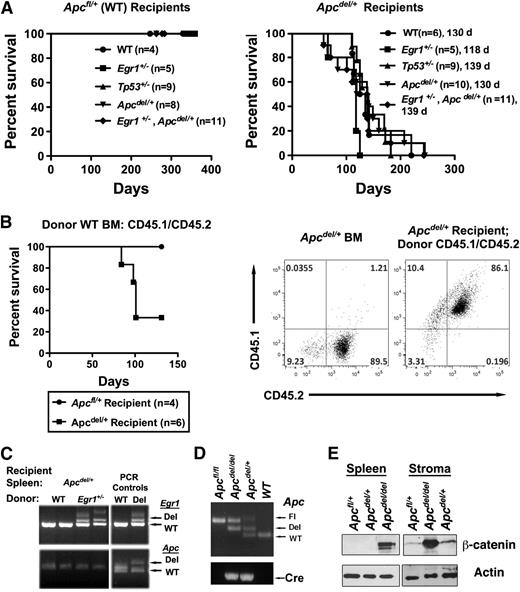
Studies with the Apcmin mice have suggested that cell extrinsic factors may contribute to the development of myeloid disease.32 To determine whether the fatal anemia in Apcdel/+ mice was cell intrinsic, we transplanted WT; Egr1+/−; Tp53+/−, Apcdel/+; or Egr1+/−, Apcdel/+ BM into lethally irradiated Apcdel/+ recipients or Apcfl/+ control recipients that are WT for Apc. Transplantation of BM cells into Apcdel/+ recipients resulted in a rapid development of anemia about 4 months after transplantation, regardless of the donor cell genotype. In contrast, transplantation into Apcfl/+ (Apc-WT) or Egr1+/− recipients (not shown) did not result in disease (Figure 6A and supplemental Figure 4). We confirmed that altered hematopoiesis occurred in donor cells, as Apcdel/+ recipient hematopoietic stem and progenitor cells are not present in recipient mice after transplantation of CD45.1/CD45.2 WT donor BM cells into Apcdel/+ (CD45.2) recipients (Figure 6B). We also showed by using PCR that the splenocytes isolated from anemic recipients were donor-derived and WT for Apc, as anticipated (Figure 6C). Together, this suggests that the fatal macrocytic anemia is induced by an Apc-haploinsufficient BM microenvironment. Supporting this conclusion, we confirmed that stromal cells isolated from Apcdel/+ mice undergo deletion of 1 Apc allele (Figure 6D). Our initial studies support a relationship between Apc dose and β-catenin levels in stroma, whereas, in splenic cells we observed β-catenin protein expression after deletion of both Apc alleles, but not after haploinsufficient loss of Apc (ie, only in Apcdel/del cells), we observed a moderate (∼1.8-fold) and a strong (∼9 fold) increase in β-catenin protein expression in Apcdel/+ and Apcdel/del stromal cells, respectively (Figure 6E).
The fatal anemia is induced by an Apc-haploinsufficient BM microenvironment (A) Kaplan-Meier survival curve of Apcfl/+ (WT) or Apcdel/+ recipients transplanted with WT, Egr1+/; Tp53+/−; Apcdel/+; or Egr1+/−, Apcdel/+BM cells. Median survival for Apcdel/+recipients was 130, 118, 139, 130, and 139 days, respectively. All Apcfl/+and Apcdel/+mice were treated with pIpC at 2 months, and recipients were lethally irradiated and transplanted 4 weeks postinduction. (B) Kaplan-Meier survival curve of Apcfl/+ (WT) or Apcdel/+ recipients transplanted with Apc-WT (CD45.1/CD45.2) BM cells. A representative dot plot shows that Apcdel/+ BM are CD45.2+, whereas Apcdel/+ mice are reconstituted with CD45.1+ CD45.2+ BM cells. (C) PCR analysis of splenocytes isolated from Apcdel/+ recipients transplanted with WT or Egr1+/− BM cells. Analysis of Egr1 and Apc clearly indicates that splenocytes are donor-derived. A PCR control on the right indicates expected sizes of deleted and WT bands. (D) Analysis of deletion of Apc, as determined by PCR analysis of DNA from stromal cells: Apcfl/fl (Control), Apcdel/del(Apc-null), Apcdel/+(Apc-het), and WT. In the sample shown, a PCR product from the floxed Apc allele was still visible in Apcdel/del and Apcdel/+ samples, in addition to the anticipated deleted and WT bands, suggesting that deletion in stromal cells may not always occur in every cell. This is in contrast to hematopoietic cells, where the deletion occurs in all cells.10 (E) Western blot analysis of extracts from spleen cells and stromal cell cultures using β-catenin antibody and actin as a loading control. Apc deletion had occurred in virtually all cells in the stromal cells used for this analysis (not shown).
The fatal anemia is induced by an Apc-haploinsufficient BM microenvironment (A) Kaplan-Meier survival curve of Apcfl/+ (WT) or Apcdel/+ recipients transplanted with WT, Egr1+/; Tp53+/−; Apcdel/+; or Egr1+/−, Apcdel/+BM cells. Median survival for Apcdel/+recipients was 130, 118, 139, 130, and 139 days, respectively. All Apcfl/+and Apcdel/+mice were treated with pIpC at 2 months, and recipients were lethally irradiated and transplanted 4 weeks postinduction. (B) Kaplan-Meier survival curve of Apcfl/+ (WT) or Apcdel/+ recipients transplanted with Apc-WT (CD45.1/CD45.2) BM cells. A representative dot plot shows that Apcdel/+ BM are CD45.2+, whereas Apcdel/+ mice are reconstituted with CD45.1+ CD45.2+ BM cells. (C) PCR analysis of splenocytes isolated from Apcdel/+ recipients transplanted with WT or Egr1+/− BM cells. Analysis of Egr1 and Apc clearly indicates that splenocytes are donor-derived. A PCR control on the right indicates expected sizes of deleted and WT bands. (D) Analysis of deletion of Apc, as determined by PCR analysis of DNA from stromal cells: Apcfl/fl (Control), Apcdel/del(Apc-null), Apcdel/+(Apc-het), and WT. In the sample shown, a PCR product from the floxed Apc allele was still visible in Apcdel/del and Apcdel/+ samples, in addition to the anticipated deleted and WT bands, suggesting that deletion in stromal cells may not always occur in every cell. This is in contrast to hematopoietic cells, where the deletion occurs in all cells.10 (E) Western blot analysis of extracts from spleen cells and stromal cell cultures using β-catenin antibody and actin as a loading control. Apc deletion had occurred in virtually all cells in the stromal cells used for this analysis (not shown).
Similar to Apcdel/+ mice, all Apcdel/+ recipient mice developed a fatal macrocytic anemia, with an arrest of erythroid maturation at the CD71+Ter119+ (R2) stage (supplemental Figure 5). Lethally irradiated Apcdel/+ recipient mice transplanted with Apcdel/+ BM developed the anemia at a significantly accelerated rate compared with Apcdel/+ mice (median survival of 130 days vs 255 days; P < .0001; comparing Figures 1 and 6), suggesting that alterations of the microenvironment after irradiation accelerated the anemic phenotype. There were 2 exceptions to the macrocytic anemia phenotype: 1 Apcdel/+ recipient that received Tp53+/− donor cells developed an immature lymphoid leukemia with an infiltration of blasts into the liver and lymph node, and another that received Egr1+/− donor cells developed a myeloproliferative disorder with an expansion of immature myeloid cells in the spleen (supplemental Figure 6). This suggests that an Apc haploinsufficient microenvironment may be conducive to developing other hematologic diseases, in addition to a fatal macrocytic anemia.
Effect of ENU on the BM microenvironment
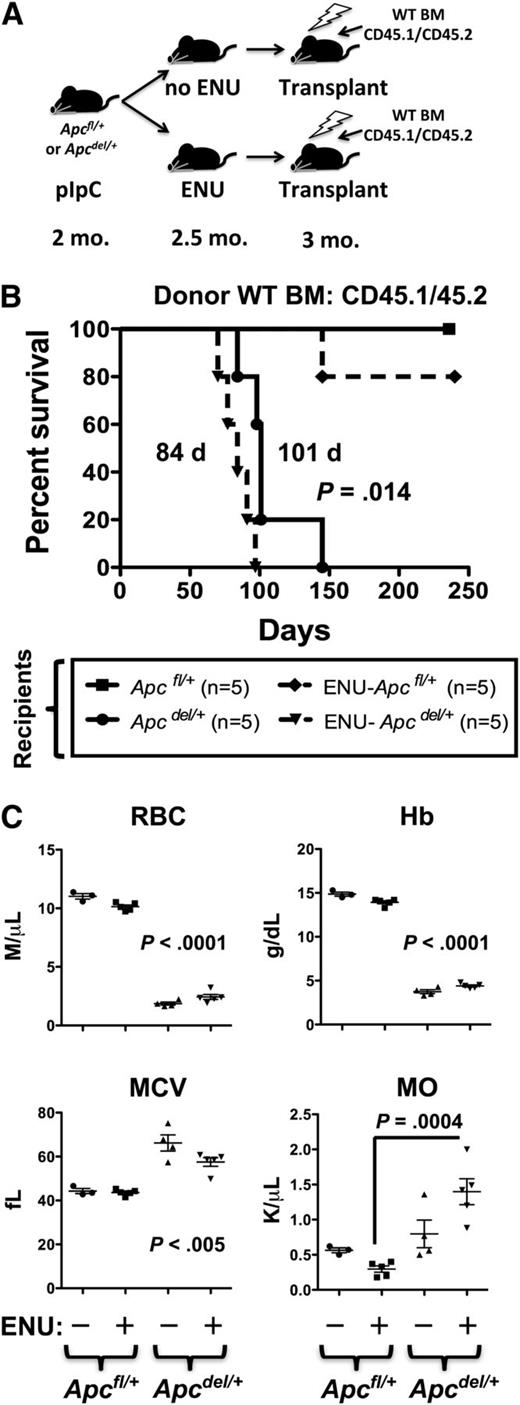
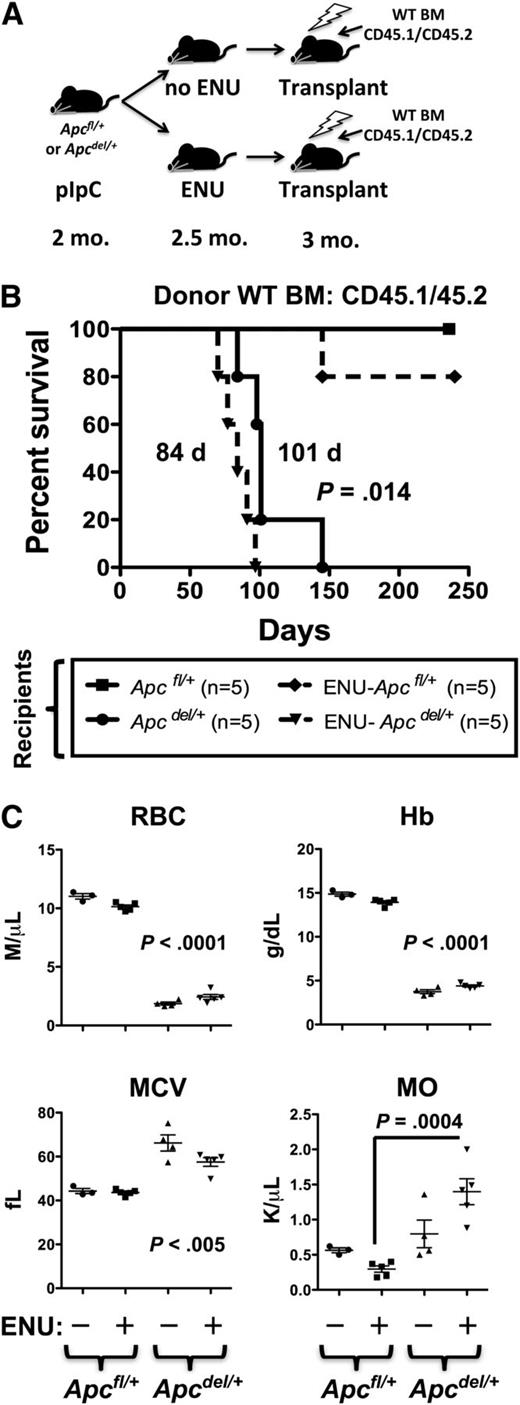
Given that ENU rapidly accelerated the onset of anemia, regardless of Egr1 or Tp53 expression, and that Apcdel/+-induced anemia was accelerated by transplantation into a lethally irradiated Apc haploinsufficient microenvironment, we considered the possibility that alkylating agent therapy and/or radiation may influence early disease progression through alterations to the BM stroma. To examine this possibility more closely, we transplanted CD45.1/CD45.2 WT BM cells into lethally irradiated Apc mice (CD45.2) that had been pretreated with both ENU (to induce mutations) and pIpC (to induce Apc deletion in Apcdel/+, but not Apcfl/+, Cre- control mice) (Figure 7). Interestingly, exposing Apcdel/+ recipients to ENU significantly accelerated the development of the fatal anemia. Median survival was 84 days for ENU-treated Apcdel/+ recipients vs 101 days for non-ENU–treated Apcdel/+ recipients (P = .014). In contrast, only 1 in 5 Apcfl/+ (WT) recipients exposed to ENU developed a T-cell lymphoma (at 145 days) within the timeframe examined (∼224 days). The accelerated rate of disease development in ENU-treated recipient mice was also observed when Tp53+/− donor BM cells were used (112 days for ENU-treated vs 141 days for non-ENU–treated recipients; P = .011) (supplemental Figure 7). To date, none of the ENU-treated Apcfl/+ (WT) recipients of Tp53+/− BM cells have developed disease (at 196 days). Together, these results suggest that exposure of the microenvironment to an alkylating agent increases the pace of disease development, in an Apc haploinsufficient recipient background, and is not influenced by donor cell genotype, under these experimental conditions.
The fatal anemia is slightly accelerated by pretreatment of Apcdel/+recipients with ENU. (A) A model showing that Apcfl/+and Apcdel/+ recipients (CD45.2+) were treated with pIpC at 2 months, ENU at 2.5 months (where indicated), and transplanted 4 weeks post-pIpC treatment with CD45.1/CD45.2 WT BM. (B) Kaplan-Meier survival curve of Apcfl/+ (WT) or Apcdel/+ recipients transplanted with CD45.1/CD45.2 WT BM. Recipients treated with ENU are indicated (dashed line). (C) CBC of untreated and ENU-treated Apcfl/+and Apcdel/+recipient mice. RBC, Hb, and MCV were significantly different for both untreated and ENU-treated Apcdel/+recipient mice compared with Apcfl/+-recipient controls. Monocytosis was significant only for the ENU-treated Apcdel/+ vs Apcfl/+-recipient mice. MO, monocytes.
The fatal anemia is slightly accelerated by pretreatment of Apcdel/+recipients with ENU. (A) A model showing that Apcfl/+and Apcdel/+ recipients (CD45.2+) were treated with pIpC at 2 months, ENU at 2.5 months (where indicated), and transplanted 4 weeks post-pIpC treatment with CD45.1/CD45.2 WT BM. (B) Kaplan-Meier survival curve of Apcfl/+ (WT) or Apcdel/+ recipients transplanted with CD45.1/CD45.2 WT BM. Recipients treated with ENU are indicated (dashed line). (C) CBC of untreated and ENU-treated Apcfl/+and Apcdel/+recipient mice. RBC, Hb, and MCV were significantly different for both untreated and ENU-treated Apcdel/+recipient mice compared with Apcfl/+-recipient controls. Monocytosis was significant only for the ENU-treated Apcdel/+ vs Apcfl/+-recipient mice. MO, monocytes.
Discussion
Therapy-related myeloid neoplasia is a complex disease; in ∼40% of cases, the disease involves loss of genetic material from the long arm of 1 chromosome 5 homolog, presumably leading to the disruption of multiple signaling networks. A prevalent hypothesis is that chemotherapy and/or radiation treatment, administered for the primary malignant disease, promotes the acquisition of recurring chromosomal abnormalities, such as del(5q) or −7/del(7q), in hematopoietic stem cells, ultimately leading to acquisition of cooperating mutations, such as TP53 mutations, and resulting in the expansion of clonal populations. In addition to the loss of 5q genes in hematopoietic stem and progenitor cells, emerging evidence in MDS and AML with myelodysplasia-related changes suggests that a permissive BM microenvironment may be required for frank malignancy to develop.33 For example, in patients with MDS with a del(5q), the beneficial effects of lenalidomide is associated with a substantial improvement of the hematopoietic supporting potential of the BM stroma.34 In this paper, we developed a genetically modified mouse model recapitulating some of the molecular defects that we hypothesize are key to the development of t-MN with a del(5q), namely haploinsufficiency of EGR1, APC, and TP53.
Apc haploinsufficient mice manifest a number of features of the early stages of t-MN, including anemia characterized by anisopoikilocytosis, macrocytosis, and an increased MCV.10 Herein, we demonstrated that Apc haploinsufficiency in mice, combined with Egr1 and/or Tp53 haploinsufficiency accelerated disease development without altering disease phenotype. These results suggest that loss of 1 copy of Egr1 or Tp53 functioned as an enhancer of the Apc haploinsufficient phenotype, possibly by introducing cross-talk with additional signaling pathways or enhancing a common pathway. Interestingly, the disease was not further exacerbated by the combined heterozygous loss of Egr1 and Tp53, suggesting that the loss of Egr1 or Tp53 may act redundantly, possibly eliciting similar molecular pathways, at least with respect to the anemia phenotype in this mouse model.
Although our genetic crosses of Egr1, Apc, and Tp53 triple heterozygous mice demonstrated cooperation between pathways induced by haploinsufficient expression, they did not address whether cooperation was occurring only within hematopoietic cells, in which loss of 5q genes is known to occur in t-MN patients. We were also interested in the effects on nonhematopoietic cells, as emerging evidence suggests that the BM microenvironment plays a crucial role in the development of myeloid neoplasms. For example, the results of characterization of Apcmin mice have suggested that haploinsufficient loss of Apc may alter the BM microenvironment and contribute to the MDS phenotype.32 To determine whether the observed cooperation between Egr1 and Apc, or Tp53 and Apc, occurred within hematopoietic or progenitor cells vs BM stromal cells, or resulted from a cooperation between these components of the BM niche, we performed transplantation studies. Regardless of the genotype of the donor cells, the Apcdel/+ recipients developed a fatal macrocytic anemia at a significantly faster rate than did the conditional Apcdel/+ mice (P < .0001). We speculate that irradiation of recipient mice may have been responsible for the faster rate of anemia development, as ionizing radiation induces multiple changes in the BM stromal cell compartment.35-37 For example, irradiated mesenchymal stromal cells have a high DNA double-strand break repair capacity, allowing them to survive radiation treatment and produce factors, such as tumor necrosis factor-α, transforming growth factor-β, and IL-6, cytokines known to influence the development of MDS and AML.38,39
Because MDS patients with a del(5q) have a loss of EGR1 and APC in hematopoietic cells, we also tested whether transplantation of Apcdel/+or Egr+/−, Apcdel/+ BM cells into WT recipients (Apcfl/+) caused a deleterious phenotype; however, none of these mice showed defects in erythropoiesis or red blood cell production or other hematopoietic defects (supplementary Figure 5). This suggests that the effects we observed were predominantly due to deregulation of Apc expression in the BM microenvironment. At first glance, the link between our mouse model and t-MN may not be apparent, as there is no strong evidence that del(5q) genes are deleted in mesenchymal stromal cells in patients. However, there is emerging evidence that WNT signaling, of which APC is a major regulator, is disrupted in BM stromal cells from patients with MDS or AML. Kode et al19 showed that 15 of 53 patients with MDS or AML, chosen at random, showed nuclear localization of β-catenin in stromal osteoblasts. Of note, 12 of these 15 patients (80%) had abnormalities of chromosomes 5 and/or 7, characteristic features of the largest genetic subgroup of t-MN patients. Consistent with the hypothesis that aberrant WNT signaling in the BM microenvironment may play a role in MDS progression, we observed an Apc dose-dependent accumulation of β-catenin in stromal cells isolated from Apc haploinsufficient or null mice (Figure 6E).
A seminal finding in our study points to the potential deleterious effects of cytotoxic therapy, particularly alkylating agents, on the BM microenvironment of patients who develop t-MN. First, we noted a rapid acceleration of the disease phenotype in ENU-treated, conditional Apc haploinsufficient mice, regardless of the germline loss of Egr1 or Tp53 in HSCs and BM stromal cells (Figure 5). Second, transplantation of BM cells from these ENU-treated Apcdel/+ mice engrafted WT recipients, but did not result in disease up to 260 days posttransplant (data not shown). Third, we showed that exposure of the BM microenvironment to ENU increased the pace of macrocytic anemia development in an Apc haploinsufficient, but not a WT, background (Figure 7 and supplementary Figure 7). Together, our data and the literature supports the hypothesis that radiation and/or chemotherapy that t-MN patients receive for their primary malignant disease leads to a deregulation of signaling networks within the BM microenvironment, possibly through a deregulation of the WNT pathway.
In the context of this current mouse model, the impact of Egr1 and Tp53 haploinsufficiency on the hematopoietic cells, stromal cells, or both is more complex. At first glance, the transplantation data supports the hypothesis that loss of Egr1 or Tp53 in the stromal cells is enhancing the effects of Apc haploinsufficiency within the microenvironment. However, it is equally plausible that the effects of radiation, needed for the transplantation process, in itself has particularly strong effects on an Apc haploinsufficient microenvironment that masks a more subtle cooperation between Egr1+/− or Tp53+/− in hematopoietic cells with Apc haploinsufficiency in stromal cells. An additional possibility that we cannot rule out at this time, is a cooperation between loss of Egr1, Tp53, and Apc in hematopoietic cells. In this regard, we have found that knock-down of Tp53 (using short hairpin RNA) in Egr1, Apc double heterozygous hematopoietic cells, when transplanted into a WT background, leads to a low frequency of myeloid leukemia (data not shown). In summary, our data point to the importance of considering the effects that radiation and/or alkylating agent therapy has not only on hematopoietic cells, but also on the microenvironment. Moreover, our data raise the possibility that loss of genetic material in hematopoietic cells, combined with the disruption of key signaling pathways within the BM niche, may synergize in the pathogenesis of myeloid disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Zhijian Qian, Rachel Bergerson, Kenan Onel, James Downing, Scott Lowe, Scott Kogan, Kevin Shannon, David Largaespada, and Richard Larson for helpful discussions, and Elizabeth M. Davis for technical assistance.
This work was supported by National Institutes of Health, National Cancer Institute (P01 CA40046) (M.M.L.B.), and the Integrated Microscopy and Cytometry and Antibody Technologies Core Facilities of the Comprehensive Cancer Center.
Authorship
Contribution: A.S. designed and supervised research, analyzed data, and wrote the manuscript; A.S., J.W., and A.A.F. performed experiments; J.A. analyzed histology data; T.K. assisted with the statistical analysis and reviewed the manuscript; and M.M.L.B. designed and supervised research and co-wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angela Stoddart, Section of Hematology/Oncology, University of Chicago, 900 E 57th St, KCBD 7th floor, Chicago, IL 60637; e-mail: astoddar@bsd.uchicago.edu.
References
Author notes
A.S. and J.W. contributed equally to this study.