Key Points
Two well-documented cases of a HIT-mimicking disorder without proximate heparin exposure (spontaneous HIT syndrome) are reported.
The definition of spontaneous HIT syndrome should include strong serum-induced platelet activation at 0 IU/mL heparin (inhibited at 100 IU/mL).
Abstract
The existence of spontaneous heparin-induced thrombocytopenia (HIT) syndrome (or autoimmune HIT), defined as a transient prothrombotic thrombocytopenic disorder without proximate heparin exposure serologically indistinguishable from HIT, is controversial. We describe 2 new cases presenting with thrombotic stroke/thrombocytopenia: one following shoulder hemi-arthroplasty (performed without heparin) and the other presenting to the emergency room without prior hospitalization, heparin exposure, or preceding infection. Both patients tested strongly positive for anti-platelet factor 4 (PF4)/heparin immunoglobulin (Ig)G in 2 different immunoassays and in the platelet serotonin-release assay. Crucially, both patients’ sera also caused strong (>80%) serotonin release in the absence of heparin, a serologic feature characteristic of delayed-onset HIT (ie, where heparin use precedes HIT but is not required for subsequent development or worsening of thrombocytopenia). We propose that a rigorous definition of spontaneous HIT syndrome should include otherwise unexplained thrombocytopenia/thrombosis without proximate heparin exposure and with anti-PF4/heparin IgG antibodies that cause strong in vitro platelet activation even in the absence of heparin.
Introduction
Heparin-induced thrombocytopenia (HIT) is a transient, autoimmune-like, prothrombotic disorder caused by platelet-activating immunoglobulin (Ig)G reactive against the self-protein, platelet factor 4 (PF4), bound to heparin.1,2 Rarely, a brief exposure to heparin triggers this adverse reaction beginning after stopping heparin (delayed-onset HIT); such patients’ sera activate platelets strongly in vitro even in the absence of pharmacologic heparin.3 Previous reports4-7 suggest that an analogous syndrome can occur without preceding heparin exposure (spontaneous HIT syndrome or autoimmune HIT). We now report 2 new cases of spontaneous HIT syndrome, review the literature, and propose a clinical-pathological definition of this disorder including characteristic in vitro platelet activation features. We also suggest that the existence of spontaneous HIT syndrome following orthopedic surgery confounds interpretation of fondaparinux-associated HIT reported in that patient population.8-12
Study design
Patient 1
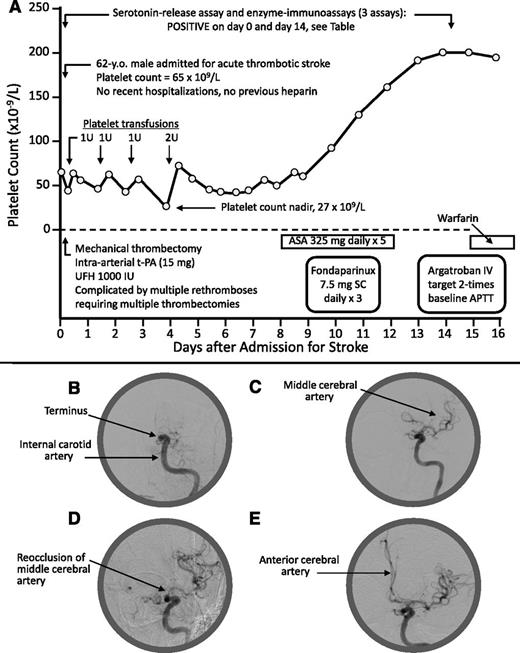
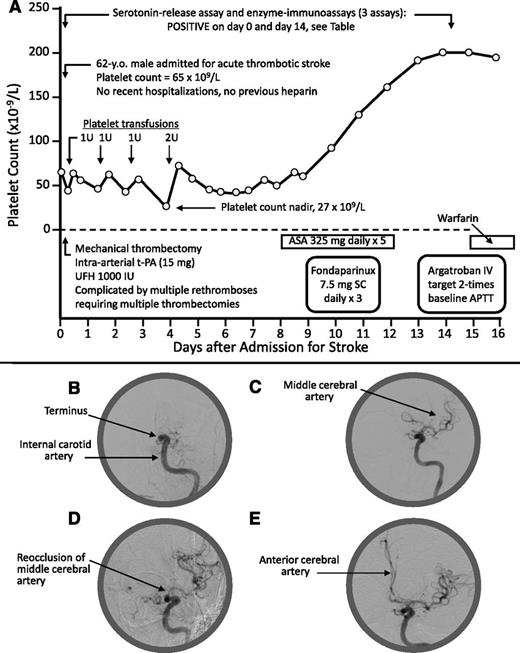
A 62-year-old man was admitted with left middle cerebral artery (MCA) thrombotic stroke and thrombocytopenia; his admission platelet count (measured prior to receiving heparin) was 65 × 109/L (Figure 1A-B). There was no previous history of thrombocytopenia, hospitalization/surgery, previous heparin, infection, or other recent acute illness. MCA recanalization was initially achieved (Figure 1C) by mechanical thrombectomy (Solitaire stent-retriever) with unfractionated heparin (UFH; 1000 IU plus an unspecified amount as heparinized saline infusion), but 5 rapid episodes of rethrombosis (Figure 1D) required 5 further passes with the Solitaire device. Finally, patency (Figure 1E) was established with tissue plasminogen activator (15 mg) administered directly into the internal carotid artery terminus. A blood sample obtained prior to heparin administration tested strongly positive for HIT antibodies (“Results and discussion”). Treatment with aspirin, fondaparinux, and argatroban was administered, with subsequent warfarin. The platelet count remained low for 1 week (nadir, 27 × 109/L) and recovered by day 14.
Patient 1: Clinical and radiological features. (A) Clinical course. (B) Thrombotic occlusion at internal carotid artery terminus. (C) Flow reestablished to MCA. (D) Reocclusion at origin of MCA (note: the unusual problem of 5 rapid rethromboses may have been related to heparin administration during the mechanical thrombectomies). (E) Flow reestablished to MCA and anterior cerebral artery (after infusion of tissue-plasminogen activator). No heparin exposure occurred as a result of the platelet transfusions (platelets were prepared using citrate anticoagulant). APTT, activated partial thromboplastin time; ASA, acetylsalicylic acid (aspirin); IU, international units; IV, intravenous; SC, subcutaneous; t-PA, tissue plasminogen activator; U, units; UFH, unfractionated heparin.
Patient 1: Clinical and radiological features. (A) Clinical course. (B) Thrombotic occlusion at internal carotid artery terminus. (C) Flow reestablished to MCA. (D) Reocclusion at origin of MCA (note: the unusual problem of 5 rapid rethromboses may have been related to heparin administration during the mechanical thrombectomies). (E) Flow reestablished to MCA and anterior cerebral artery (after infusion of tissue-plasminogen activator). No heparin exposure occurred as a result of the platelet transfusions (platelets were prepared using citrate anticoagulant). APTT, activated partial thromboplastin time; ASA, acetylsalicylic acid (aspirin); IU, international units; IV, intravenous; SC, subcutaneous; t-PA, tissue plasminogen activator; U, units; UFH, unfractionated heparin.
Patient 2
A 54-year-old woman developed right leg swelling, left-upper extremity weakness/paresthesias, and thrombocytopenia (61 × 109/L) 15 days after shoulder hemiarthroplasty; no intra-/postoperative heparin had been given, and no central/invasive lines were used. Brain magnetic resonance imaging demonstrated acute infarct in the left posterior inferior cerebellar artery territory; angiography showed nonvisualization of the left vertebral artery (V2 segment). Ultrasound venography showed right lower-extremity deep vein thrombosis. An echocardiographic saline bubble contrast study revealed no abnormalities. UFH treatment resulted in a further platelet count fall to 37 × 109/L (nadir). Subsequent treatment with argatroban followed by fondaparinux was associated with gradual platelet count recovery to >150 × 109/L by day 39.
Patient/control sera were tested for PF4-dependent antibodies using 3 enzyme immunoassays (EIAs): an in-house IgG-specific EIA13 and 2 commercial (Immucor GTI Diagnostics, Waukesha, WI)—one IgG-specific (Lifecodes PF4 IgG)13 the other a polyspecific EIA detecting IgG/IgA/IgM (Lifecodes PF4 Enhanced).14 We also performed the platelet serotonin release assay (SRA), as previously described.15 Both patients provided informed consent to report their cases. This study was conducted in accordance with the Declaration of Helsinki.
Results and discussion
Both patients’ admission sera tested strongly positive for HIT antibodies by all 3 EIAs and in the SRA. Moreover, both patients’ admission sera (obtained before heparin administration) caused strong platelet activation at 0.1 and 0.3 IU/mL UFH (100% serotonin release), as well as in the absence of heparin (>80% serotonin release), with no platelet activation at 100 IU/mL heparin (Table 1). Heparin-dependent platelet activation was confirmed using diluted patient sera (Table 1). Similar serological features have been reported for patients with delayed-onset HIT,3 as well as in our initial report4 describing spontaneous HIT syndrome. Antibody reactivity declined markedly by 2 to 4 weeks (including disappearance of platelet-activating properties at 0 IU/mL heparin), in keeping with the usual transience of HIT antibodies,16 and paralleling both patients’ platelet count recovery. Laboratory testing did not support a diagnosis of antiphospholipid syndrome in either patient (Table 1; both antiphospholipid syndrome and spontaneous HIT syndrome are potential explanations for thrombotic storm17 ).
These 2 cases further support spontaneous HIT syndrome as an unusual explanation for acute thrombosis and thrombocytopenia. Preceding orthopedic surgery is a possible trigger of these antibodies, as per patient 2 (present report) and as previously reported.5-7 Indeed, of the 7 previously reported cases of spontaneous HIT syndrome in which patients presented with thrombocytopenia and thrombosis in the absence of proximate heparin exposure, 5 occurred after orthopedic surgery (all with warfarin anticoagulation given after knee replacement surgery), whereas the remaining 2 cases occurred after infection. In patient 1 (present report), however, we could not identify a potentially causal proximate event. Including our 2 new cases (present report), plus 2 previously reported cases (patients 1 and 2 in our initial report4 ), there have now been 4 patients with putative spontaneous HIT syndrome investigated in the McMaster Platelet Immunology Laboratory. All 4 sera yielded substantial platelet activation (mean, 76%) in the absence of pharmacologic heparin. This is a crucial observation, as it may explain how anti-PF4/heparin antibodies can induce intravascular platelet activation at a time when no heparin is being administered.
Spontaneous HIT syndrome should not be confused with a related clinical picture resembling rapid-onset HIT triggered by therapeutic dose heparin administration in which, however, no preceding heparin exposure can be identified, yet the patients have circulating anti-PF4/heparin antibodies. This situation has been documented in 2 patients4,18 (without previous heparin exposure) who initially presented with normal platelet counts but then developed acute thrombocytopenia on receiving a therapeutic dose of low-molecular-weight heparin4 or UFH.18 Importantly, neither serum caused platelet activation at 0 IU/mL heparin, consistent with serum-induced platelet activation that occurs in the absence of heparin (ie, at buffer control), which is important in explaining spontaneous HIT syndrome that presents with thrombocytopenia.
We believe our observations could also have relevance to the understanding of the pathogenesis of fondaparinux-associated HIT. To date, 6 relatively well-documented cases of HIT following fondaparinux thromboprophylaxis have been reported,8-12 without any apparent preceding heparin exposure. Notably, 5 of the 6 patients had preceding orthopedic surgery (all but 1 post-knee replacement), raising the possibility that fondaparinux could be an innocent bystander, ie, the orthopedic surgery itself could have been the trigger of the subsequent anti-PF4/heparin immune response. Indeed, only minor enhancement of platelet activation in vitro in the presence of fondaparinux has been observed in these patients.10,12 It is plausible that nonheparin triggers of the anti-PF4/heparin immune response, such as glycosaminoglycans released during orthopedic surgery, or bacterial antigens in the case of preceding infection, could account for these unusual clinical observations (bacterial cell walls19 and RNA/DNA nucleotides20 bind to PF4, recapitulating HIT antigens, and murine19 and human bacterial infection21 are associated with anti-PF4/heparin antibody formation).
We propose that a definitive diagnosis of spontaneous HIT syndrome should be based on the following criteria: thrombocytopenia (without alternate explanation), thrombosis, lack of proximate heparin exposure, strong positive PF4-dependent EIAs (≥2 different assays), a strong positive platelet activation assay (>80% peak serotonin release) featuring strong heparin-independent platelet activation (>50% serotonin release at 0 IU/mL heparin), as well as heparin-dependent platelet activation (evident on serum dilution), and exhibiting also the other characteristic features of HIT sera (inhibition at 100 IU/mL heparin and with Fc receptor-blocking monoclonal antibody). Although fulfilling all of these aforementioned criteria will require serum referral for specialized laboratory investigations (namely, the SRA or another functional assay such as the heparin-induced platelet activation test,22 performed using neat and diluted serum and also including buffer control), initial treatment of presumptive spontaneous HIT syndrome should not be delayed; moreover, our proposed rigorous definition will avoid potential overdiagnosis that could occur if a positive EIA alone was used to detect anti-PF4/heparin antibodies in a patient who presented with otherwise unexplained thrombocytopenia.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jo-Ann I. Sheppard for technical assistance and for help in the preparation of the figure.
This work was supported by grant T6950 (T.E.W.) from the Heart and Stroke Foundation of Ontario (Toronto, ON, Canada).
Authorship
Contribution: T.E.W. designed and supervised the experiments, analyzed the data, and interpreted the results; P.A.B., J.K., and R.A.B. identified the 2 patients, referred acute and follow-up patient sera to the McMaster Platelet Immunology Laboratory, and obtained the clinical data; and T.E.W., P.A.B., J.K., and R.A.B. reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.E.W. has received lecture honoraria from Pfizer Canada and Instrumentation Laboratory and research funding from GlaxoSmithKline and Immucor GTI Diagnostics and has provided expert witness testimony relating to HIT. The remaining authors declare no competing financial interests.
Correspondence: Theodore E. Warkentin, Hamilton Regional, Laboratory Medicine Program, Rm 1-270B, Hamilton Health, Sciences (General Site), 237 Barton St E, Hamilton, ON, Canada L8L2X2; e-mail: twarken@mcmaster.ca.