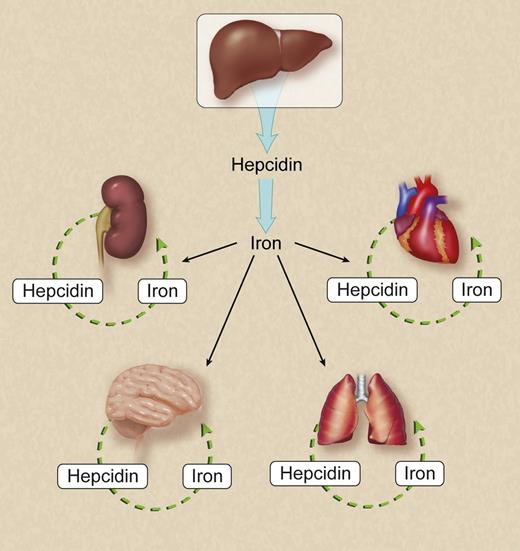
In this issue of Blood, Zumerle et al elegantly demonstrate that hepatocytes are the key site of synthesis of hepcidin, a central regulator of iron homeostasis deficient or aberrantly expressed in hereditary hemochromatosis, β-thalassemia, anemia of inflammation, and other iron-related conditions.1
Hepatocytes are the key source of hepcidin in the regulation of systemic iron homeostasis. Hepcidin synthesized in the kidney, central nervous system, lung, heart and other organs may be essential for regulation of local iron homeostasis but have a negligible effect on systemic iron homeostasis. For simplicity, not all known or putative sources of hepcidin are shown. Professional illustration by Debra T. Dartez.
Hepatocytes are the key source of hepcidin in the regulation of systemic iron homeostasis. Hepcidin synthesized in the kidney, central nervous system, lung, heart and other organs may be essential for regulation of local iron homeostasis but have a negligible effect on systemic iron homeostasis. For simplicity, not all known or putative sources of hepcidin are shown. Professional illustration by Debra T. Dartez.
Hepcidin is a peptide hormone secreted predominantly by the liver.2 Originally presumed to function largely as an antimicrobial factor (hence its full genetic name, hepcidin antimicrobial peptide), hepcidin inhibits absorption of dietary iron and release of iron from macrophages. It accomplishes this by regulating levels of ferroportin, the only known cellular iron export protein identified to date in vertebrates. Ferroportin is expressed on the basolateral cell surface of duodenal enterocytes and the cell surface of macrophages responsible for scavenging iron from red blood cells. Hepcidin binding to ferroportin initiates the internalization and degradation of ferroportin. In this manner, hepcidin limits uptake of iron from the gut and release of iron recycled from red blood cells. Given this critical role of hepcidin in homeostasis of iron, an essential yet potentially toxic nutrient, it is not surprising that hepcidin is regulated by many processes in the body, including iron abundance, inflammation, erythropoietic drive, and hypoxia.
Although hepcidin is synthesized largely by the liver, its expression has been documented in cell types other than hepatocytes, as referenced in the article at hand. Determining the physiologically relevant site of hepcidin synthesis is a critical step in understanding hepcidin’s role in iron biology. By constructing and characterizing mice with hepatocyte-specific hepcidin deficiency, Zumerle et al convincingly demonstrate that hepatocytes are the relevant source of hepcidin synthesis for systemic iron homeostasis (see figure). Their mouse model develops predicted characteristics of hereditary hemochromatosis, a common inherited disease of iron overload attributable to hepcidin deficiency: iron overload in the liver, pancreas, and plasma; iron deficiency in macrophages in the spleen; and increased ferroportin expression in the duodenum and spleen. What is particularly striking about their mouse model is that serum hepcidin levels are undetectable, indicating that cell types other than hepatocytes contribute negligibly to circulating hepcidin levels.
Zumerle et al’s finding that hepcidin synthesis by other cell types cannot compensate for deficiency of hepatocyte-derived hepcidin does not exclude the possibility that hepcidin derived from cells other than hepatocytes plays a role in local iron homeostasis. For example, the potential role of locally derived hepcidin in modulating cardiac iron biology may be clinically relevant given that cardiac dysfunction is a leading cause of mortality in patients with β-thalassemias, a group of inherited anemias characterized by inappropriately low hepcidin levels and iron loading in the heart and other organs.3 The potential role of brain-derived hepcidin in regulating distribution of iron to and within the brain may also be relevant given that aberrant brain iron homeostasis has been strongly implicated in neurodegenerative diseases such as Alzheimer disease and Parkinson disease.4 The use of the mouse model established by Zumerle et al to generate mice with tissue-specific hepcidin deficiencies now permits investigators to ask targeted questions about the relevance of hepcidin synthesized in other organs. For example, does hepcidin synthesized in the heart play an essential role in baseline cardiac physiology or the response of mice to cardiac iron overload, hypoxia, and inflammation? What effect does hepcidin deficiency in neurons and other resident cell types have on iron transport across the blood-brain barrier and the choroid plexus–cerebrospinal fluid barrier or on distribution of iron to specific regions within the central nervous system?
Zumerle et al’s report also has therapeutic implications. If the liver is the origin of systemically active hepcidin, then targeting hepcidin expression by the liver should be sufficient to impact systemic iron homeostasis. Indeed, pharmacologic antagonism of TMPRSS6, a physiological inhibitor of hepcidin expression present mainly in the liver, markedly attenuates the disease characteristics of mouse models of hereditary hemochromatosis and β-thalassemia.5,6 Other therapeutic means of modulating hepcidin expression are actively being explored, including hepcidin agonists and antagonists. Results of their application to models of diseases of systemic hepcidin deficiency or excess are encouraging.7 Although these novel potential treatments for iron-related diseases are tremendously exciting, such advances all originated when fundamental, mechanism-based questions about physiological processes underlying iron biology were asked and addressed by researchers such as Zumerle et al.
Conflict-of-interest disclosure: The author declares no competing financial interests.