Key Points
Selective myeloma cell killing and enhanced effector function of a novel anti-BCMA antibody conjugated with MMAF via noncleavable linker.
Specific multiple myeloma antigen for monoclonal antibody-based immunotherapy.
Abstract
B-cell maturation antigen (BCMA), highly expressed on malignant plasma cells in human multiple myeloma (MM), has not been effectively targeted with therapeutic monoclonal antibodies. We here show that BCMA is universally expressed on the MM cell surface and determine specific anti-MM activity of J6M0-mcMMAF (GSK2857916), a novel humanized and afucosylated antagonistic anti-BCMA antibody-drug conjugate via a noncleavable linker. J6M0-mcMMAF specifically blocks cell growth via G2/M arrest and induces caspase 3–dependent apoptosis in MM cells, alone and in coculture with bone marrow stromal cells or various effector cells. It strongly inhibits colony formation by MM cells while sparing surrounding BCMA-negative normal cells. J6M0-mcMMAF significantly induces effector cell-mediated lysis against allogeneic or autologous patient MM cells, with increased potency and efficacy compared with the wild-type J6M0 without Fc enhancement. The antibody-dependent cell-mediated cytotoxicity and apoptotic activity of J6M0-mcMMAF is further enhanced by lenalidomide. Importantly, J6M0-mcMMAF rapidly eliminates myeloma cells in subcutaneous and disseminated mouse models, and mice remain tumor-free up to 3.5 months. Furthermore, J6M0-mcMMAF recruits macrophages and mediates antibody-dependent cellular phagocytosis of MM cells. Together, these results demonstrate that GSK2857916 has potent and selective anti-MM activities via multiple cytotoxic mechanisms, providing a promising next-generation immunotherapeutic in this cancer.
Introduction
Although there is no monoclonal antibody (mAb)–based targeted therapy approved to treat patients with multiple myeloma (MM), many mAbs targeting different antigens have been preclinically and clinically evaluated.1,2 For example, following promising preclinical results of elotuzumab targeting CS1,3,4 encouraging activity was subsequently reported in derived clinical trials when combined with lenalidomide/dexamethasone or bortezomib.2,5 Another mAb currently in phase 1/2 clinical development for MM, daratumumab targeting CD38,6 shows an acceptable safety profile with signs of single-agent activity in refractory MM.7 A phase 1 clinical trial of Milatuzumab (CD74) demonstrated stable disease but no responses, supporting further study of this mAb in combination with other anti-MM drugs.8 Several antibody-drug conjugate (ADC) molecules with classical or novel drug payloads to directly kill MM cells without effector-mediated activity (ie, CD56-maytansinoid [DM1; Lorvotuzumab/IMGN901],9 CD138-DM1/DM4 [BT062],10,11 CD74-doxorubicin [IMMU-110]12 ) were either moved toward or remain in clinical development based on encouraging results from preclinical studies. However, these antigens still lack specificity and are also expressed in other normal tissues including natural killer (NK) or other effectors, which could limit their clinical utility. Therefore, novel therapeutic mAbs to achieve improved MM selectivity, simultaneously targeting cytotoxic drugs to MM cells, are urgently needed.
B-cell maturation antigen (BCMA), a member of the tumor necrosis factor receptor superfamily (TNFRSF17), is selectively induced during plasma cell differentiation and is nearly absent on naive and memory B cells.13,14 Upon binding to its ligands B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), the survival of bone marrow (BM) plasma cells and plasmablasts is promoted.15,16 BCMA does not maintain normal B-cell homeostasis, but is required for the survival of long-lived plasma cells.17 In MM, BCMA messenger RNA (mRNA) is commonly expressed at high levels in malignant plasma cells.18-20 Using chromatin immunoprecipitation in the KMS12 MM cell line, BCMA is coimmunoprecipitated with interferon regulatory factor 4 (IRF-4), a master transcription factor mediating myeloma cell survival, indicating BCMA as a direct IRF4 target.21 Elevated serum BCMA in MM patients further correlates with disease status, response to therapy, and overall survival.22 Also, BAFF and APRIL, predominantly produced by osteoclasts in the BM microenvironment, were detected at increased levels in the circulation of MM patients and further stimulate MM cell growth and survival.20,23-26 These results define an active BAFF/APRIL-BCMA axis in the pathophysiology of MM. Additionally, MM patients in remission with graft-versus-tumor response post–allogenic stem cell transplantation developed BCMA antibodies that may contribute to tumor cell lysis in vivo.27 Most recently, adoptive transfer of anti-BCMA–chimeric antigen receptor–transduced T cells binds and kills MM cells.28,29 In-depth analysis of gene and protein expression further demonstrated lack of BCMA expression in other normal tissues.29 However, to date, there are no clinical trials targeting BCMA with mAb-based therapeutics for MM.
We have developed a novel, afucosylated, and humanized antagonistic anti-BCMA IgG1 mAb. The afucosylation significantly increases the binding affinity of the Fc domain of this anti-BCMA mAb to the FcγR (FcγRIIIa) expressed on effector cells, thereby enhancing the antibody-dependent cell-mediated cytotoxicity (ADCC) activity of the molecule. Conjugation of this anti-BCMA mAb J6M0 to the potent microtubule disrupting monomethyl auristatin E (MMAE) or F (MMAF) with protease cleavable valine-citrulline (vc) or uncleavable maleimidocaproyl (mc) linkers30,31 was next evaluated in MM cells, alone and in the BM microenvironment in vitro and in vivo. We found that J6M0-mcMMAF (GSK2857916) not only specifically inhibits MM cell viability but also induces superior effector cell-mediated autologous patient MM cell lysis. Importantly, it rapidly eliminates MM tumors in subcutaneous and disseminated MM models. Moreover, it induces macrophage-mediated phagocytosis of MM cells. Therefore, J6M0-mcMMAF directly and indirectly targets MM cells via multiple mechanisms of action while sparing nearby BCMA-negative BM stromal cells (BMSCs) and effector cells, providing the preclinical rationale for clinical trials of this next-generation immunotherapeutic to treat MM and other BCMA-expressing B-cell malignancies.
Materials and methods
Information concerning cell culture and patient sample, real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR), nuclear factor κB (NFκB) signaling, Meso Scale Discovery analysis, flow cytometry, cytotoxicity assays, ADCC assay, xenograft, and disseminated models for human MM, immunohistochemistry, and statistics is provided in the supplemental Methods (available on the Blood Web site). Patient MM and normal donor samples were obtained after informed consent was provided, in accordance with the Declaration of Helsinki and under the auspices of a Dana-Farber Cancer Institute Institutional Review Board approved protocol. All animal studies were ethically reviewed and carried out in accordance with Animals (Scientific Procedures) Act 1986 and the GlaxoSmithKline (GSK) policy on the care, welfare, and treatment of animals.
Generation of anti-BCMA antibody J6M0 and derived ADC
The murine clone 118G03 and humanized anti-BCMA antibody J6M0 with defucosylated Fc were generated and purified by GSK, Biopharm Research & Development. ADCs of J6M0 were generated by conjugating to either vc (protease cleavable linker) MMAE or mc (noncleavable) MMAF (Seattle Genetics Technology).31 ADC concentration was determined by UV spectroscopy. Antibody-drug conjugates were run on reverse-phase high-performance liquid chromatography (HPLC) to determine the percentage of unlabeled antibody and the drug to antibody conjugation ratio. ADCs were also run by size exclusion chromatography (SEC)–HPLC to determine the percentage of antibody aggregation. Endotoxin testing was performed by kinetic chromogenic Limulus Amebocyte Lysate method and only endotoxin-negative ADCs (<0.2 EU/mg) were used. All ADCs were diluted in phosphate-buffered saline (PBS).
Results
BCMA expression is highly restricted in patient MM cells and novel anti-BCMA mAbs bind BCMA on the cell membrane
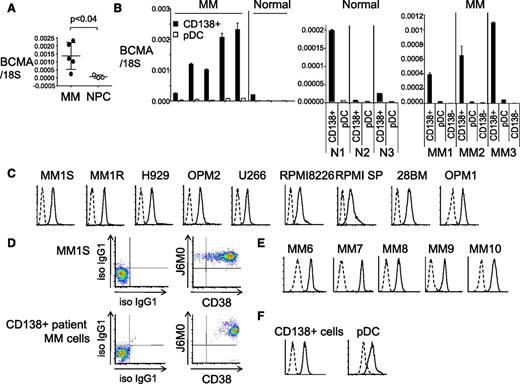
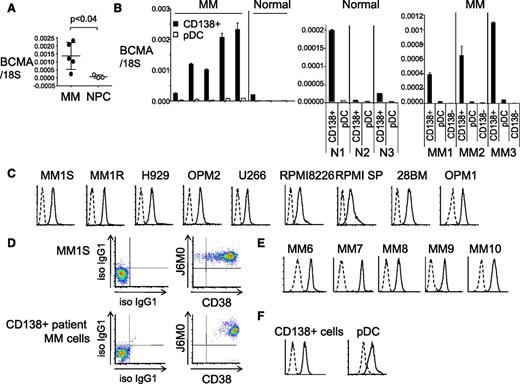
Using real-time qRT-PCR, we first showed that BCMA mRNA was upregulated in CD138-purified plasma cells from MM patients (n = 5) compared with normal donors (n = 3) (Figure 1A, P < .04), consistent with high and restrictive BCMA expression in plasma cells but not other normal tissues and their derived tumors by gene expression profiling and immunohistochemistry (IHC) (supplemental Figure 1A-B; Carpenter et al29 ). We further found that plasmacytoid dendritic cells (pDCs; CD138−/BDCA-4+), which support MM cell growth and survival in the BM microenvironment,32 have detectable BCMA mRNA at significantly (ninefold to 50-fold) lower levels than CD138+ plasma cells (P < .005 for each paired sample) from either MM patients or normal donors (Figure 1B; supplemental Figure 1C). As seen in CD138+ plasma cells, BCMA transcript is increased in pDCs from MM patients vs normal donors (P < .03, supplemental Figure 1C). In contrast, BCMA is nearly undetectable in CD138-negative cells from BM aspirates of MM patients (Figure 1B).
BCMA is specifically and highly expressed on the cell membrane of patient MM cells. (A) BCMA mRNA was quantitated by real-time qRT-PCR in CD138+ cells from 5 MM patients and 3 normal donors (NPC). 18S is an internal control for normalization. P < .04 (B) BCMA mRNA was measured in paired CD138+ and pDCs from MM patients and normal donors (N1-3) (left and middle panel), as well as matched CD138+ cells, pDCs, and CD138-negative (CD138−) cells from 3 MM patients (MM1-3, right panel). (C) MM cell lines were immunostained with a novel humanized anti-BCMA J6M0 mAb (solid line) or isotype control human IgG (dashed line). (D) Dual staining by J6M0-PerCP-Cy5.5 and CD38-PE Cy7 was done in MM1S and CD138+ patient MM cells. Immunostaining with J6M0 mAb (solid line) or isotype control human IgG (iso IgG1, dashed line) was performed in CD138+ cells from additional MM patients (E), as well as paired CD138+ cells and pDCs from a MM patient (F). qRT-PCR, quantitative RT-PCR.
BCMA is specifically and highly expressed on the cell membrane of patient MM cells. (A) BCMA mRNA was quantitated by real-time qRT-PCR in CD138+ cells from 5 MM patients and 3 normal donors (NPC). 18S is an internal control for normalization. P < .04 (B) BCMA mRNA was measured in paired CD138+ and pDCs from MM patients and normal donors (N1-3) (left and middle panel), as well as matched CD138+ cells, pDCs, and CD138-negative (CD138−) cells from 3 MM patients (MM1-3, right panel). (C) MM cell lines were immunostained with a novel humanized anti-BCMA J6M0 mAb (solid line) or isotype control human IgG (dashed line). (D) Dual staining by J6M0-PerCP-Cy5.5 and CD38-PE Cy7 was done in MM1S and CD138+ patient MM cells. Immunostaining with J6M0 mAb (solid line) or isotype control human IgG (iso IgG1, dashed line) was performed in CD138+ cells from additional MM patients (E), as well as paired CD138+ cells and pDCs from a MM patient (F). qRT-PCR, quantitative RT-PCR.
Flow cytometry analysis showed that an anti-BCMA mAb (clone 118G03) bound to the cell membrane of MM lines (n = 12), CD138+ patient MM cells (n = 12), and pDCs from MM patients (n = 3) (supplemental Figure 1D). Importantly, the humanized and afucosylated anti-BCMA mAb J6M0 also bound to all CD138+ MM cell lines and patient MM cells (Figure 1C-F). Dual CD38 and BCMA membrane expression was confirmed in CD138+ patient MM cells. J6M0 mAb, like the murine antibody (118G03), also bound to pDCs, although with lesser mean fluorescence intensity (MFI) when compared with CD138+ cells from the same patient (Figure 1F; supplemental Figure 1D). Thus, BCMA membrane expression is universally detected by the novel anti-BCMA J6M0 mAb in MM cell lines and CD138+ patient MM cells.
J6M0-mcMMAF specifically blocks MM cell proliferation and colony formation via induction of G2/M arrest and induction of apoptosis
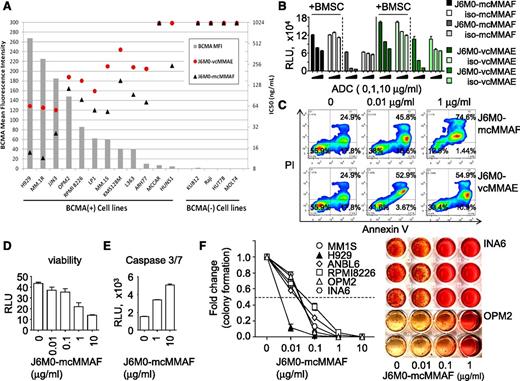
We next determined direct and selective cytotoxicity of J6M0 ADCs (J6M0-mcMMAF and -vcMMAE) against MM cells. J6M0 competes for binding to BCMA with its 2 ligands BAFF and APRIL, and inhibits ligand-induced NFκB signaling with an half maximal inhibitory concentration (IC50) of 0.91 and 2.43 μg/mL, respectively (supplemental Figure 2). J6M0-mcMMAF (GSK2857916) binds to human BCMA protein with a Kd of ∼1nM, as measured by surface plasmon resonance (SPR) at 25°C, but does not bind to related receptors BAFF-R or transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) (data not shown).
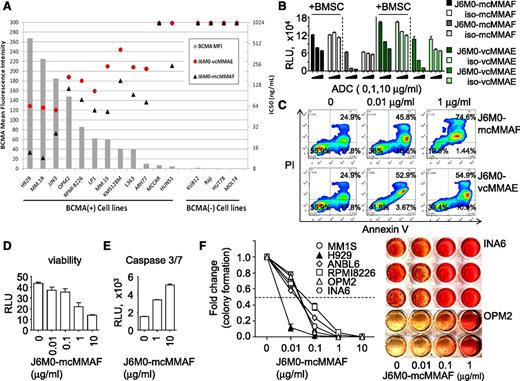
BCMA cell-surface protein expression for each cell line was compared with the IC50 of J6M0 ADCs in each cell line to determine whether BCMA expression level correlates with drug activity (Figure 2A). BCMA cell-surface expression was undetectable in 4 non-MM cell lines with no BCMA mRNA, confirming that J6M0 binding is BCMA-specific. The IC50 for J6M0-mcMMAF was lower than those of J6M0-vcMMAE for all cell lines, ranging from 11.5 ng/mL to >1000 ng/mL and 55.8 ng/mL to >1000 ng/mL for J6M0-mcMMAF and J6M0-vcMMAE, respectively. The highest BCMA-expressing cell lines (NCI-H929, MM.1R, and JJN3) were most sensitive to both J6M0 ADCs and the lowest expressing cell lines (MC/CAR and HuNS1) were least sensitive. Neither J6M0 ADCs induced toxicities against 4 BCMA-negative cells. Isotype IgG1 control ADCs iso-mcMMAF and iso-vcMMAE also showed no significant activity in any of these 16 cell lines (data not shown). Thus, both J6M0-ADCs block MM cell growth specifically dependent on BCMA.
J6M0-mcMMAF selectively inhibits MM cell viability and colony formation via caspase 3/7-dependent apoptosis. (A) J6M0 ADCs (-mcMMAF or -vcMMAE) were added in the culture for 6 days followed by luminescence-based viability assays. Gray bars represent the normalized mean fluorescence intensity of BCMA expression in each cell line. Black triangles and red circles represent the growth inhibitory IC50 for J6M0-mcMMAF and J6M0-vcMMAE, respectively, calculated from triplicate measurements. (B) ANBL6 cells were treated for 3 days with J6M0-mcMMAF/J6M0-vcMMAE, or iso-mcMMAF/iso-vcMMAE (isotype control ADCs), in the presence of absence of BMSCs. (C) CD138+ patient MM cells were incubated for 3 days with indicated ADCs followed by annexin V/PI staining and flow cytometry analysis. CD138+ cells from additional MM patients were treated with J6M0-mcMMAF for 3 days, followed by viability (D) and caspase 3/7 activity (E) assays. (F) J6M0-mcMMAF was added to MM cell culture in methylcellulose for 21 days to determine effects on colony formation. Representative colony formation of INA6 and OPM2 cells are shown on the right.
J6M0-mcMMAF selectively inhibits MM cell viability and colony formation via caspase 3/7-dependent apoptosis. (A) J6M0 ADCs (-mcMMAF or -vcMMAE) were added in the culture for 6 days followed by luminescence-based viability assays. Gray bars represent the normalized mean fluorescence intensity of BCMA expression in each cell line. Black triangles and red circles represent the growth inhibitory IC50 for J6M0-mcMMAF and J6M0-vcMMAE, respectively, calculated from triplicate measurements. (B) ANBL6 cells were treated for 3 days with J6M0-mcMMAF/J6M0-vcMMAE, or iso-mcMMAF/iso-vcMMAE (isotype control ADCs), in the presence of absence of BMSCs. (C) CD138+ patient MM cells were incubated for 3 days with indicated ADCs followed by annexin V/PI staining and flow cytometry analysis. CD138+ cells from additional MM patients were treated with J6M0-mcMMAF for 3 days, followed by viability (D) and caspase 3/7 activity (E) assays. (F) J6M0-mcMMAF was added to MM cell culture in methylcellulose for 21 days to determine effects on colony formation. Representative colony formation of INA6 and OPM2 cells are shown on the right.
J6M0-mcMMAF, also more potently than J6M0-vcMMAE, induced cytotoxicity against ANBL6 (Figure 2B) and MM1S cells cocultured with BMSCs (supplemental Figure 3A). In addition, control iso-mcMMAF, but not iso-vcMMAE, had no toxicity against ANBL6 MM cells, regardless of the presence of BMSCs (Figure 2B). These data confirmed that J6M0-mcMMAF conjugates, once rapidly internalized, are cleaved and exclusively retained inside target cells, which minimizes toxin/drug leaking to nearby BCMA-negative cells. These data are consistent with previously published studies with the other mcMMAF ADC targeting CD70.30,31,33-35 Both ADCs induced apoptosis of CD138+ patient MM cells following 3 days’ treatment, and greater dose-dependent killing was seen by J6M0-mcMMAF than J6M0-vcMMAE (Figure 2C). Percentages of annexin V/propidium iodide (PI) cells combined were 45.8% ± 0.5% and 74.5% ± 1.2% at 0.01 μg/mL and 1 μg/mL J6M0-mcMMAF, respectively. We therefore focused subsequent studies on J6M0-mcMMAF (GSK2857916).
Importantly, 3-day treatment with J6M0-mcMMAF decreased viability and induced caspase 3/7 activity in CD138+ cells from 2 representative MM patients (Figure 2D-E). In a 3-week colony formation assay in methylcellulose, J6M0-mcMMAF produced more potent cytotoxicity against MM cell lines (Figure 2F; supplemental Figure 3C) as compared with short-term 2-day or 3-day luminescent viability assays (supplemental Figure 3A-B). IC50 (6-70 ng/mL vs 45-750 ng/mL) were ∼eightfold to 10-fold lower in colony formation than cell viability assays. Further cell-cycle analysis by flow cytometry showed that J6M0-mcMMAF induced dose- and time-dependent G2/M arrest in MM cells (supplemental Figure 3D-E). Therefore, J6M0-mcMMAF blocks MM cell growth and survival via induction of G2/M cell-cycle arrest followed by apoptosis.
J6M0-mcMMAF selectively induces anti-MM toxicity in the cocultures while sparing BMSCs and various effector cells
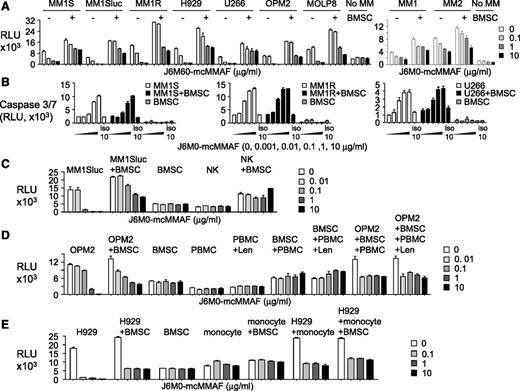
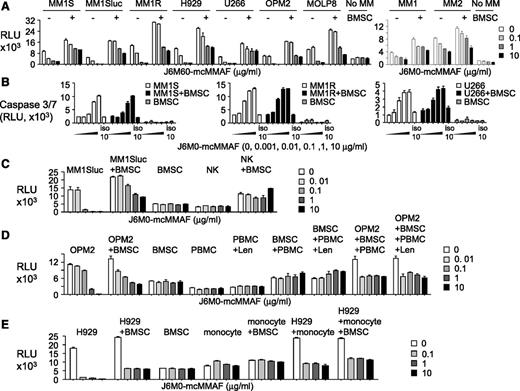
Because the BM microenvironment confers MM cell growth and resistance to current treatment, we next investigated effectiveness of J6M0-mcMMAF on MM cells in the cocultures with BMSCs or other BM accessory cells. Three-day treatment with J6M0-mcMMAF blocked BMSC-increased growth and viability in MM cell lines (n = 7) and patient MM cells (n = 2) in a dose-dependent fashion, as seen in MM cells cultured alone (Figure 3A). Neither IL-6 nor pDC overcame J6M0-mcMMAF–inhibited MM cell viability (supplemental Figure 4A-B). In contrast, J6M0-mcMMAF did not alter viability of BMSCs derived from multiple samples. Specifically, J6M0-mcMMAF, but not iso-mcMMAF, activated caspase 3/7 in MM cells, alone and cocultured with BMSCs (Figure 3B). It did not induce caspase 8 activity in the same cell cultures (supplemental Figure 4C). As expected, it did not activate any caspases in BMSCs alone.
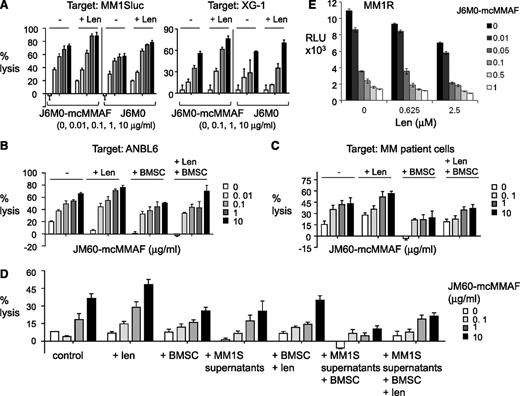
J6M0-mcMMAF broadly inhibits MM cell growth and survival without targeting BMSCs and effector cells. (A) Various MM cell lines (left panel) and patient MM cells (right panel) were cultured for 3 days, alone or with BMSCs, in the presence of J6M0-mcMMAF followed by luminescent viability assays. (B) Three MM cell lines were treated with serial dilutions of J6M0-mcMMAF or 10 μg/mL iso-mcMMAF (iso 10) overnight followed by caspase 3/7 activity assays. (C) NK cells were treated with J6M0-mcMMAF for 4 days, alone or with BMSCs. MM1Sluc cells, alone or with BMSCs, were included to show differential toxicity. (D) PBMCs, pretreated with or without lenalidomide (Len, 2μM), were treated for 3 days with J6M0-mcMMAF with or without BMSCs and in the presence or absence of OPM2 cells. (E) Monocytes (CD14+) were treated with J6M0-mcMMAF for 4 days, in the presence or absence of BMSCs or H929 MM cells.
J6M0-mcMMAF broadly inhibits MM cell growth and survival without targeting BMSCs and effector cells. (A) Various MM cell lines (left panel) and patient MM cells (right panel) were cultured for 3 days, alone or with BMSCs, in the presence of J6M0-mcMMAF followed by luminescent viability assays. (B) Three MM cell lines were treated with serial dilutions of J6M0-mcMMAF or 10 μg/mL iso-mcMMAF (iso 10) overnight followed by caspase 3/7 activity assays. (C) NK cells were treated with J6M0-mcMMAF for 4 days, alone or with BMSCs. MM1Sluc cells, alone or with BMSCs, were included to show differential toxicity. (D) PBMCs, pretreated with or without lenalidomide (Len, 2μM), were treated for 3 days with J6M0-mcMMAF with or without BMSCs and in the presence or absence of OPM2 cells. (E) Monocytes (CD14+) were treated with J6M0-mcMMAF for 4 days, in the presence or absence of BMSCs or H929 MM cells.
Importantly, 4-day J6M0-mcMMAF treatment did not affect viability of NK, BMSCs, and coculture of NK with BMSCs (Figure 3C), while 0.1 μg/mL of this ADC completely blocked cell viability of MM1Sluc, alone and with BMSCs. J6M0-mcMMAF consistently showed no cytotoxicity against peripheral blood mononucleated cell (PBMC), pretreated with or without lenalidomide for 5 days and alone or in coculture with BMSCs, conditions at which significant toxicities against OPM2 MM cells were seen (Figure 3D). Similarly, toxicity was not observed in monocytes (CD14+), alone or with BMSCs (Figure 3E), but was restricted to H929 MM cells. Thus, J6M0-mcMMAF has minimal bystander killing on surrounding BCMA-negative BMSCs and various effector cells, whereas it selectively and strongly blocks viability of MM cells.
J6M0-mcMMAF greatly enhances potency and efficacy of effector cell-mediated MM cell lysis
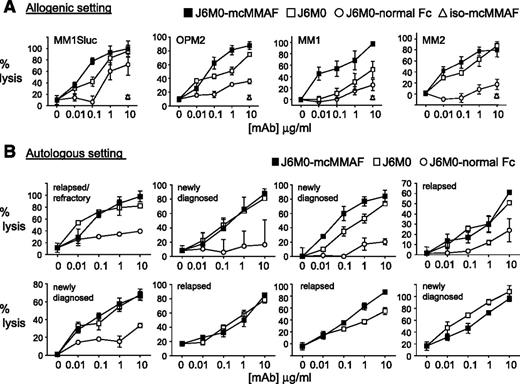
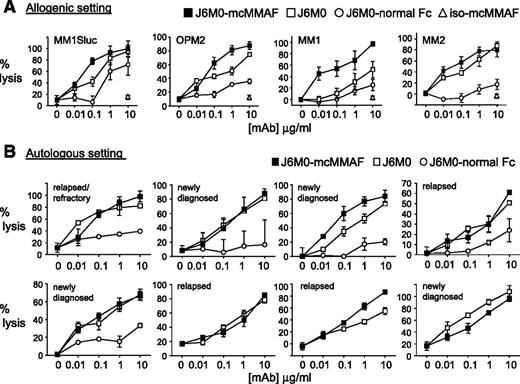
Because J6M0-mcMMAF and naked J6M0 have defucosylated Fc, we next determined whether increased binding of Fc-bearing effector cells (NK or PBMC) translated to greater antibody-dependent MM cell lysis. In calcein-AM–based ADCC assays, J6M0-mcMMAF triggered NK-mediated cell lysis against MM cell lines (n = 9) (half maximal effective concentration range, 5-25 ng/mL for all cell lines except 85.63 ng/mL and 62.31 ng/mL for XG-1 and 28BM), while iso-mcMMAF did not induce any ADCC (supplemental Figure 5A). Significantly, J6M0-mcMMAF and J6M0 enhanced cell lysis against all MM cell lines and patient cells assayed, when compared with J6M0 with normal Fc (J6M0-normal Fc) (Figure 4A; supplemental Figure 5B). Significantly enhanced potency by J6M0-mcMMAF or J6M0 was even prominent against MM cells which are relatively resistant to ADCC: maximal lysis by J6M0-mcMMAF and J6M0 reached 85% to 100% (Figure 4B). J6M0-mcMMAF slightly increased cell lysis against MM cell lines and patient cells compared with J6M0, due to direct cytotoxicity against MM cells induced by J6M0-mcMMAF but not J6M0. Importantly, such augmented ADCC induced by J6M0-mcMMAF (and J6M0) vs J6M0-normal Fc was evident when autologous patient MM cells were assayed (Figure 4B, n = 5), regardless of disease status. Consistently, J6M0-mcMMAF and J6M0 markedly enhanced maximal autologous MM cell lysis. Thus, J6M0-mcMMAF and J6M0 significantly improved potency (lower half maximal effective concentration) and efficacy (superior maximal lysis) of ADCC against MM cells.
J6M0-mcMMAF significantly improved potency and efficacy of ADCC against autologous MM cells. (A) Target cells (MM1Sluc, OPM2, and patient MM cells MM1-2) were labeled with calcein-AM, washed, and incubated with indicated mAbs and NK effector cells in triplicates. Percent lysis was calculated at the end of ADCC assay, based on calcein-AM release. (B) Autologous ADCC activity against CD138+ patient cells was determined using PBMCs or CD138-negative cells from the same MM patient at an E:T ratio of 20:1. All data represent mean percent lysis ± standard deviation.
J6M0-mcMMAF significantly improved potency and efficacy of ADCC against autologous MM cells. (A) Target cells (MM1Sluc, OPM2, and patient MM cells MM1-2) were labeled with calcein-AM, washed, and incubated with indicated mAbs and NK effector cells in triplicates. Percent lysis was calculated at the end of ADCC assay, based on calcein-AM release. (B) Autologous ADCC activity against CD138+ patient cells was determined using PBMCs or CD138-negative cells from the same MM patient at an E:T ratio of 20:1. All data represent mean percent lysis ± standard deviation.
Lenalidomide further enhances J6M0-mcMMAF–induced direct and indirect killing of MM cells
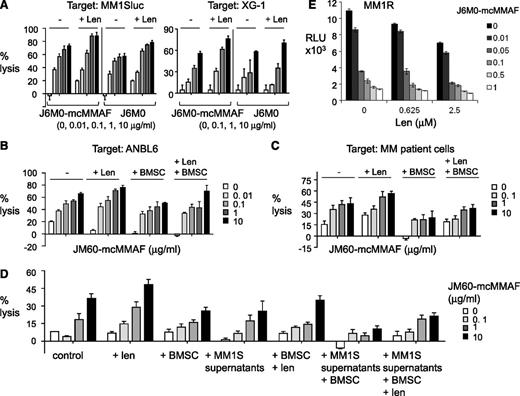
To investigate the potential enhancement by immunomodulatory drug lenalidomide of J6M0-mcMMAF (or J6M0)–induced MM cell lysis, PBMC was preincubated with lenalidomide (Len) prior conducting ADCC assays. Len-pretreated PBMCs enhanced ADCC of J6M0-mcMMAF or J6M0 against MM1Sluc and XG-1 cells (Figure 5A). Len-enhanced J6M0-mcMMAF–induced ADCC was also observed when ANBL6 cells or patient MM cells were targeted with patient PBMC effectors even in the presence of BMSCs (Figure 5B-C). Because soluble BCMA was detected in MM cell culture supernatants and patient sera, ADCC assays were next conducted in MM cell culture supernatants, with or without BMSCs. J6M0-mcMMAF still induced significant MM1S cell lysis in the presence of MM1S culture supernatant (Figure 5D). Pretreatment of PBMC effectors with Len still enhanced cell lysis by J6M0-mcMMAF against MM1S cells cocultured with BMSCs and in the presence of MM1S supernatant. Furthermore, Len augmented direct toxicities induced by J6M0-mcMMAF in MM1R cells (dex-resistant) (Figure 5E).
Lenalidomide further enhances J6M0-mcMMAF–induced MM cell lysis. PBMCs were preincubated with or without Len (2 μM) before adding into calcein-AM–based ADCC assay with J6M0-mcMMAF or J6M0. Target cells included MM1Sluc, XG-1 (A), ANBL6 (B), CD138+ patient MM cells (C), and MM1S cells (D) in the presence or absence of BMSCs. J6M0-mcMMAF–induced ADCC against MM1S cells was also assayed in the supernatant from MM1S cell culture (D). (E) MM1R cells were treated with each drug, alone or together, for 3 days followed by a luminescent viability assay.
Lenalidomide further enhances J6M0-mcMMAF–induced MM cell lysis. PBMCs were preincubated with or without Len (2 μM) before adding into calcein-AM–based ADCC assay with J6M0-mcMMAF or J6M0. Target cells included MM1Sluc, XG-1 (A), ANBL6 (B), CD138+ patient MM cells (C), and MM1S cells (D) in the presence or absence of BMSCs. J6M0-mcMMAF–induced ADCC against MM1S cells was also assayed in the supernatant from MM1S cell culture (D). (E) MM1R cells were treated with each drug, alone or together, for 3 days followed by a luminescent viability assay.
J6M0-mcMMAF inhibits tumor growth and prolongs survival of mice in xenograft and disseminated SCID models of human MM
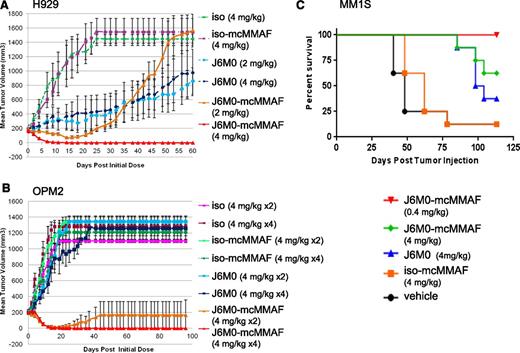
J6M0-mcMMAF was next tested in both H929 and OPM2 subcutaneous mouse xenograft models (Figure 6A-B, n = 5 per group). J6M0-mcMMAF significantly inhibited H929 tumor growth at both 2 mg/kg and 4 mg/kg doses, as compared with iso-mcMMAF control (P < .0001, Figure 6A). The 4 mg/kg dose resulted in complete tumor eradication in all mice for the entire 60-day study whereas tumors regrew after an initial response in all mice receiving 2 mg/kg J6M0-mcMMAF. The unconjugated/naked J6M0 also had significant activity in the H929 xenografts. J6M0-mcMMAF dosed at 4 mg/kg ×4 also resulted in complete tumor eradiation in all mice harboring OPM2 tumor out to 100 days (Figure 6B). As few as 2 doses of J6M0-mcMMAF (4 mg/kg ×2) resulted in complete tumor elimination up to 100 days in 4 of 5 mice treated. In addition, J6M0-mcMMAF treatment significantly reduced tumor volume compared with the equivalent dose of naked J6M0 mAb (P < .001), demonstrating superiority of the ADC in both models.
J6M0-mcMMAF rapidly eradicates MM tumors established in mice and significantly prolongs survival. (A) SCID mice with palpable H929 tumors (∼150 mm3) were randomized into groups (n = 5 each) and then treated twice weekly for 4 total doses by intraperitoneal injection. (B) SCID mice with palpable OPM2 tumors were randomized into groups (n = 5 each) and then treated twice weekly for a total of 2 or 4 doses by intraperitoneal injection. (C) NK-deficient SCID-beige mice were inoculated IV with MM1Sluc cells. At a mean bioluminescence (BLI) of 3E ± 06 indicating MM1Sluc tumor growth, mice were randomized into groups (n = 8 each) and treated with J6M0-mcMMAF, J6M0, iso-mcMMAF, or vehicle (PBS), twice weekly for a total of 9 doses by intraperitoneal injection. Survival of mice was examined using log-rank (Mantel-Cox) analysis. *P < .0002.
J6M0-mcMMAF rapidly eradicates MM tumors established in mice and significantly prolongs survival. (A) SCID mice with palpable H929 tumors (∼150 mm3) were randomized into groups (n = 5 each) and then treated twice weekly for 4 total doses by intraperitoneal injection. (B) SCID mice with palpable OPM2 tumors were randomized into groups (n = 5 each) and then treated twice weekly for a total of 2 or 4 doses by intraperitoneal injection. (C) NK-deficient SCID-beige mice were inoculated IV with MM1Sluc cells. At a mean bioluminescence (BLI) of 3E ± 06 indicating MM1Sluc tumor growth, mice were randomized into groups (n = 8 each) and treated with J6M0-mcMMAF, J6M0, iso-mcMMAF, or vehicle (PBS), twice weekly for a total of 9 doses by intraperitoneal injection. Survival of mice was examined using log-rank (Mantel-Cox) analysis. *P < .0002.
The in vivo anti-MM activity of J6M0-mcMMAF was further examined in a SCID-beige orthotopic MM model with establishment of diffuse MM bone lesions mimicking human disease.36 J6M0-mcMMAF rapidly abolished detectable tumor burden in mice after only 2 doses at 0.4 mg/kg and 4 mg/kg (supplemental Figure 6A). Tumor burden in J6M0-mcMMAF–treated mice stayed below baseline levels throughout the study and mice remained tumor-free for 3.5 month follow-up, resulting in significantly prolonged survival (P < .0001, Figure 6C) with no weight loss (supplemental Figure 6B). There were no significant differences in tumor growth and survival between the vehicle and iso-mcMMAF (P = .08 for survival). All other groups showed significantly diminished tumor growth and extended survival vs 2 control groups. Naked J6M0 mAb is less effective than J6M0-mcMMAF but still significantly blocked tumor growth and prolonged survival (P = .0002 for J6M0 vs vehicle or iso-mcMMAF; P = .0004 for J6M0 vs J6M0-mcMMAF). Median survival is 101, 62, and 48 days for J6M0-, iso-mcMMAF-, and vehicle-treated groups, respectively (Figure 6C).
J6M0-mcMMAF further confers anti-MM activity via macrophage-mediated phagocytosis
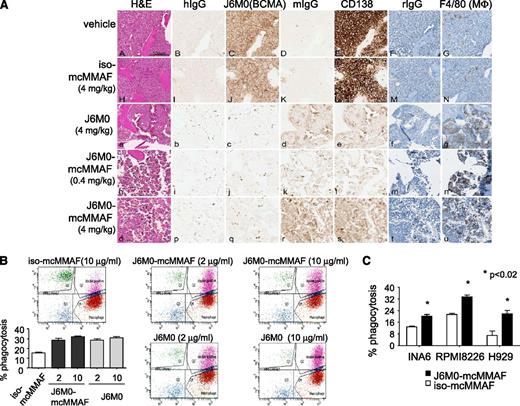
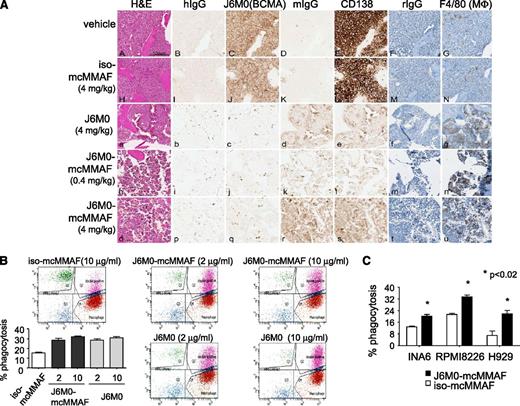
IHC studies for BCMA and CD138 on long bones from mice postsacrifice verified the anti-MM effect seen with in vivo luminescent imaging (Figure 7A; supplemental Figure 7). Treatment with J6M0-mcMMAF or J6M0 significantly increased macrophage infiltration, as evidenced by much greater F4/80 staining surrounding MM1Sluc tumor remains. Because SCID-beige mice lack NK cells, these results indicate a potential involvement of FcγR-expressing monocytes or macrophages in J6M0-mcMMAF– and J6M0-induced anti-MM activity in vivo.
J6M0-mcMMAF promotes phagocytosis of MM cells by macrophages. (A) Long bone tissue sections from vehicle (A-G), iso-mcMMAF (4 mg/kg) (H-N), J6M0 (4 mg/kg) (a-g), J6M0-mcMMAF (0.4 mg/kg) (h-n), and J6M0-mcMMAF (4 mg/kg) (o-u) treated animals stained by H&E (A,H,a,h,o) and by IHC with human IgG (hIgG: B,I,b,i,p), J6M0 (C,J,c,j,q for BCMA), murine IgG (mIgG: D,K,d,k,r), murine anti-human CD138 antibody (E,L,e,l,s), rat IgG, and hematoxylin counterstain (rIgG: F,M,f,m,t), or F4/80 and hematoxylin counterstain (G,N,g,n,u for MΦ). Scale bar = 100 µm. ADCP at 4 hours was determined by flow cytometry using PKH67-labeled MM cells (B, MM1S; C, INA6, RPMI8226, H929) as targets and monocyte-derived macrophages as effector cells at an E:T ratio of 4:1. Experiments were done in triplicates. Percent phagocytosis was measured as the number of dual-positive (PKH67+CD11b+) cells divided by the total number of PKH67+ cells. Data shown are mean ± standard deviation from 2 independent experiments. *P < .02.
J6M0-mcMMAF promotes phagocytosis of MM cells by macrophages. (A) Long bone tissue sections from vehicle (A-G), iso-mcMMAF (4 mg/kg) (H-N), J6M0 (4 mg/kg) (a-g), J6M0-mcMMAF (0.4 mg/kg) (h-n), and J6M0-mcMMAF (4 mg/kg) (o-u) treated animals stained by H&E (A,H,a,h,o) and by IHC with human IgG (hIgG: B,I,b,i,p), J6M0 (C,J,c,j,q for BCMA), murine IgG (mIgG: D,K,d,k,r), murine anti-human CD138 antibody (E,L,e,l,s), rat IgG, and hematoxylin counterstain (rIgG: F,M,f,m,t), or F4/80 and hematoxylin counterstain (G,N,g,n,u for MΦ). Scale bar = 100 µm. ADCP at 4 hours was determined by flow cytometry using PKH67-labeled MM cells (B, MM1S; C, INA6, RPMI8226, H929) as targets and monocyte-derived macrophages as effector cells at an E:T ratio of 4:1. Experiments were done in triplicates. Percent phagocytosis was measured as the number of dual-positive (PKH67+CD11b+) cells divided by the total number of PKH67+ cells. Data shown are mean ± standard deviation from 2 independent experiments. *P < .02.
We finally investigated whether J6M0-mcMMAF induced macrophage-mediated phagocytosis of MM cells. Using human macrophages derived from macrophage colony-stimulating factor (M-CSF)–stimulated monocytes and MM cell lines (n = 4) at an E:T ratio of 4:1 in antibody-dependent cellular-mediated phagocytosis (ADCP) assays, J6M0-mcMMAF (or J6M0) significantly promoted phagocytosis of MM cells (P < .02; Figure 7B-C). In contrast, iso-mcMMAF did not increase macrophage phagocytic activity above baseline levels.
Discussion
Prior clinical experiences with a variety of therapeutic mAbs for MM indicated that these mAbs were safe with good tolerability, but lacked activity as a single agent. The first mAb starting to show direct anti-MM effect is daratuzumab which is currently being tested in several clinical trials. However, this antibody could also target a large number of CD38+ normal cells, especially activated lymphocytes and CD34+ hematopoietic progenitor cells. Thus, it is urgent to develop therapeutic mAbs targeting more specific and highly expressed MM antigens. BCMA is highly expressed in normal and malignant plasma cells and the novel anti-BCMA mAb J6M0 investigated here selectively binds to BCMA receptor on the surface of these cells while simultaneously recruiting immune effector cells and thereby effectively destroying tumor cells. By further conjugating this novel afucosylated anti-BCMA mAb to the cytotoxic drug MMAF via an uncleavable mc linker, J6M0-mcMMAF directly inhibits patient MM cell viability and augments BCMA-specific tumor cell lysis via ADCC. It further recruits macrophages to produce ADCP of MM cells. This is the first therapeutic ADC for MM and with 3 distinct mechanisms of action against a very specific MM antigen.
Recently, an anti-BCMA SG1 with triple Fc mutations (S293D:A330L:I332E) which significantly increase binding for FcγRIIIa and FcγRIIa also showed greater potency for ADCC, compared with the normal Fc antibody.37 However, point mutations in the Fc could potentially result in undesired effects such as increased immunogenicity or decreased stability. In contrast, removal of fucose does not affect mAb stability and is unlikely to change the immunogenicity in vivo.38 Defucosylation of Fc glycans could also circumvent the inhibitory effects both of plasma IgG on the binding of FcγRIIIa on NK cells and of fucosylated counterparts on the binding of the antigen on target cells.39-41 Importantly, J6M0-mcMMAF and naked J6M0 significantly enhanced ADCC in vitro and anti-MM activity in vivo, when compared with its fucosylated normal Fc version. To our knowledge, J6M0-mcMMAF would be the first therapeutic ADC with defucosylated oligosaccharides entering clinical trials in MM.
In our current investigation, both J6M0-mcMMAF and naked J6M0 equivalently exhibit increased (>1 log) potency and have superior efficacy compared with the J6M0-normal Fc analog in inducing ADCC against patient MM cells from multiple samples. In contrast, SG1 was neither evaluated against primary patient MM cells, nor in settings with primary patient MM cells and effector cells derived from the same patients. Furthermore, no nonspecific growth inhibition of iso-mcMMAF was seen, whereas iso-vcMMAE slightly inhibited viability against MM cells, alone and cocultured with BMSCs. Because minor amounts of vcMMAE would still be cleaved extracellularly even before J6M0-vcMMAE is bound and internalized into the target MM cells, the toxicity of residual MMAE to surrounding BCMA-negative proliferative normal cells cannot be completely prohibited. These results suggest that J6M0-mcMMAF would likely have a better therapeutic window than J6M0-vcMMAE or SG1-vcMMAF8, where cleavable linker vc was used to conjugate drugs to anti-BCMA mAb. Indeed, J6M0-mcMMAF did not alter growth and survival of BMSCs, PBMCs, NK, and monocytes, alone and in the cocultures, indicating negligible by-standard killing against BCMA-negative cells that surround MM cells in the BM milieu. Importantly, this minimum in vitro killing of normal nontarget cells by J6M0-mcMMAF was extended to 3 in vivo MM models. Mice in the diffuse MM model were healthy without any sign of toxicities even after a total of 9 doses of drug treatment and at 3.5-month follow-up. These results indicate that free mcMMAF, which is liberated from the dying BCMA-positive human MM cells, is not toxic to surrounding normal cell populations in the BM and elsewhere. Indeed, this is one of the advantages of MMAF as an antibody drug conjugate payload because it is non-cell-permeable as a free drug, unlike MMAE. It should be noted that J6M0-mcMMAF does not bind to murine BCMA so mice are not a suitable species for evaluating the on-target toxicity of the intact J6M0-mcMMAF ADC. The in vivo efficacy of SG1-vcMMAF is unknown because no in vivo study was reported.37 On the other hand, because BCMA is absent in memory B cells, any possible immunosuppressive effects by J6M0-mcMMAF would be likely to be transient and reversible.
BCMA is significantly elevated in patient MM cells as compared with normal plasma cells from healthy donors. Gene expression profiling from published databases also indicate that elevated BCMA levels in malignant vs normal plasma cells is as significant as in the cases of CS1 and CD138, 2 MM antigens recently being targeted with mAb. However, CS1 is expressed in NK cells, the key ADCC mediator for elotuzumab. In addition, elotuzumab cannot directly inhibit MM cell growth, which is likely related to the lack of single-agent activity with this mAb in follow-up clinical trials.2-5 CD138 expression is also found in other normal cells, that is, endothelial cells, renal rubules.42 BT062 (CD138 maytansinoid ADC), which is currently in clinical trial in MM, has bystander effect on surrounding cells and lacks any effector-mediated cytotoxicities.10 Although CD38 expression is higher in patient MM vs normal plasma cells, it is more broadly expressed on progenitor and activated hematopoietic cells. Nonetheless, daratuzumab (a naked anti-CD38 mAb), which induces MM cell death when cross-linked and has ADCC and complement-dependent cytotoxicity activities in vitro, shows encouraging single-agent activity in ongoing clinical trials.7,43 Again, because NK, CD34+ stem cells, and other normal tissues also express CD38, nonspecific toxicities might occur. In contrast, intensive investigations of BCMA mRNA and protein levels confirm selective BCMA expression in plasma cells, suggesting a most favorable therapeutic index.
Interestingly, low BCMA is detected in pDCs, which stimulates MM cell growth, survival, and drug resistance in the BM microenvironment.32 We further show significantly higher BCMA in pDCs derived from MM patients vs normal donors, although BCMA expression level is considerably lower in pDCs than in plasma cells from the same MM patients. J6M0-mcMMAF might therefore also target MM pDCs, which warrants further investigation.
J6M0-mcMMAF potently blocks MM cell growth in 3 different in vivo models and significantly extends overall survival. The efficacy of J6M0-mcMMAF (0.4 mg/kg), as evidenced by prolonged survival, in the disseminated MM model was superior to the effects of bortezomib tested in the same model where MM1S tumors grow and home to the bones in the mice.44 J6M0-mcMMAF–treated MM1S tumor-bearing mice not only extended their lifespan (more than threefold to fivefold), but also showed no weight loss when compared with archived bortezomib-treated groups. After just 2 doses of J6M0-mcMMAF at 0.4 mg/kg (∼20 μg in total), tumor load was completely eliminated and remained undetectable in all mice (8 of 8) for the remainder of the study of 120 days. Although 3 mice (3 of 8) in the 4 mg/kg group died after 84 days without MM1Sluc tumor burden, autopsy suggested that thymic lymphosarcomas appeared to have occurred in these mice. They are prone to develop such tumors which often disseminate. The possibility of myeloma masquerading as lymphosarcoma cannot be completely ruled out, but is unlikely. In addition, Fc-bearing macrophages significantly increased in tumor tissues harvested from both J6M0-mcMMAF– and J6M0-treated mice. Macrophages derived from human monocytes induced phagocytosis of MM cells via J6M0-mcMMAF and J6M0, confirming ADCP as an additional Fc-dependent effector mechanism of J6M0-mcMMAF or J6M0 to target MM.
In summary, J6M0-mcMMAF (GSK2857916) is a highly specific antagonistic anti-BCMA mAb that delivers a potent anti-mitotic drug MMAF to MM cells in vitro and in vivo. Moreover, the enhanced potency and efficacy of J6M0-mcMMAF in inducing ADCC and ADCP will ensure that all MM patients even those with low to moderate BCMA expression are targeted. Rapid and sustained elimination of MM tumors by J6M0-mcMMAF (GSK2857916) in 3 different mice models further supports rapid bench-to-bedside translation of this novel ADC to improve patient outcome in MM.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Daniel Tannenbaum, Komal Sane for technical support. We thank the nursing staff and clinical research coordinators of the LeBow Institute for Myeloma Therapeutics and The Jerome Lipper Multiple Myeloma Center of the Dana-Farber Cancer Institute for support and help in providing primary tumor specimens for this study.
This work was supported by National Institutes of Health Research Project Grant Program grants RO-1 50947, PO1-78378, and the Dana-Farber/Harvard Cancer Center Specialized Program in Research Excellence (DF/HCC SPORE) in Multiple Myeloma P50CA100707.
K.C.A. is an American Cancer Society Clinical Research Professor.
Authorship
Contribution: Y.-T.T., J.C., P.A.M., and K.C.A. conceptualized research and formed the hypothesis of this paper; Y.-T.T., P.A.M., J.C., and A.L.K. designed, performed experiments, and analyzed data; P.A.M., C.A., M.Y.Z., M.C., A.C., J.C., J.Y., L.G., W.F., and J.T. performed the in vitro research and collected data; P.A.M., A.L.C., A.L.K., Y.-T.T., L.G., W.F., B.H. and J.T. designed, performed, and analyzed animal work; P.A.M. and J.T. performed and analyzed IHC studies; P.A.M. and J.C. provided reagents and analytic tools; N.C.M., P.R., and K.C.A. provided MM patient samples; Y.-T.T. wrote the manuscript; and Y.-T.T., P.A.M., and K.C.A. critically evaluated and edited the manuscript.
Conflict-of-interest disclosure: P.A.M., J.C., J.Y., L.G., W.F., B.H., and J.T. are employees of GlaxoSmithKline, whose product was used in this research. P.R. serves on advisory boards to Millennium, Celgene, Novartis, Johnson & Johnson, and Bristol-Myers Squibb. N.C.M. serves on advisory boards to Millennium, Celgene, and Novartis. K.C.A. serves on advisory boards to Onyx, Celgene, Gilead, and Sanofi-Aventis. Y.-T.T. is a consultant to Onyx. The remaining authors declare no competing financial interests.
Correspondence: Yu-Tzu Tai, Department of Medical Oncology, Dana-Farber Cancer Institute, M551, 450 Brookline Ave, Boston, MA 02215; e-mail: yu-tzu_tai@dfci.harvard.edu; and Kenneth C. Anderson, Department of Medical Oncology, Dana-Farber Cancer Institute, M557, 450 Brookline Ave, Boston, MA 02215; e-mail: kenneth_anderson@dfci.harvard.edu.