Abstract
Hematopoiesis in general is demand driven and adaptive, but in contrast to erythropoiesis or thrombocytopoiesis, our knowledge on how neutrophil production is adapted to individual needs remains incomplete. Recently, neutrophil homeostasis has been shown to depend on danger receptors, macrophages, and even circadian rhythms. Puzzle pieces for a broader view of neutrophil homeostasis accumulate, and we will herein try to put seemingly contradictory evidence in a perspective of neutrophil homeostasis and emergency granulopoiesis determined by innate immunologic signaling.
Introduction
Disorders of peripheral neutrophil counts are a common medical problem. Chemotherapy-induced neutropenia predisposes to infectious complications, and febrile neutropenia is still an unwanted effect of cytotoxic treatment. Conversely, increased white blood cell levels are known to correlate with all-cause and cardiovascular mortality in epidemiologic studies of apparently healthy individuals.1
Human recombinant granulocyte colony-stimulating factor (G-CSF) has been introduced to the clinic almost 20 years ago. Despite its widespread use in the clinic, and although the importance of neutrophils has been understood decades ago, there is still no universally accepted hypothesis of how neutrophil production is regulated to meet individual needs. Recent evidence points to a role of the innate immune system in sensing and regulating granulopoiesis.
G-CSF and its upstream humoral regulators
Soon after the discovery of G-CSF and its reciprocal correlation with neutrophil counts, a sensor (or “neutrostat”) was postulated that would regulate G-CSF levels to adapt granulopoiesis to prevailing needs in both health and disease (ie, infection).2 However, genetically modified mouse models showed that granulopoiesis was reliable even in the absence of myeloid cytokines: G-CSF–deficient mice still harbored between 20% and 30% of normal neutrophil numbers. The term “emergency granulopoiesis” has been coined to denote the massive rise in neutrophils following acute infection in mouse models such as cecal ligation and puncture or parenteral application of bacteria or fungi. Interestingly, G-CSF and granulocyte-macrophage colony-stimulating factor double-deficient mice are capable of mounting an emergency granulopoiesis response in respective experiments. This observation led to the concept of emergency granulopoiesis being a G-CSF–independent process.3 Reciprocal correlation of neutrophil numbers and G-CSF concentrations was not found in adhesion-molecule–deficient mice, whose myeloid cells cannot transmigrate into peripheral tissues; these animals display peripheral blood neutrophilia and increased G-CSF. This phenomenon was explained by disinhibited, macrophage (MΦ)- or dendritic cell (DC)-dependent negative feedback: peripheral MΦs/DCs engulf apoptotic neutrophils, which decreases their secretion of interleukin-23 (IL-23), thereby reducing the production of IL-17 by innate lymphoid cells, natural killer T cells, γδ-T cells, or TH17 cells. Finally, reduced IL-17 levels account for low G-CSF expression. Thus, the model proposed a turnstile-like neutrostat, where transmigrating neutrophils are quantified by phagocytes, for example in the gastrointestinal tract, and adapt G-CSF accordingly (Figure 1).4 Involvement of the “IL-23–IL-17–G-CSF axis” in the regulation of granulopoiesis was confirmed in several independent murine models,5-7 and an interplay of TH17 cells and neutrophils was also reproducible in humans.8
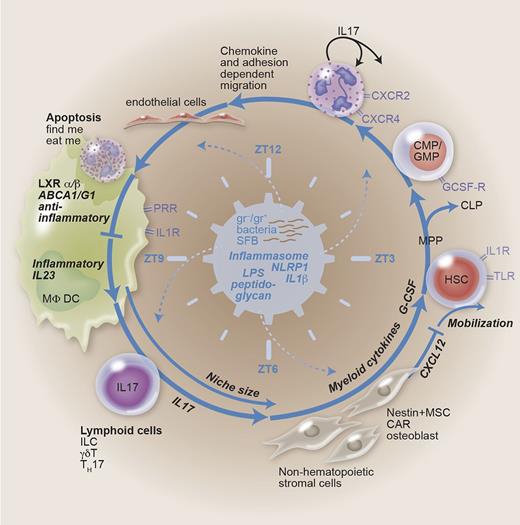
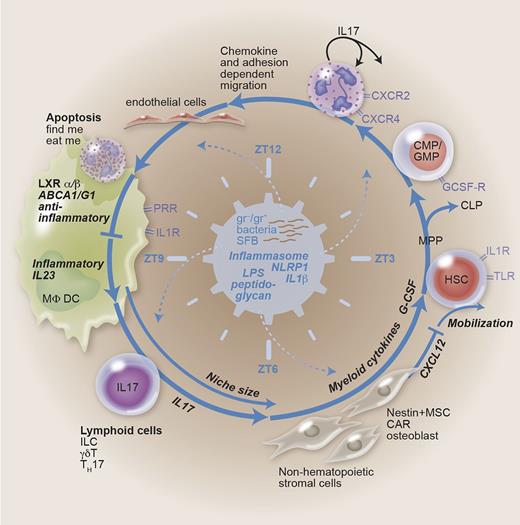
Feedback-loop regulation of granulopoiesis. Granulopoiesis is demand driven in the steady state and in case of emergency. The feedback loop contains HSCs proliferating and differentiating into the myeloid lineage (common myeloid progenitor/granulocyte-monocyte progenitor) at the expense of lymphoid cells. Bone marrow emigration, demargination, and extravasation are dependent on chemokine signaling and adhesion molecules. Engulfment of aged apoptotic neutrophils generates an anti-inflammatory signal. Phagocytes like MΦs and DCs thus generate a negative feedback on granulopoiesis. Disinhibition (lack of neutrophils, enhanced danger signaling) may lead to IL-23, IL-17, and G-CSF secretion. Hematopoiesis is regulated by niche-forming cells, with myeloid cytokines, chemokines, and size as determining factors. The microbiome-generating PAMPs, the inflammasome integrating danger signaling, and circadian rhythms symbolized by zeitgeber time are suggested to represent the central, sine qua non regulators. CAR, CXC ligand 12–abundant reticular cell; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; HSC, hematopoietic stem cell; ILC, innate lymphoid cell; LXR, liver X receptor; MPP, multipotent progenitor; MSC, mesenchymal stem cell; ZT, zeitgeber time.
Feedback-loop regulation of granulopoiesis. Granulopoiesis is demand driven in the steady state and in case of emergency. The feedback loop contains HSCs proliferating and differentiating into the myeloid lineage (common myeloid progenitor/granulocyte-monocyte progenitor) at the expense of lymphoid cells. Bone marrow emigration, demargination, and extravasation are dependent on chemokine signaling and adhesion molecules. Engulfment of aged apoptotic neutrophils generates an anti-inflammatory signal. Phagocytes like MΦs and DCs thus generate a negative feedback on granulopoiesis. Disinhibition (lack of neutrophils, enhanced danger signaling) may lead to IL-23, IL-17, and G-CSF secretion. Hematopoiesis is regulated by niche-forming cells, with myeloid cytokines, chemokines, and size as determining factors. The microbiome-generating PAMPs, the inflammasome integrating danger signaling, and circadian rhythms symbolized by zeitgeber time are suggested to represent the central, sine qua non regulators. CAR, CXC ligand 12–abundant reticular cell; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; HSC, hematopoietic stem cell; ILC, innate lymphoid cell; LXR, liver X receptor; MPP, multipotent progenitor; MSC, mesenchymal stem cell; ZT, zeitgeber time.
Interestingly, there is IL-23–independent stimulation of IL-17 secreted by innate immune cells, which is inducible by inflammatory signals such as IL-1β.9 In addition, because lymphoid cell–derived IL-17–independent feedback regulation of granulopoiesis was observed in several models of neutropenia, there must be significant redundancy in granulopoiesis-stimulating signals.5,10,11 For example, an autocrine feedback loop of IL-17A secreted by a subset of neutrophils during fungal infection was identified recently.12
In the meantime, there is a large body of evidence from studies in mice with dysfunctional or deficient Mϕs/DCs that demonstrates a role for phagocytes in controlling granulopoiesis (reviewed in Bugl et al13 ). What the phagocyte-dependent models of neutrophil regulation have in common is the observation that granulopoiesis increases to an exaggerated level as soon as there is missing negative feedback from phagocytes. This, however, implies that tonic stimulatory signals acting on MΦs/DCs and the hematopoietic marrow constantly trigger granulopoiesis. Upon engulfment of apoptotic neutrophils expressing find-me and eat-me signals,14 MΦs receive anti-inflammatory signals via nuclear peroxisome-proliferator–activated receptor γ and liver X receptor.15 It is tempting to speculate that this signal could represent a counterbalance for the above-mentioned stimulatory signal that constantly drives granulopoiesis.
TLR4- and G-CSF–dependent positive feedback upon neutrophil depletion
Based on the assumption that steady-state neutrophil homeostasis should be a highly conserved and demand-driven process, we analyzed blood and bone marrow of mice after complete peripheral neutrophil depletion utilizing anti-neutrophil antibodies. Upon antibody-induced neutropenia, robust G-CSF–dependent positive feedback was generated on the marrow level including massive expansion of the lin−/Sca-1+/c-kit+ and granulocyte macrophage progenitor compartments at the expense of thrombopoietic and red cell precursors. Interestingly, this feedback signal was also observable in NSG mice, which are devoid of all lymphocyte subsets. These mice did not display concomitant elevations of IL-17 upon induced neutropenia. However, when peripheral blood neutrophils were depleted in Toll-like receptor 4 (TLR4)-deficient mice, they were not able to generate this previously characterized feedback response.5 We concluded from these results that the lipopolysaccharide (LPS) receptor TLR4 was an essential prerequisite of steady-state neutrophil homeostatic regulation.
A plausible candidate stimulator of myelopoiesis is microbial confrontation, and several lessons can be learned from demand-driven emergency granulopoiesis. Infectious dangers are presented to the innate immune system by pathogen-associated molecule patterns (PAMPs) detected by their respective receptors (pathogen recognition receptors [PRRs]). On the one hand, hematopoietic stem cells (HSCs) are equipped not only with cytokine receptors like IL-1R and interferon-R but also with PRRs, including TLRs, and enter the cell cycle upon infections, receiving instructive signals for myeloid differentiation (reviewed in detail in Takizawa et al16 ). Loss of specific signaling pathways does not impair emergency granulopoiesis in sepsis models utilizing MyD88-, TRIF-, interferon-IR–, or IL-1R–deficient mice.17 On the other hand, indirect effects of LPS and other danger signals on nontransplantable and/or niche-forming cell types may be even more important.18,19 Further, direct effects of TLR2 and TLR4 agonists on the migration of mature neutrophils and their function and survival are well described. Of note, microbiota-derived Nod1 ligands systemically induce functional maturation of neutrophils under homeostatic conditions.20
Evidence for an essential role of microbiota: old, new, and hidden
Simple steady-state laboratory values obtained in germ-free–kept mice contribute important additional information on a possible role of microbiota in neutrophil homeostasis. Neutrophil levels in these mice are considerably lower than in G-CSF−/− animals, and their G-CSF levels are extremely low.5 Enteric microbiota were postulated to represent the source of LPS-triggering granulopoiesis even prior to the discovery of pattern-recognition receptors: oral polymyxin, an antibiotic that reduced fecal LPS content to 10% of pretreatment levels, was effective in reducing femoral marrow colony-forming unit granulocyte macrophage in specific-pathogen-free, but not germ-free–kept, mice. Moreover, recovery of granulopoiesis after treatment with Ara-C took longer in antibiotic-treated mice.21,22 Interestingly, very early data in germ-free mice showed reduced colony-stimulating factor activity (which was not further subdivided into lineage-specific growth factors at the time),23 and in vitro data show LPS as a candidate upstream regulator.24
These results suggest that microbiota may indeed represent the major positive signal not only in emergencies but also in the steady state, thereby representing the setpoint device of the neutrostat. Because even germ-free mice were able to generate a positive feedback signal upon neutrophil depletion, intrinsic TLR agonists or minute amounts of LPS contaminants in autoclaved food may be sufficient to maintain permanent positive signals.5 Clinical practice guidelines suggest prophylactic antibiotic gut decontamination with oral antibiotics in neutropenic patients after chemotherapy, which may contribute to prolonged neutropenia. Moreover, chemotherapy or radiotherapy by themselves may induce alterations of the intestinal flora that contribute to delayed neutrophil regeneration. With accumulating evidence of a correlation among neutrophils, nutrition, and intestinal flora, clinical studies of interventions aimed at promoting timely regeneration of neutrophils by modulating the intestinal microflora are warranted.
Recently, with a better understanding of both the adaptive and innate immune system and its cellular counterparts, the mutual influence of immune cells and intestinal microbiota has been described in more detail. IL-17–secreting TH17 cells, for example, are to be found in large numbers in enteric mucosal tissues (intestinal lamina propria) only in the presence of commensal germs, but not in germ-free mice, and to a much lesser degree in animals that receive antibiotics early in life. Specific bacterial components of the microflora, namely segmented filamentous bacteria, were identified to induce TH17 cells in the intestinal lamina propria.25
CXC chemokine ligand receptor 2 (CXCR2) deficiency has been demonstrated to cause mild peripheral blood neutrophilia and markedly increased marrow myeloid hyperplasia. The CXCR2 ligand CXCL5 is expressed on enterocytes, and intestinal-mucosa–derived CXCL5 is essential in attracting CXCR2-positive neutrophils to the intestinal mucosa, thereby inhibiting IL-17 production.6 Thus far, this study supports the above-mentioned turnstile hypothesis.4 Interestingly, however, commensal bacteria were shown to be required for activation of IL-17/G-CSF in CXCR2-deficient mice: treatment of these animals with antibiotics was sufficient to downregulate their increased levels of G-CSF, IL-17, and peripheral blood as well as marrow neutrophils. Germ-free–kept CXCR2−/− mice lose their neutrophil phenotype as well.6
Evidence for circadian rhythms of neutrophil migration and hematopoietic niche size
Circadian variations in neutrophil numbers in humans are well known and correlate with cortisol and G-CSF levels.26,27 Adrenergic signals regulate rhythmic recruitment of leukocytes to tissues.28 Moreover, intrinsic circadian rhythms in the migration of inflammatory Ly6Chi monocytes,29 release of HSCs,30 and inflammatory phenotype of MΦs have previously been described.31
In a recently published, sophisticated analysis of parabiotic mice, CXCR4hi CD62Llo aged circulating neutrophils were found to significantly fluctuate in a circadian rhythm32 with preferential homing and degradation of these aged neutrophils in spleen, liver, and bone marrow, as previously reported.33 Interestingly, the authors observed that aged neutrophils entering the marrow reduce size and function of the hematopoietic niche, ie, CXCL12-abundant reticular cells and Nestin+ cells. Vice versa, antibody-mediated depletion of neutrophils resulted in an expansion of niche cells.32 Of note, homing of aged neutrophils to the bone marrow also synchronized with marrow MΦs and their cholesterol-sensing receptors (liver X receptor), with CXCL12 signaling by niche-forming nonhematopoietic cells, and finally with egress of HSCs.32
In conclusion, the authors showed that rhythmic removal of dying neutrophils dictates oscillating myelopoiesis. However, causes and effects may not be easy to distinguish; it is tempting to speculate that inflammation sensed by nonhematopoietic cells may augment niche capacity as well. Indeed, mobilization of inflammatory monocytes from the marrow by remote infections depends on niche-forming cells, which sense low concentrations of TLR ligands in the marrow’s bloodstream.34
These results, although they may seem contradictory to a strictly demand-driven process regulating neutrophil homeostasis, have to be put into perspective with the circadian nature of signals provided by the gastrointestinal tract and food intake. Lessons learned from the study of eosinophils and basophils may help understand these phenomena: eosinophils, found abundant in lungs and intestinal tract, preferentially control parasitic infections. Intestinal type 2 innate lymphoid cells constitutively express IL-5 and IL-13, thereby maintaining eosinophil numbers. Circadian cycling of intestinal type 2 innate lymphoid cell cytokine secretion has recently been described to be dependent on food intake (metabolic cycling) and the circadian master regulator vasoactive intestinal peptide.31 Similarly, basophil hematopoiesis and immunoglobulin E depend on proper intestinal colonization. Germ-free mice display excessive marrow basophil granulopoiesis via IL-3 signaling, which in turn depends on MyD88 expression in immunoglobulin E–secreting B cells.35
Interestingly, response of the innate immune system to microbial compounds is circadian in nature as well: endogenous cortisol levels predetermine cytokine response to LPS in humans,36 and a MΦ circadian clockwork controls their inflammatory response.37 Of note, the circadian-regulated response to PAMPs is not limited to hematopoietic cells but has been characterized in detail in the mutual relationship of intestinal epithelial cells and microbiota.38
In summary, circadian fluctuations in neutrophil migration influence hematopoiesis via marrow MΦs and niche-forming cells. These phenomena may, however, reflect circadian clockworks inherent to MΦs and/or even be a function of commensal microbiota setting the circadian clock of glucocorticoid-producing intestinal epithelial cells.
Discussion
In summary, the published literature provides ample evidence for the role of cytokines and growth factors, such as IL-23, IL-17, and G-CSF, in regulating neutrophil homeostasis. This is achieved by modulations of niche size, including consecutive effects on hematopoietic stem/progenitor cells and their fate decision. The cellular sources of the above-mentioned humoral factors are represented by MΦs, DCs, and innate immune cells. Moreover, TLR4 seems to be an essential sensor of neutropenia, and removal of aged neutrophils is coupled to the reproduction of neutrophils in a rhythmic, circadian fashion. Numerous redundancy mechanisms on all levels of this modulation cascade have been described and represent a safeguard for granulopoiesis when distinct cell types or mechanisms are knocked out in genetically modified mice.
We suggest that a common denominator of neutrophil granulopoiesis or setpoint device of the neutrostat is represented by the more or less pathogenic microbiome that efficiently triggers innate immune receptors such as TLR4 or may even signal via the noncanonical, TLR-independent inflammasome pathway (Figure 1).39 Further work will be needed to understand the bigger picture of neutrophil homeostasis, but we predict that ancient signaling pathways depending on PAMPs/PRRs and functions of the inflammasome are an integral part: a recent study revealed that constitutive activation of the inflammasome pathway is sufficient to induce profound neutrophilia even in germ-free mice.40
Authorship
Contribution: S.W., S.B., and H.-G.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hans-Georg Kopp, Department of Hematology/Oncology, Eberhard-Karls University, Otfried-Mueller-Strasse 10, D-72076 Tuebingen, Germany; e-mail: hans-georg.kopp@med.uni-tuebingen.de.
References
Author notes
S.W., S.B., and H.-G.K. contributed equally to this work.