Key Points
Allodepleted-T-cells containing the iC9 safety gene persist long-term in vivo, promote immune recovery, and protect against infections.
GvHD caused by iC9-T cells can be permanently controlled by a single administration of AP1903 without abrogating immune reconstitution.
Abstract
Adoptive transfer of donor-derived T lymphocytes expressing a safety switch may promote immune reconstitution in patients undergoing haploidentical hematopoietic stem cell transplant (haplo-HSCT) without the risk for uncontrolled graft versus host disease (GvHD). Thus, patients who develop GvHD after infusion of allodepleted donor-derived T cells expressing an inducible human caspase 9 (iC9) had their disease effectively controlled by a single administration of a small-molecule drug (AP1903) that dimerizes and activates the iC9 transgene. We now report the long-term follow-up of 10 patients infused with such safety switch-modified T cells. We find long-term persistence of iC9-modified (iC9-T) T cells in vivo in the absence of emerging oligoclonality and a robust immunologic benefit, mediated initially by the infused cells themselves and subsequently by an apparently accelerated reconstitution of endogenous naive T lymphocytes. As a consequence, these patients have immediate and sustained protection from major pathogens, including cytomegalovirus, adenovirus, BK virus, and Epstein-Barr virus in the absence of acute or chronic GvHD, supporting the beneficial effects of this approach to immune reconstitution after haplo-HSCT. This study was registered at www.clinicaltrials.gov as #NCT00710892.
Introduction
Haplo-identical donors are an alternative source of hematopoietic stem cells (HSCs) for patients without a more closely matched donor or who need an urgent allogeneic hematopoietic stem cell transplant (HSCT).1,2 Because the donor graft for such haploidentical transplants (haplo-HSCT) has a high frequency of alloreactive T cells, recognizing the non-shared HLA haplotype, extensive T-cell depletion (usually by positive selection of HSCs), remains a fundamental prerequisite if the graft is not to cause fatal acute graft-versus-host-disease (GvHD). Although extensive T-cell removal of the graft effectively prevents GvHD, the process also causes prolonged and profound posttransplant immunodeficiency for a year or more,3,4 with compromised antiviral immunity.5-7 As a consequence, infectious morbidity and mortality remain high and are a frequent cause of treatment failure.8-10
Other groups and our own have shown that the posttransplant infusion of small numbers of donor T lymphocytes that have been depleted of recipient-reactive T cells can improve immune reconstitution and antiviral immunity.6,11-13 Engineered T cells with safety switches have been developed to increase the feasibility of infusing higher numbers of donor-derived T cells while providing a tool to control the increased risk for acute GvHD that may be associated with incomplete abrogation of alloreactivity against the recipient. Thus, adoptive transfer of donor-derived T cells engineered with the herpes simplex virus thymidine kinase (HSV-TK) gene can enhance immune recovery posttransplant, and resultant acute GvHD has been controlled by administration of the ganciclovir prodrug.14 Because HSV-TK is potentially immunogenic and requires activation by a drug that remains a crucial pharmacologic agent for the treatment of cytomegalovirus infection,15 we tested an alternative approach based on the expression of an inducible human caspase 9 transgene (iC9),16-18 which is dimerized, and hence activated, by administration of an otherwise bio-inert small molecule drug, AP1903. We studied 5 patients and showed that infused iC9-T cells engrafted in all 5 and contributed to short-term immune recovery. When GvHD occurred, the iC9-T cells were more than 90% eliminated within 2 hours of dimerizer administration, and GvHD was rapidly and apparently permanently reversed.19 Here we report the long-term follow-up (at 3.5 years) of all 10 patients enrolled in this phase 1 study and show the effect of iC9-T cell infusions and dimerizer drug administration on short- and long-term immune recovery and resistance to opportunistic viral infections.
Methods
Patients and study design
This phase 1 clinical study (CASPALLO trial [A Phase I Study Evaluating the Use of Allodepleted T Cells Transduced With Inducible Caspase 9 Suicide Gene After Haploidentical Stem Cell Transplantation], investigational new drug [IND] 13813) was approved by the institutional review board of Baylor College of Medicine and the US Food and Drug Administration and reviewed by the Recombinant DNA Advisory Committee. This study was conducted in accordance with the Declaration of Helsinki. It was designed to assess the safety and efficacy of infusing escalating doses of allodepleted donor-derived T cells genetically modified to express the iC9 transgene (iC9-T cells) in patients undergoing haplo-HSCT. Briefly, patients meeting eligibility criteria received donor-derived iC9-T cells between 30 to 90 days after the infusion of CD34+ cells after a dose-escalation protocol: dose level 1, 1 × 106 iC9-T cells/kg; dose level 2, 3 × 106 iC9-T cells/kg; and dose level 3, 1 × 107 iC9-T cells/kg.20 No immunosuppression was used after haplo-HSCT. Patients who developed acute GvHD grade I or II after infusion of iC9-T cells received 0.4 mg/kg of the dimerizing agent AP1903 (Bellicum Pharmaceuticals, Inc.) as a 2-hour infusion.19,21
Generation of iC9-T cells
The iC9-T cells were generated as previously described.6,18,19 Cell manipulation was performed under good manufacturing practice conditions at the Center for Cell and Gene Therapy, using approved standard operating procedures. In brief, donor-derived peripheral blood mononuclear cells (PBMCs) were cocultured with irradiated recipient Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines at a responder-to-stimulator ratio of 40:1 in serum-free medium. After 72 hours, activated T cells that express CD25 were depleted from coculture by overnight incubation with a recombinant immunotoxin consisting of the anti-CD25 monoclonal antibody RFT5 fused to the deglycosylated ricin A-chain (dgA) (RFT5-SMPT-dgA).18,19,22 Allodepleted donor T cells were then activated by anti-CD3 antibody (Miltenyi Biotech) and transduced with a retroviral vector encoding the iC9 and the selectable marker ΔCD19 transgenes.17,18 Four days after transduction, cells were selected on the basis of the expression of CD19 by using the CliniMacs Plus selection device (Miltenyi Biotech). After selection, T cells were expanded for up to an additional 4 days before cryopreservation. Lack of alloreactivity measured in a conventional mixed lymphocyte reaction was included as a release criterion for iC9-T cells for infusion.19
Flow cytometry analysis
The iC9-T cells were characterized using a panel of fluorochrome-conjugated monoclonal antibodies, including CD3, CD4, CD8, CD19, CD20, CD56 (BD Biosciences), CD45RA/RO, and CD62L (Beckman Coulter). Cells were acquired on a FACSCalibur flow cytometer (BD Biosciences). Nontransduced control cells were used to set the negative gate for CD19 expression. T-cell receptor Vβ repertoire analysis was performed with IO Test β Mark kit (Beckman Coulter). For intracellular interferon (IFN)-γ staining, PBMCs were incubated with virus-derived peptide antigens (see following list) overnight in the presence of brefeldin A at a 1:1000 dilution (BD Biosciences) before the cells were harvested, washed, and stained with CD3/CD8/CD19 antibodies. After permeabilization with Cytofix/Cytoperm solution, the cells were incubated with 10 µL phycoerythrin-anti-IFN-γ antibody (BD Biosciences) and washed with phosphate-buffered saline/fetal bovine serum containing 0.1% saponin. Flow cytometry data were analyzed using Cell Quest software (Becton Dickinson) and Kaluza software (Beckman Coulter).
Real-time quantitative polymerase chain reaction of iCasp9.2A.ΔCD19 transgene
The iC9 transgene was measured in PBMCs by quantitative polymerase chain reaction (Q-PCR), as previously described.19
Detection of pathogen-specific T cells
IFN-γ release from PBMCs collected at different times after T-cell infusions was evaluated either by enzyme-linked immunospot (ELISpot) assay or intracellular staining, as previously described.18 Peptide libraries (pepmixes, 15-mer peptides overlapping by 11 amino acids) spanning the adenovirus (AdV) proteins hexon and penton; the cytomegalovirus (CMV) antigens pp65 and IE1; the EBV antigens BZLF1, LMP1, LMP2, EBNA1, EBNA3a, EBNA3b, and EBNA3c; and the BK virus (BKV) antigens LT, ST, VP-1, VP-2, and VP-3 were used to stimulate the PBMCs. The responses to Aspergillus fumigatus were detected as previously described.23 Staphylococcal enterotoxin B or phytohemagglutinin were used as positive controls. ELISpots were independently enumerated by Zellnet consulting and compared with input cell numbers to obtain the frequency of cells responding to each stimulus.
Monitoring of infections
Viral (CMV, AdV, BKV) reactivation or infections were monitored by Q-PCR assays (ViraCor-IBT Laboratories Inc) on blood PBMCs, plasma, urine, or stool, as noted. EBV-DNA viral load was determined by quantitative PCR of PBMCs, using specific primers and probes targeting the EBER gene.24 Fungitell assay on blood samples was performed by ViraCor Laboratories.
Statistical analysis
Summary statistics including mean and standard error of mean are reported. Immunologic follow-up data (mean ± standard error of mean) were plotted against time (month after infusion) by T-cell subtypes. Circulating T cells were plotted for individual patients for those who received AP1903 and those who did not. Nonparametric Wilcoxon rank-sum test was used to compare the difference between groups. A P value < .05 was considered statistically significant.
Results
Patient details
Patient details are provided in Table 1. In brief, we enrolled 10 pediatric patients (median age, 8 years; range, 3-17 years) with high-risk malignancies who received haplo-HSCT on the CASPALLO study (IND 13813). Conditioning regimens were as previously described.25 All patients were infused with iC9-T cells, using interpatient dose escalation (from 1 × 106 to 1 × 107 cells/kg) between 30 to 90 days after transplant, with the exception of 1 patient infused at 124 days with US Food and Drug Administration approval.19 Six patients received a single T-cell infusion, and 4 patients (patients 2, 7, 9, and 10) received a second T-cell infusion in an effort to eradicate persistent mixed chimerism (Table 1). There were no immediate toxicities related to infusion, but 4 patients (1, 2, 4, and 5) developed GvHD from 2 to 6 weeks after their first iC9-T-cell infusion and received a single dose of the iC9 dimerizing drug AP1903.19 There was no subsequent recurrence of GvHD. One patient relapsed (myelodysplastic syndrome) and had a second transplant, 3 patients (all transplanted for relapsed acute lymphoblastic leukemia) died of relapse, and 1 patient died of complications after autoimmune hemolytic anemia. Five patients are alive and disease-free at a median follow-up of 1016 days after transplant (range, 835-1440 days) (Table 1).
Short- and long-term engraftment of iC9-T cells
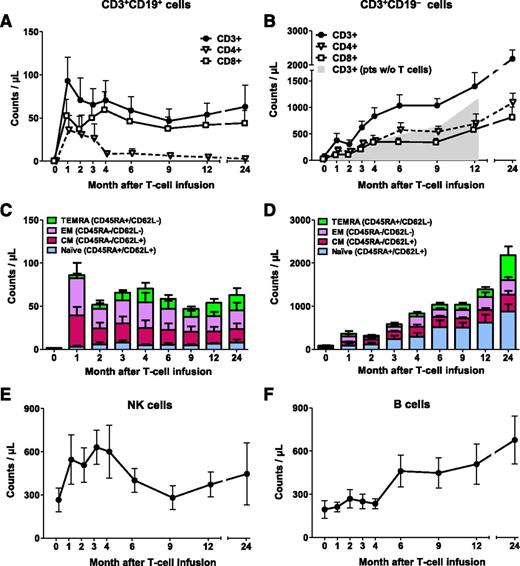
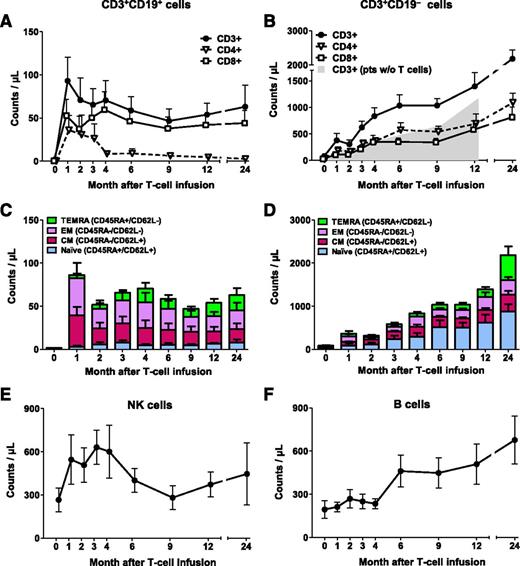
We previously reported the short-term engraftment of iC9-T cells (ie, CD3+CD19+ T cells) in 5 patients.19 We have now extended the follow-up to a median of 493 days (range, 125-1355 days) and included 5 additional patients. Infused CD3+CD19+ T cells were detectable in the PB as early as 7 days after infusion and were engrafted long-term in all patients, as assessed by flow cytometry for CD3+CD19+ dual expression (Figure 1A) and Q-PCR for the iC9 transgene (supplemental Figure 1, available on the Blood Web site). At 12 and 24 months, CD3+CD19+ T-cell counts were 54 ± 13 cells/µL (n = 7; patients 1, 2, 3, 6, 7, 8, and 10) and 63 ± 25 cells/µL (n = 4; patients 1, 3, 6, and 7), respectively (Figure 1A). Infused iC9-T cells contained both CD4+ and CD8+ T cells (supplemental Table 1), and both subsets were detected long-term in the PB. However, the CD4:CD8 ratio of CD3+CD19+ T cells was initially low and progressively declined with time, from 0.3 to 0.11 in median value at 2 and 9 months, respectively, with the ratio falling further to 0.09 in median value at 24 months (Figure 1A; supplemental Figure 2). Detailed phenotypic analysis of CD3+CD19+ T-cell subsets revealed that long-term engrafted cells were predominantly central-memory and effector-memory (Figure 1C).
Overall immune reconstitution in patients infused with iC9-T cells. Counts of circulating CD3+CD19+ (A) and CD3+CD19− T cells (B). Shaded area indicates counts of circulating CD3+ T cells in 4 control patients who underwent haplo-HSCT without T-cell add-back, and time point 0 that corresponds to month 3 posttransplant. Subset composition of circulating CD3+CD19+ (C) and CD3+CD19− T cells (D). Counts of circulating natural killer cells (E) and B lymphocytes (F). The number of evaluable patients at each points is 10 (month 1), 9 (months 2 and 3), 8 (months 4, 6, and 9), 7 (month 12), and 4 (month 24). Data show means ± standard error of mean of the patients infused with iC9-T cells.
Overall immune reconstitution in patients infused with iC9-T cells. Counts of circulating CD3+CD19+ (A) and CD3+CD19− T cells (B). Shaded area indicates counts of circulating CD3+ T cells in 4 control patients who underwent haplo-HSCT without T-cell add-back, and time point 0 that corresponds to month 3 posttransplant. Subset composition of circulating CD3+CD19+ (C) and CD3+CD19− T cells (D). Counts of circulating natural killer cells (E) and B lymphocytes (F). The number of evaluable patients at each points is 10 (month 1), 9 (months 2 and 3), 8 (months 4, 6, and 9), 7 (month 12), and 4 (month 24). Data show means ± standard error of mean of the patients infused with iC9-T cells.
Effects of the infusion of iC9-T cells on total T-cell engraftment
Because the donor stem cell graft is intensively depleted (4-5 logs) of T cells, donor T lymphocytes may not appear for 6 months or longer after haplo-HSCT, and the delay may be even longer for the CD4+ subset.3,6,7 In this study, however, the early rise in the infused CD3+CD19+ T cells was rapidly followed by recovery of endogenous CD3+CD19− T cells compared with patients who underwent haplo-HSCT without T-cell add-back at concurrent points posttransplant (Amrolia et al6 ; Figure 1B). At the time of T-cell infusion, CD3+CD19− cells were 36 ± 19 cells/µL but had increased by a mean 25-fold within 6 months and 42-fold by 12 months. Thus, the mean absolute counts of CD3+CD19− were greater than 500 per µL at 3 months after iC9-T-cell infusion (5 months posttransplantation), in contrast to delays of up to 12 months posttransplant in patients without T-cell add-back (Figure 1B). The phenotype of the recovering endogenous CD3+CD19− cells was, however, quite distinct from that of the infused CD3+CD19+ T cells, and the populations retained this difference with time. Thus, CD3+CD19− cells were predominantly CD4+, rather than CD8+ T cells, so that in contrast to the data in Figure 1A, the CD4:CD8 ratios of CD3+CD19− cells were 2.8, 1.5, and 1.3 in median value at months 2, 9, and 24, respectively (P values: .014, .026, and .067, respectively, compared with their CD3+CD19+ counterparts; median, 0.3, 0.11, and 0.09, respectively, Wilcoxon rank-sum test) (Figure 1B; supplemental Figure 2). Moreover, 49% and 44% of the CD3+CD19− cells expressed markers typical of naive T cells (CD45RA and CD62L) at 6 and 12 months, respectively (Figure 1D). As anticipated, numbers of natural killer cells and B lymphocytes also normalized over time, but there was no evident acceleration relative to previously reported values at any given time after haplo-HSCT (Figure 1E-F).
Effects of AP1903 on GvHD and on subsequent T-cell recovery
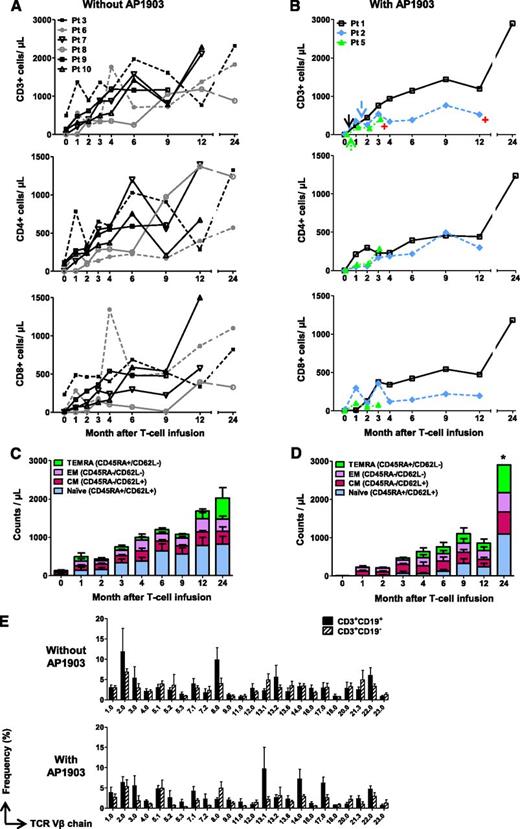
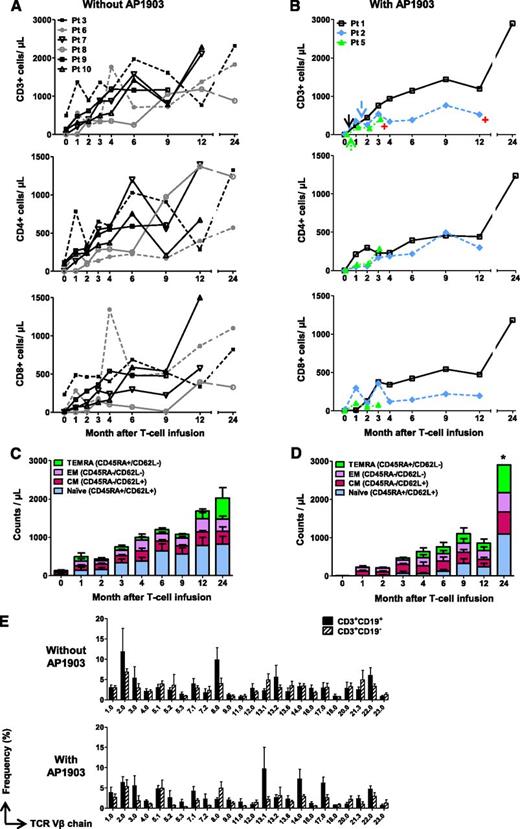
We previously reported that administration of a single dose of AP1903 promptly reversed acute GvHD in all 4 treated patients (patients 1, 2, 4, and 5).19 We observed that the subsequent reexpansion of CD3+CD19+ T cells quantified by either flow cytometry (Figure 2B) or Q-PCR for the iC9 transgene (supplemental Figure 1) in these patients was not associated with the reappearance of GvHD, indicating sustained functional allodepletion. We have also determined the effects of such AP1903 administration on the subsequent long-term T-cell immune reconstitution in the 3 patients (patients 1, 2, and 5) who are longer-term survivors. In these 3 patients, the kinetics of T-cell reconstitution appeared identical to that of patients who did not develop GvHD and did not receive AP1903 (Figure 2A-B). Similarly, the subsequent composition of the recovering T-cell subsets was unaffected (Figure 2C-D). Of note, the T-cell receptor Vβ repertoire usage in the 3 AP1903-treated patients remained as polyclonal during the ensuing months as in the 6 patients who had not received AP1903, and polyclonality was found in both CD3+CD19+ and CD3+CD19− fractions for all patients (Figure 2E). Hence, there was no apparent predisposition to develop T-cell oligoclonality in patients who were T-lymphodepleted in vivo by activation of the iC9 transgene.
Administration of AP1903 does not adversely affect T-cell immune recovery. Counts of circulating T cells in 6 patients who did not receive AP1903 (A) and 3 patients who received AP1903 and had follow-up more than 6 weeks (B). Arrows and red symbols indicate the administration of AP1903 and the time when patients went off-study, respectively. Counts of specific T-cell subsets in patients who did not receive AP1903 (C) and those who received AP1903 (D). *Data from 1 patient. (E) T-cell receptor Vβ receptor repertoire in untreated patients (upper) or in those who received AP1903 (lower); repertoires of CD3+CD19+ and CD3+CD19− T cells are shown. The analysis was performed in samples collected at a median of 7 months (range, 2-12 months) after iC9-T cell infusion for patients who had received AP1903 and a median of 6 months (range, 4-12 months) for patients who did not receive AP1903.
Administration of AP1903 does not adversely affect T-cell immune recovery. Counts of circulating T cells in 6 patients who did not receive AP1903 (A) and 3 patients who received AP1903 and had follow-up more than 6 weeks (B). Arrows and red symbols indicate the administration of AP1903 and the time when patients went off-study, respectively. Counts of specific T-cell subsets in patients who did not receive AP1903 (C) and those who received AP1903 (D). *Data from 1 patient. (E) T-cell receptor Vβ receptor repertoire in untreated patients (upper) or in those who received AP1903 (lower); repertoires of CD3+CD19+ and CD3+CD19− T cells are shown. The analysis was performed in samples collected at a median of 7 months (range, 2-12 months) after iC9-T cell infusion for patients who had received AP1903 and a median of 6 months (range, 4-12 months) for patients who did not receive AP1903.
Effects of iC9-T-cell infusions on antiviral immune reconstitution
Patients 3, 6, 7, and 9 had evidence of virus reactivation or disease before infusion of iC9-T cells. We assessed the ability of iC9-T cell infusions to control virus replication by sequential measurement of viral load and by the numbers of T cells reactive to overlapping peptide libraries specific for the viral antigens; LT, ST, and VP1, VP2, and VP3 (BK virus); pp65 and IE1 (CMV); LMP1, LMP2, BZLF1, EBNA3a, EBNA3b, EBNA3c, and EBNA1 (EBV); and hexon and penton (AdV). We enumerated virus-reactive T cells by measuring secreted or intracellular IFN-γ production by ELISpot or flow cytometry, respectively.
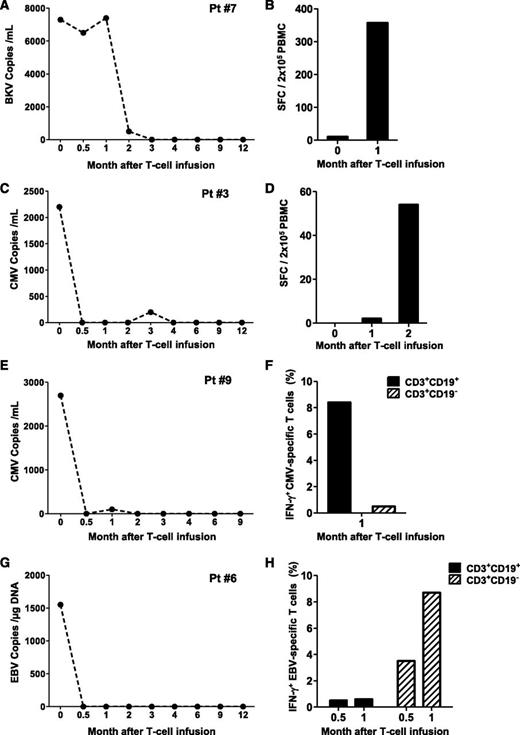
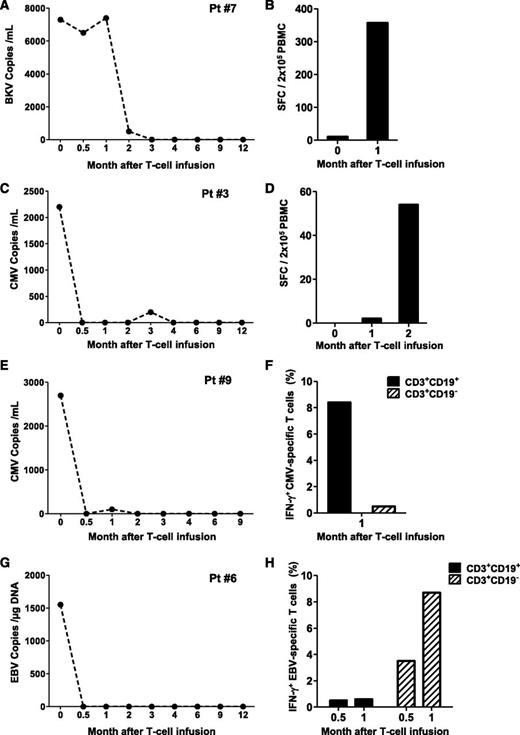
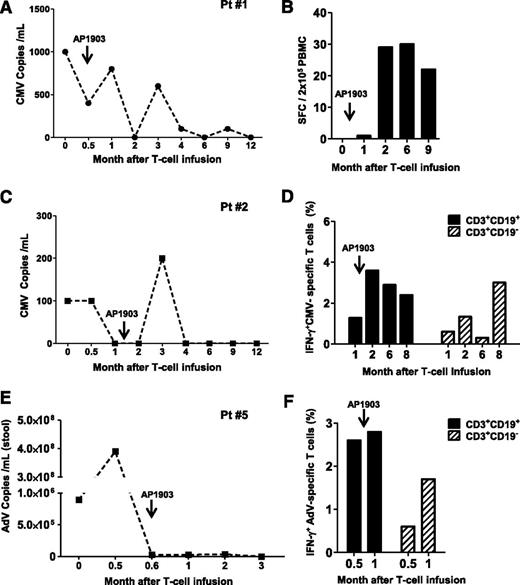
Patient 7 developed asymptomatic BK viremia (7.4 × 103 DNA copies/mL) (Figure 3A) and BK viruria (1 × 1010 copies/mL) nonresponsive to more than 10 days of treatment with cidofovir. The appearance of BK-specific T-cell precursors was detected by IFN-γ ELISpot 30 days after the infusion of iC9-T cells (spot-forming cells 357 ± 9/2 × 105 PBMC) (Figure 3B), and this correlated with the subsequent progressive elimination of the virus.
Antiviral immune reconstitution after iC9-T-cell infusion. Patients 7 (A-B), 3 (C-D), 9 (E-F), and 6 (G-H) had viral reactivation before iC9-T cell infusion and did not receive AP1903. Left panels (A,C,E,G) illustrate the viral load for each single patient at different times; right panels (B,D,F,H) illustrate the quantification of pathogen-specific T cells detected either by IFN-γ ELIspot or intracellular staining in the CD3+CD19+ and CD3+CD19− T cells at multiple times.
Antiviral immune reconstitution after iC9-T-cell infusion. Patients 7 (A-B), 3 (C-D), 9 (E-F), and 6 (G-H) had viral reactivation before iC9-T cell infusion and did not receive AP1903. Left panels (A,C,E,G) illustrate the viral load for each single patient at different times; right panels (B,D,F,H) illustrate the quantification of pathogen-specific T cells detected either by IFN-γ ELIspot or intracellular staining in the CD3+CD19+ and CD3+CD19− T cells at multiple times.
Patient 3 had a 2-month history of persistent CMV antigenemia in PB before infusion of iC9-T cells, despite treatment with ganciclovir. One week after iC9-T cell infusion, CMV viral load fell from 2200 to 500 DNA copies/mL and became undetectable 2 weeks later (Figure 3C). There was a corresponding increase in the CMV-specific IFN-γ ELISpot, from 2 ± 1/2 × 105 cells at month 1 to 54 ± 0/2 × 105 cells at month 2 after infusion (Figure 3D). A similar benefit was obtained in patient 9, who had a 6-week history of CMV viremia resistant to ganciclovir/foscarnet, which resolved within 2 weeks of the infusion of iC9-T cells (Figure 3E). Clearance of CMV viremia was associated with an increase in CMV-reactive T cells in the PB in both the infused CD3+CD19+ population and in endogenous CD3+CD19− cells, as assessed by flow cytometry for intracellular IFN-γ (8.4% IFN-γ+ cells in CD3+CD19+ fraction vs 0.5% in CD3+CD19− fraction per 2 × 105 cells) (Figure 3F).
The infusion of iC9-T cells also benefited patients infected with EBV. Patient 6 had a 2-month history of EBV reactivation. At the time of T-cell infusion, viral load was 1551 copies/µg DNA and rapidly reduced to 204 copies/µg DNA after 3 days, before becoming undetectable by week 2 after the infusion of iC9-T cells (Figure 3G). EBV-reactive T cells were detected by flow cytometric analysis of intracellular IFN-γ staining on PBMCs collected 16 days after adoptive transfer, and these EBV-reactive cells were present in both the infused CD3+CD19+ and the endogenous CD3+CD19− T cells (0.5% and 3.5% of IFN-γ+ per 2 × 105 cells, respectively). These EBV-specific T-cell precursors represented 0.6% and 8.7% of circulating CD3+CD19+ and CD3+CD19− cells, respectively, by day 30 after infusion (Figure 3H).
Effects of AP1903 on antiviral immune reconstitution
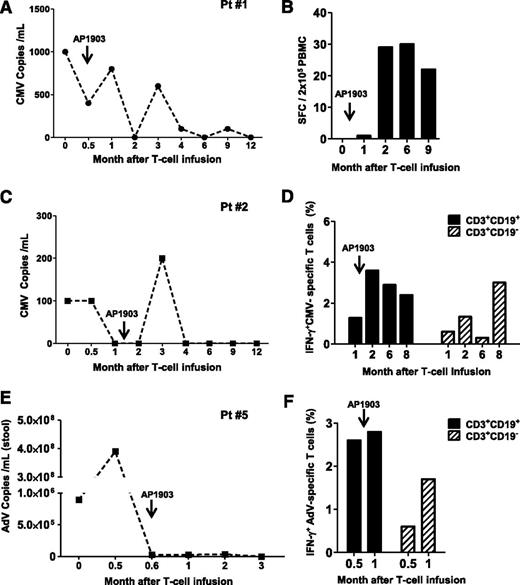
Three patients who received AP1903 to control acute GvHD (patients 1, 2, and 5) also had virus reactivation. Patients 1 and 2 had CMV reactivation that incompletely responded to ganciclovir and foscarnet, but in both patients, there was a recovery of CMV-reactive T cells and control of CMV-viremia, despite the administration of AP1903. Patient 1 was CMV-seropositive and had received a transplant from a CMV-seronegative donor and developed CMV antigenemia (Figure 4A). This was controlled after infusion of iC9-T cells but rose briefly after AP1903 administration before falling again (Figure 4A). This second period of control of CMV viremia was associated with the rapid recovery and expansion of CMV-specific T cells after AP1903 administration (Figure 4B). Patient 2 was a CMV-seropositive recipient with a CMV-seropositive donor and had a similar pattern of response (Figure 4C), developing low-level CMV viremia initially controlled by iC9-T-cell infusion, with a subsequent brief rise in viremia after AP1903 administration, followed by sustained control thereafter (Figure 4C). Sustained control of CMV reactivation in this recipient was also associated with expansion of CMV-reactive T cells within 2 weeks of AP1903 administration (Figure 4D). In this patient, sufficient CMV-reactive cells were available before and after dimerizer administration to analyze their phenotype by flow cytometry. We found CMV-reactive cells in both the CD3+CD19+ and CD3+CD19− T cells, with the latter increasing in preponderance over the course of several months (Figure 4D). Finally, patient 5, who had an AdV infection with diarrhea refractory to foscarnet and cidofovir showed resolution of symptoms and a decline of the viral load in stool within 3 weeks of iC9-T-cell infusion (Figure 4E). Virus levels remained low even after administration of AP1903 (Figure 4E), and flow cytometric analysis confirmed the recovery of AdV-specific precursors within 2 weeks of AP1903 administration (Figure 4F). AdV-reactive cells were present in both the CD3+CD19+ and CD3+CD19− T cells before and after administration of the dimerizer drug (Figure 4F). Hence, the ability of iC9-T cells to respond to and help control viral infections are not compromised by AP1903-mediated T-cell destruction in patients who develop acute GvHD.
Recovery of antiviral immune responses after iC9-T-cell infusion and administration of AP1903. Patients 1 (A-B), 2 (C-D), and 5 (E-F) had viral reactivation and also received AP1903 to control acute GvHD. Left panels (A,C-D) illustrate the viral load for each single patient at multiple times; right panels (B,D-F) illustrate the quantification of pathogen-specific T cells detected either by IFN-γ ELIspot or intracellular staining in the CD3+CD19+ and CD3+CD19− T cells at each time.
Recovery of antiviral immune responses after iC9-T-cell infusion and administration of AP1903. Patients 1 (A-B), 2 (C-D), and 5 (E-F) had viral reactivation and also received AP1903 to control acute GvHD. Left panels (A,C-D) illustrate the viral load for each single patient at multiple times; right panels (B,D-F) illustrate the quantification of pathogen-specific T cells detected either by IFN-γ ELIspot or intracellular staining in the CD3+CD19+ and CD3+CD19− T cells at each time.
Effect of AP1903 on antifungal immune responses
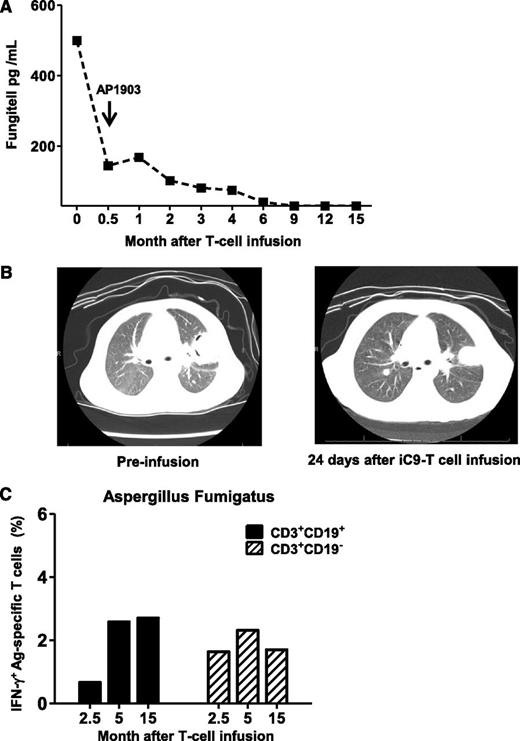
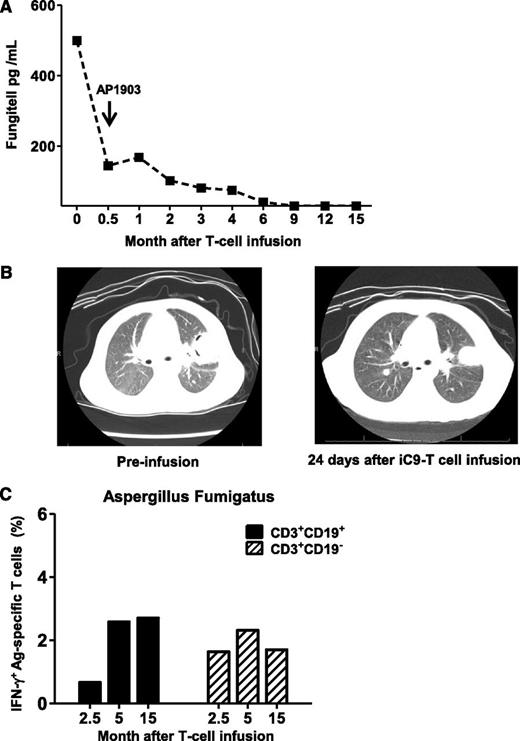
Patient 1 entered transplant with pulmonary Aspergillosis; reactivation of disease was detected 5 days posttransplant by computed tomography scan and by increasing serum levels of galactomannan and β-D glucan. Despite 2 months of treatment with broad-spectrum antifungal drugs (caspofungin, micafungin, voriconazole, posaconazole), β-D glucan levels remained high (Figure 5A) and computed tomography scan showed progressive cavitation (Figure 5B). Administration of iC9-T cells led to a rapid fall in β-D glucan, which continued even after administration of AP1903 after the onset of acute GvHD (Figure 5A), and was associated with progressive improvement in the appearance of the computed tomography scan (Figure 5B). T cells reactive to Aspergillus fumigatus extracellular extract could be detected in both CD3+CD19+ and CD3+CD19− T cells by 2 months after administration of AP1903, and these reactive cells persisted long-term (Figure 5C). This patient remains free of active fungus disease at 48 months posttransplant (Table 1) and is receiving no antifungal agents.
Anti-Aspergillus fumigatus immune-response after iC9-T-cell infusion and administration of AP1903. Patient 1 entered haplo-HSCT with pulmonary Aspergillosis. (A) Fungitell levels at different times after infusion of iC9-T cells. Arrow indicates the administration of AP1903 to control acute GvHD. (B) Computed tomography scan of chest showing left upper lung lobe lesion from Aspergillus fumigatus, before iC9-T cell infusion and 24 days after infusion. Images show a decrease in size of the largest nodule within the left upper lobe (left) at 24 days after iC9-T-cell infusion with a loss of cavitary appearance (right). (C) Detection of Aspergillus fumigatus specific T-cells by intracellular IFN-γ release from both CD3+CD19+ and CD3+CD19− T cells in samples collected at different times after AP1903 administration.
Anti-Aspergillus fumigatus immune-response after iC9-T-cell infusion and administration of AP1903. Patient 1 entered haplo-HSCT with pulmonary Aspergillosis. (A) Fungitell levels at different times after infusion of iC9-T cells. Arrow indicates the administration of AP1903 to control acute GvHD. (B) Computed tomography scan of chest showing left upper lung lobe lesion from Aspergillus fumigatus, before iC9-T cell infusion and 24 days after infusion. Images show a decrease in size of the largest nodule within the left upper lobe (left) at 24 days after iC9-T-cell infusion with a loss of cavitary appearance (right). (C) Detection of Aspergillus fumigatus specific T-cells by intracellular IFN-γ release from both CD3+CD19+ and CD3+CD19− T cells in samples collected at different times after AP1903 administration.
Discussion
Improving immune reconstitution and reducing opportunistic infections after haplo-HSCT without causing GvHD remains a challenge to the success of these profoundly T-cell-depleted stem cell transplants. We have previously shown in 5 patients that acute GvHD caused by the infusion of donor-derived T cells can be efficiently controlled if these cells are modified with the iC9 safety switch.19 We now report long-term follow-up of 10 treated patients to show that infused iC9-T cells persist more than 2 years, contribute to the control of opportunistic infections, and promote the recovery of endogenous T lymphocytes. Moreover, control of GvHD by the activation of iC9 transgene by AP1903 is permanent while sparing sufficient pathogen-specific T cells for continued protection against common posttransplant virus infections.
In our previous study, we demonstrated that T lymphocytes engineered to express an inducible human caspase 9 protein that activates the cell apoptosis cascade does not impair the capacity of these cells to rapidly expand in vivo.17,19,26 This evidence strongly supports the tightly regulated function of the engineered iC9 with negligible basal activity in the absence of the dimerizing drug.18 We now show that iC9-T cells persist in vivo for more than 2 years, indicating that the iC9.2A.ΔCD19 cassette used to engineer iC9-T cells has little significant spontaneous proapoptotic activity and does not induce a destructive immune response. These in vivo observations are in line with our recent ex vivo data indicating that even the “2A” junction sequence derived from Thosea Asigna insect virus within the otherwise human iC9.2A.ΔCD19 cassette does not elicit functional T-cell responses against iC9-T cells.27
Long-term persisting iC9-T cells (CD3+CD19+) in vivo are mostly CD8+ memory T cells. Of note, a fraction of them retain the expression of CD62L as central memory cells.28 Consistent with data previously published in clinical studies with HSV-TK gene-modified T cells, we observed that donor T-cell infusions accelerated the recovery of endogenous (defined as CD3+CD19−) T cells after haplo-HSCT.6,14 Patients infused with iC9-T cells had a rapid recovery of CD3+CD19− T cells, as 3 months after adoptive transfer, the absolute count of CD4+CD19− T cells was greater than 300 cells/µL, whereas similar T-cell counts are reached only by month 9 to 12 after haplo-HSCT without T lymphocyte add-back.6,7,14,29-31 In contrast to infused CD3+CD19+ T cells, the CD3+CD19− detected in vivo may originate either from expansion of residual donor-derived T cells contaminating the graft preparation of CD34+ selected HSCs or from newly formed T lymphocytes within the thymus.32-34 Generation of naive CD4+ T cells after haplo-HSCT has been demonstrated in pediatric patients in whom residual thymic function is preserved.35-39 In line with these data, we observed that although infused CD3+CD19+ iC9-T cells expanding in vivo are predominantly CD8+ memory T cells (which preferentially expand compared with memory CD4+ T cells in the first 6 months postallogeneic HSCT40,41 ), the reconstituting CD3+CD19− cells contain a significant fraction of CD4+ naive T cells, which are likely newly differentiated cells of thymic origin.34,35,40-42 Thus, infused CD3+CD19+ iC9-T cells appear to provide a previously described “helper” effect promoting the repopulation of endogenous naive T cells that is usually severely delayed in the absence of adoptive transfer of T lymphocytes after haplo-HSCT.14,34
The improved recovery of immune T cells after haplo-HSCT leads to a significant effect on control of infection. More than 40% of nonrelapse-related mortality after haplo-HSCT is caused by CMV or Aspergillus infection alone, and other viral infections also contribute to mortality and significant morbidity.9 Our current data demonstrate that infusion of iC9-T cells can promote the control of CMV, EBV, AdV, BKV, and (in 1 patient) Aspergillus infections after haplo-HSCT. All patients with these infections quickly reduced their antigen loads and clinical symptoms, paralleling an increase in the frequency of T-cell precursors specific for pathogenic antigens. Notably, both infused memory iC9-T cells and endogenously reconstituting T cells contributed in pathogen control, as we detected specific T-cell precursors in both CD3+CD19+ and CD3+CD19− T cells isolated in vivo from these patients.
We previously reported that in 4 patients who developed acute GvHD, a single administration of AP1903 resolved signs and symptoms related to acute GvHD.19 We can now conclude that the resolution of their GvHD was permanent. No recurrence or emergence of chronic GvHD subsequently occurred in these patients even though there was subsequent re-expansion of the residual and polyclonal population of iC9-T cells. Hence, a single treatment with AP1903 can permanently ablate residual alloreactive T cells in vivo. Of note, however, is that in patients with viral reactivation and concomitant occurrence of GvHD, the infusion of AP1903 did not permanently delete pathogen-specific iC9-T cells, as these cells recovered, eliminated the pathogen, and remained detectable for months. The mechanism responsible for this differential effect of AP1903 in vivo on alloreactive versus pathogen-specific T cells remains to be elucidated and may indeed simply be fortuitous and a reflection of the small numbers of patients so far treated. If such an effect is consistently seen, however, then it should be possible to replace the time-consuming in vitro allodepletion used in this protocol with an in vivo allodepletion mediated by the administration of the dimerizering drug as and when GvHD occurs.
Although we found that T cells recognizing tumor-associated antigens can be reactivated ex vivo from the peripheral blood both before and after AP1903 infusion (supplemental Figure 3), 3 of the 4 patients receiving AP1903 for control of GvHD subsequently relapsed compared with only 1 of 6 patients who were not so treated. This difference in relapse rate raises the concern that elimination of GvHD with AP1903 can concomitantly eliminate a graft-versus-leukemia effect from the donor T cells. For the moment, we believe such a conclusion would be premature: the patient numbers are small, the trial was uncontrolled, and the great majority of enrolled patients had lymphoid malignancies or biphenotypic leukemia, for which there is little good evidence for a substantive contribution from a graft-versus leukemia effect of donor T cells.8,9,43-47 This possibility will, however, need to be considered in the design of large-scale studies.
In conclusion, our study shows the safety and clinical benefits of infusing iC9-T cells in patients after haplo-HSCT, providing permanent control of GvHD and overcoming the opportunistic infections that still impede the success of haplo-HSCT.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are thankful to the patients and their families for their cooperation. We are grateful to Swati Naik for clinical data assistance; Melissa Gates, Keli Sharpe, and Sarah Driedger for flow cytometry; and Enli Liu, Olga Dakhova, Rong Cai, and Yijiu Tong for follow-up sample support.
This clinical protocol (IND13813) was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grant U54HL08100, and development of the caspase system was supported by grants P01CA094237 and P50CA126752. The clinical trial also received support from the Clinical Research Center at Texas Children’s Hospital and shared resources of the Dan L. Duncan Cancer Center support grant P30CA125123.
Authorship
Contribution: G.D., H.E.H., C.M.R., and M.K.B. designed the study; X.Z., A.D.S., and A.M.L. performed experiments; X.Z. and A.D.S. analyzed data; X.Z. and G.D. wrote the manuscript; A.D.S., H.E.H., and M.K.B. contributed to the preparation of the manuscript; R.A.K., C.M., and K.S.L. enrolled patients and monitored clinical responses; A.D.S., S.-K.T., B.S., G.D., A.P.G., and C.M.R. developed good manufacturing practice protocols; A.G.D. performed flow cytometry on patient samples; A.D.S. performed cell manufacturing; X.Z. and M.-F.W. performed the statistical analysis; H.L. designed biostatistical analysis for study; Y.-F.L. coordinated the study; and B.J.G. and H.E.H. ensured compliance with regulatory requirements for the clinical trial.
Conflict-of interest disclosure: D.M.S. is an employee of Bellicum Pharmaceuticals Inc., which provided the dimerizing agent AP1903. The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene.
Correspondence: Malcolm K. Brenner, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1660, Feigin Center, Houston, TX 77030; e-mail: mbrenner@bcm.edu; and Gianpietro Dotti, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1660, Feigin Center, Houston, TX 77030; e-mail: gdotti@bcm.edu.
References
Author notes
X.Z. and A.D.S. contributed equally to this study.