Key Points
Cold agglutinin–driven classical pathway activity terminates prior to the initiation of the terminal cascade in CAD patient blood.
By inhibiting cold agglutinin–mediated complement deposition on the cellular membrane, TNT003 prevents RBCs from being phagocytosed.
Abstract
Activation of the classical pathway (CP) of complement is often associated with autoimmune disorders in which disease pathology is linked to the presence of an autoantibody. One such disorder is cold agglutinin disease (CAD), an autoimmune hemolytic anemia in which autoantibodies (cold agglutinins) bind to red blood cells (RBCs) at low temperatures. Anemia occurs as a result of autoantibody-mediated CP activation on the surface of the erythrocyte, leading to the deposition of complement opsonins that drive extravascular hemolysis in the liver. Here we test the effects of TNT003, a mouse monoclonal antibody targeting the CP-specific serine protease C1s, on CP activity induced by cold agglutinins on human RBCs. We collected 40 individual CAD patient samples and showed that TNT003 prevented cold agglutinin–mediated deposition of complement opsonins that promote phagocytosis of RBCs. Furthermore, we show that by preventing CP activation, TNT003 also prevents cold agglutinin–driven generation of anaphylatoxins. Finally, we provide evidence that CP activity in CAD patients terminates prior to activation of the terminal cascade, supporting the hypothesis that the primary route of RBC destruction in these patients occurs via extravascular hemolysis. Our results support the development of a CP inhibitor for the treatment of CAD.
Introduction
The complement system is a family of plasma proteins that mediates humoral immune surveillance. Complement activation results in an enzymatic cascade leading to the production of opsonins and anaphylatoxins responsible for clearing pathogens and initiating inflammation. Complement can be triggered by one of three distinct pathways: the classical pathway (CP), alternative pathway (AP), or lectin (LP) pathway. Activation of the different pathways is mediated by pathway-specific proteins that recognize molecular patterns on pathogens. In the case of the CP, activation is driven by immune complexes containing complement-fixing antibodies. Although complement is one of the first lines of defense against infection, hyperactivity of specific pathways has been described in numerous autoimmune and inflammatory disease settings.1-3 In such indications, therapeutic intervention by preventing complement activation may have clinical utility.4
Cold agglutinin (CA) disease (CAD) is a rare form of autoimmune hemolytic anemia (AIHA) comprising approximately 15% of all AIHA cases with a reported incidence rate of ∼1/1 000 000.5 In addition to anemia, patients often present with fatigue, hemoglobinuria, and Raynaud’s phenomenon.6-10 The disease is driven by CAs, monoclonal immunoglobulin M (IgM) antibodies that bind to the I antigen on the red blood cell (RBC) surface in accordance to their thermal amplitude—the temperature below which the CA binds.11 C1 complex, a multimeric assembly consisting of the pattern recognition receptor C1q and the serine proteases C1r and C1s, binds to the CA-RBC immune complex to activate the CP leading to surface deposition of complement opsonins.9,11-13 Complement-coated RBCs are then engulfed by cells of the mononuclear phagocyte system, a process termed extravascular hemolysis (EVH).6,13,14 On rare occasions, such as during a heightened immune response, the activation of the CP can also lead to the formation of the membrane attack complex (MAC) and the rupturing of the RBCs, a process known as intravascular hemolysis (IVH).15,16 In this manner, a hyperactive CP driven by CAs is believed to be responsible for the RBC loss and anemia suffered by patients with CAD.
Evidence for CP activity in CAD patients comes from the analysis of complement proteins in their circulation. Compared with healthy individuals, CAD patient serum samples are hypocomplementemic for components in the CP such as C4, suggesting the consumption of these substrates due to pathway activity.8 Additionally, unlike in healthy individuals, circulating RBCs in CAD patients are coated with C3 fragments, evidence that complement activation is occurring on the surface of RBCs.8,9,11,12 Indeed, a clinical marker used to diagnose patients with CAD is C3d deposition on RBCs using the direct antiglobulin test.6,7,17
Here we describe the effects of TNT003, an immunoglobulin G2a (IgG2a) mouse monoclonal antibody (mAb) targeting the CP-specific serine protease C1s in ex vivo hemolysis assays with 40 unique CAD patient plasma samples. We found that CAD patient autoantibodies induce significant complement fragment deposition on human RBCs that leads to RBC engulfment by macrophage-like THP-1 cells. We show that TNT003 prevents CA-driven complement deposition on RBCs, and that these RBCs are rescued from phagocytosis. Furthermore, anaphylatoxin generation and hemolysis driven by CA activation of complement are also prevented in the presence of TNT003. Finally, analysis of patient plasma revealed that CAD samples contain reduced levels of upstream CP components but not C5, providing evidence of CP activity in the plasma of CAD patients that terminates prior to the initiation of the terminal cascade. Our results support the therapeutic use of a selective upstream CP inhibitor for the treatment of CAD.
Materials and methods
CAD patient plasma samples
CAD patient plasma samples (n = 40; average patient age of 65 ± 11 years; supplemental Table 1, available at the Blood Web site) were obtained after approval of patient consent forms and sample collection protocol by Western Institutional Review Board. Verification of CAD diagnosis was obtained from patients’ physicians. Samples were collected by PrecisionMed Inc. (Solana Beach, CA) in accordance with the Declaration of Helsinki. Blood was drawn in prewarmed (37°C) 10 mM EDTA-containing tubes to minimize RBC agglutination and complement activation and was immediately centrifuged to collect CA-containing plasma. Samples were frozen, shipped in dry ice to True North Therapeutics, Inc., and stored at −80°C. CA titers and thermal amplitudes were determined by LifeShare Blood Centers (see supplemental Methods).
TNT003 identification and generation
Mouse mAb TNT003 (isotype IgG2a) was identified by screening a hybridoma library from mice immunized with human-activated C1s protein (EMD Millipore, Billerica, MA) by using standard techniques. TNT003 was purified from hybridoma supernatants by using Protein A chromatography and was buffer exchanged into phosphate-buffered saline (PBS). All lots of TNT003 contained <1 EU/mg protein.
Ex vivo hemolysis assay
Human RBCs (hRBCs; blood type O negative; Allcells, Alameda, CA) were washed 3 times with gelatin veronal buffer (GVB; Complement Technology, Tyler, TX) and resuspended at 1 × 109 cells per milliliter in GVB plus 10 mM EDTA. To sensitize hRBCs with CAs, 10 μL CAD plasma containing 10 mM EDTA was mixed with 20 μL hRBC solution at 4°C for 45 minutes. To induce complement activation, CA-sensitized hRBCs were resuspended in 200 μL of 25% normal human serum (NHS) diluted 1:4 with GVB++ (GVB + 0.15 mM CaCl2 + 0.5 mM MgCl2) and incubated at 17°C for 1 hour. To determine the effect of TNT003 or isotype control (IC) antibody, experiments were performed in the presence of various concentrations of the same. To determine lysis, the optical density (OD) of the supernatant was measured at 540 nm. Complete lysis was determined by incubating hRBCs in ammonia lysis buffer. Nonsensitized RBCs plus 25% NHS were used as background. CA-sensitized hRBCs plus 25% NHS plus 10 mM EDTA were used to determine the occurrence of complement-mediated lysis. Percent lysis = 100 × (ODsample/ODcomplete lysis).
C3 fragment detection by flow cytometry
Cell-bound C3 fragments were detected by staining hRBCs at 4°C for 45 minutes with a murine mAb recognizing human C3/C3b/iC3b (clone 6C9; 20 μg/mL; Thermo Scientific, Rockford, IL). After 3 washes in fluorescence-activated cell sorter (FACS) buffer (PBS plus 0.5% bovine serum albumin plus 0.1% NaN3), Alexa Fluor 488 goat anti-mouse IgG1 (1.5 μg/mL; Life Technologies, Grand Island, NY) was added at room temperature (RT) for 30 minutes. Cells were washed 3 times in FACS buffer and were analyzed by flow cytometry. FlowJo software, version 7.6.5 (Tree Star, Inc., Ashland, OR) was used for analysis. Nonsensitized hRBCs plus 25% NHS were used as the background to gate CA-mediated C3 fragment deposition; 30 000 events were acquired for each sample. For experiments comparing C3 fragment deposition across CAD samples, all samples (n = 40) were run on the same plate using the same NHS as a complement source and the same hRBC source.
hRBC preparation for phagocytosis
hRBCs were washed 3 times with cold PBS and then stained with 20 μM CellTracker Green 5-chloromethylfluorescein diacetate (Life Technologies) for 2 hours at 37°C. Labeled hRBCs were washed and incubated in PBS at 37°C for 1 hour. hRBCs were washed and resuspended with GVB plus EDTA at 1 × 109 cells per milliliter. Cells were then sensitized in CAD plasma as described above and resuspended in 20 μL PBS at 4°C overnight. The following day, 25% NHS was added to induce complement activation. To assess the effect of complement inhibitors on phagocytosis, NHS was added in the presence of TNT003, anti-C5 mAb (Quidel, San Diego, CA), or IC. Then, 50 μL cold GVB was added to stop the reaction, and the plate was centrifuged. RBCs were washed, resuspended in 25 μL PBS, then gently dispersed after incubation at 37°C for 30 minutes. Each treatment condition was run in duplicate.
THP-1
Human monocytic THP-1 cells (ATCC, Manassas, VA) were cultured in RPMI-1640 plus 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid plus 10% fetal bovine serum. Four days prior to the phagocytosis assay, cells were differentiated by using 3 nM retinoic acid. Immediately prior to the phagocytosis assay, differentiated cells were centrifuged and resuspended in RPMI-1640 without serum and were seeded at 10 000 cells per well in two 96-well round-bottom plates. Cells were incubated with 5 μL human TruStain Fc receptor blocking solution (human TruStain FcX; BioLegend, San Diego, CA) per well at RT for 10 minutes to prevent FcR-mediated phagocytosis.
Phagocytosis
Then, 10 μL labeled hRBCs was incubated with differentiated THP-1 cells at 37°C for 2 hours. A duplicate plate was incubated at 4°C as a negative control. Cells were then washed in PBS and centrifuged. Nonphagocytosed hRBCs were lysed by incubating cells in hypotonic buffer (0.2% NaCl) for 1 minute. Cells were then washed 3 times and resuspended in 80 μL FACS buffer for flow cytometry. Phagocytosis was quantified as the percentage of CellTracker Green–positive THP-1 cells (OD = 488 nm); 60 000 events were acquired for each sample. C3 fragment deposition was performed on hRBCs in the duplicate well as described above.
Enzyme-linked immunosorbent assay (ELISA)
To quantify C1s levels in plasma, high-binding enzyme immunosorbent assay plates (Costar) were coated with 100 ng anti-C1s antibody per well (Abcam, Cambridge, MA) and incubated overnight at 4°C. Protein solution was then removed, and the plate was blocked with 250 μL Dulbecco’s phosphate-buffered saline (DPBS) plus 1% casein at RT for 2 hours, then washed 2 times with 300 μL DPBS. Plasma samples were diluted 1:400 in DPBS containing 0.1% polyoxyethylene (20) sorbitan monolaurate and 20 mM EDTA (DPBS-TE). A 24-point standard curve of inactive C1s (Complement Technology) was prepared by twofold serial dilutions of 1 μg/mL inactive C1s in DPBS-TE. Next, 100 μL of standard or plasma was added to the plate, incubated at RT for 2 hours, then washed 5 times with 300 μL DPBS-TE. Subsequently, 100 ng biotinylated TNT003 was added to each well at RT for 1 hour and then washed 5 times. Then, 100 μL streptavidin horseradish peroxidase (1:2000 dilution; Southern Biotech) was added to each well at RT for 1 hour. Plates were washed 5 times, and 100 μL ultra 3,3′,5,5′-tetramethylbenzidine substrate (Thermo Scientific) was added. The color was developed for 5 minutes, and the reaction was stopped with 100 μL 1 N sulfuric acid. Absorbance (OD = 450 nm) was read on a spectrophotometer, and plasma C1s concentration was interpolated from the standard curve.
Levels of C3a, C4a, and C5a in hemolysis assay supernatants were quantified by ELISA (OptEIA; BD Biosciences). Quantified levels of endogenous anaphylatoxins in the NHS were subtracted to generate TNT003 concentration response curves. ELISAs were used to quantify C4 (Abnova, Taipei City, Taiwan), C2 (Abcam), and C5 (Abnova) in CAD and healthy plasma. ELISAs were performed according to manufacturer’s protocol.
Statistical analysis
A normal distribution of the concentrations of plasma complement proteins within cohorts (healthy and CAD) and CA titers in CAD plasma samples was assessed on the basis of visual inspection of histograms and Q-Q plots. Values were natural log transformed to yield a more normal distribution for statistical analyses. All data were expressed as the mean ± standard error. P < .05 was considered significant.
Results
TNT003 inhibition of C3 fragment deposition induced by CAD autoantibodies
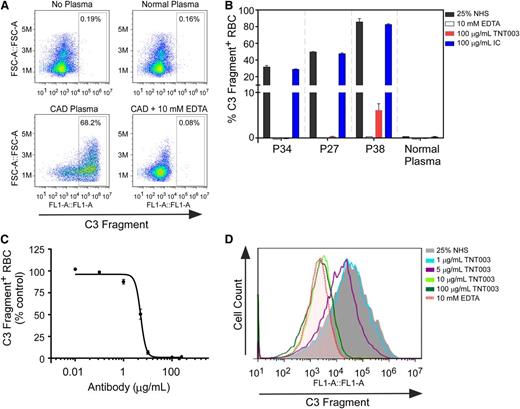
We collected plasma samples from 40 clinically diagnosed CAD patients to test the hypothesis that CP inhibition would prevent CA-mediated complement activation and deposition. Flow cytometry analysis revealed that hRBCs sensitized in CAD plasma, but not healthy plasma, deposited complement on the hRBC surface after exposure to 25% NHS (Figure 1A-B). Of the 40 CAD samples collected, 28 (70%) deposited C3 fragments detectable by using our protocol. C3 fragment deposition did not occur when cells were exposed to NHS chelated with 10 mM EDTA (Figure 1A-B). We found that 100 µg/mL TNT003, but not IC, prevented C3 fragment deposition by 98.7% ± 2.4% (n = 28 samples) on average. Furthermore, TNT003 potently inhibited CAD plasma–mediated C3 fragment deposition in a concentration-dependent manner with an average 50% inhibition concentration (IC50) of 5.0 ± 1.0 µg/mL (33 nM) across all patient samples (supplemental Table 1).
CAD patient plasma samples induce C3 fragment deposition on normal hRBCs that is inhibited by TNT003. (A) Flow cytometry plots from hRBCs exposed to the specified plasma sample (sensitization) and subsequently exposed to 25% NHS (complement activation). All plasma samples contained 10 mM EDTA; “CAD + 10 mM EDTA” refers to NHS exposure in the presence of EDTA. Y-axis: forward scatter (FCS, as defined in the y-axis); x-axis: cell surface C3 fragments. FL1-A, the fluorescence intensity measured, which corresponds to the amount of cell surface C3 fragments as defined in x-axis. (B) Bar graphs of C3 fragment deposition on hRBCs exposed to 3 representative CAD patient plasma samples (patient 34 annotated as P34, and so on) or a normal plasma sample, and subsequently exposed to NHS in the presence of 10 mM EDTA, TNT003, or an IC IgG2a antibody. (C) Averaged concentration response curve for TNT003 inhibition of C3 fragment deposition for all CAD patient samples. IC50 = 5.0 ± 1.0 μg/mL (33 ± 6 nM); n = 27 samples. Data were normalized across different patient samples by setting C3 fragment deposition occurring in the presence of NHS at 100%. Individual IC50 values for each patient sample are provided in supplemental Table 1. (D) Histograms showing the effect of increasing TNT003 concentration on CA-mediated C3 fragment deposition. The shaded histogram depicts C3 fragment deposition in the absence of TNT003.
CAD patient plasma samples induce C3 fragment deposition on normal hRBCs that is inhibited by TNT003. (A) Flow cytometry plots from hRBCs exposed to the specified plasma sample (sensitization) and subsequently exposed to 25% NHS (complement activation). All plasma samples contained 10 mM EDTA; “CAD + 10 mM EDTA” refers to NHS exposure in the presence of EDTA. Y-axis: forward scatter (FCS, as defined in the y-axis); x-axis: cell surface C3 fragments. FL1-A, the fluorescence intensity measured, which corresponds to the amount of cell surface C3 fragments as defined in x-axis. (B) Bar graphs of C3 fragment deposition on hRBCs exposed to 3 representative CAD patient plasma samples (patient 34 annotated as P34, and so on) or a normal plasma sample, and subsequently exposed to NHS in the presence of 10 mM EDTA, TNT003, or an IC IgG2a antibody. (C) Averaged concentration response curve for TNT003 inhibition of C3 fragment deposition for all CAD patient samples. IC50 = 5.0 ± 1.0 μg/mL (33 ± 6 nM); n = 27 samples. Data were normalized across different patient samples by setting C3 fragment deposition occurring in the presence of NHS at 100%. Individual IC50 values for each patient sample are provided in supplemental Table 1. (D) Histograms showing the effect of increasing TNT003 concentration on CA-mediated C3 fragment deposition. The shaded histogram depicts C3 fragment deposition in the absence of TNT003.
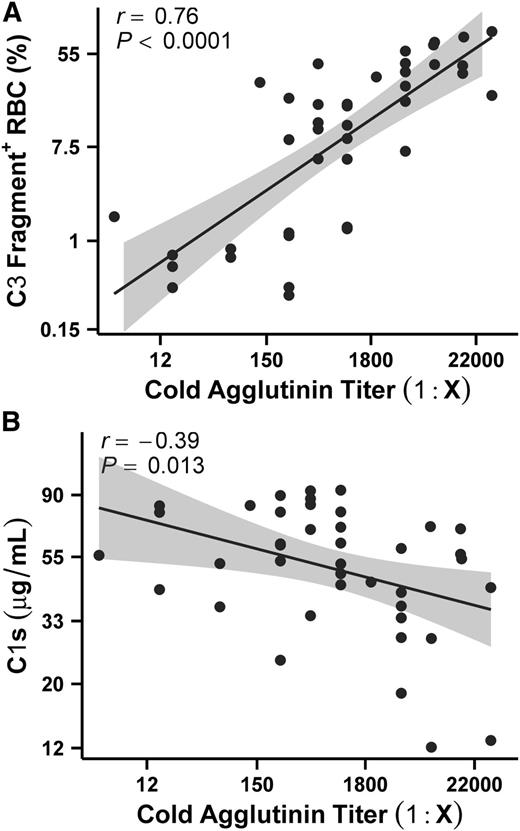
These experiments revealed that the degree to which C3 fragments deposited on the hRBC surface differed from sample to sample (Figure 1B). We hypothesized that if CAs were responsible for complement activation, then CA titers should correlate with the degree of complement deposition on the hRBCs. We used the same source of NHS for all CAD plasma samples, thereby isolating the CAs as the only variable for complement activation. We found that the percentage of hRBCs staining positive for C3 fragments was positively correlated with the CA titer in the plasma at 4°C (Figure 2A; supplemental Table 1). We next hypothesized that if the CP was specifically responsible for driving complement activation, then consumption of a CP-specific protein such as C1s may also correlate with CA titers. Indeed, we found that C1s plasma concentrations correlated with CA titers (Figure 2B). These findings support the role of CAs driving CP complement activation and demonstrate that TNT003 prevents CAD autoantibody–mediated complement deposition on hRBCs.
CA titers in CAD patient plasma correlate with the extent of C3 fragment staining on the RBC surface. (A) Complement deposition, quantified as the percentage of hRBCs staining positive for surface C3 fragments after sensitization in CAD plasma and subsequent NHS exposure, is correlated with CA titers in the plasma sample. Correlation analyses were performed on natural log transformed values (see “Materials and methods”) but are depicted as 1:Ab titer for simplicity. All 40 CAD samples were run on the same plate using the same source of NHS and hRBCs. Line represents the line of best fit. n = 40 samples with 2 independent experiments per sample; see also supplemental Table 1. (B) C1s levels were found to correlate with CA titers. As in (A), correlation analyses were performed on natural log transformed values but graphed as 1:Ab titer. n = 40 samples with 2 independent experiments per sample.
CA titers in CAD patient plasma correlate with the extent of C3 fragment staining on the RBC surface. (A) Complement deposition, quantified as the percentage of hRBCs staining positive for surface C3 fragments after sensitization in CAD plasma and subsequent NHS exposure, is correlated with CA titers in the plasma sample. Correlation analyses were performed on natural log transformed values (see “Materials and methods”) but are depicted as 1:Ab titer for simplicity. All 40 CAD samples were run on the same plate using the same source of NHS and hRBCs. Line represents the line of best fit. n = 40 samples with 2 independent experiments per sample; see also supplemental Table 1. (B) C1s levels were found to correlate with CA titers. As in (A), correlation analyses were performed on natural log transformed values but graphed as 1:Ab titer. n = 40 samples with 2 independent experiments per sample.
TNT003 inhibits phagocytosis of hRBCs exposed to CAs and NHS
To test the hypothesis that CA-induced complement deposition on hRBCs promotes erythrophagocytosis, we used the phagocytic THP-1 cell line. We found that hRBCs presensitized in normal human plasma and exposed to NHS showed little complement deposition on the cell surface (red bars) and little uptake (black bars) by THP-1 cells (Normal Plasma plus 25% NHS; Figure 3A). Similar levels of complement deposition and phagocytosis were observed in cells presensitized in CA patient plasma, but not exposed to NHS (No NHS). In contrast, CA-presensitized hRBCs exposed to NHS exhibited a significant amount of C3 fragment deposition, and showed approximately fourfold increase in uptake by THP-1 cells compared with controls (Figure 3A-B).
TNT003 prevents phagocytosis of hRBCs exposed to CAD patient plasma samples and NHS. (A) Representative example of the phagocytosis assay run by using a single patient sample. CellTracker Green–labeled hRBCs sensitized in plasma and subsequently exposed to 25% NHS were incubated in the presence of retinoic acid–treated THP-1 cells to induce phagocytosis. hRBCs sensitized in the presence of normal human plasma and subsequently exposed to NHS showed little C3 fragment deposition on the cell surface (red bars, right y-axis) and were minimally taken up by THP-1 cells (black bars, left y-axis), similar to what was observed with CA-sensitized hRBCs not exposed to NHS. In contrast, CA-sensitized hRBCs exposed to NHS exhibited significant C3 fragment deposition on their cell surface and were readily phagocytosed by THP-1 cells. NHS exposure in the presence of an IC IgG2 mAb or an anti-C5 mAb (both 100 μg/mL) did not prevent C3 fragment deposition or RBC phagocytosis. In contrast, TNT003 (100 μg/mL) prevented both C3 fragment deposition and hRBC phagocytosis. (B) Bar graph depicting data generated from 10 different experiments using 5 separate CAD patient samples (2 experiments per patient sample). Data are normalized to control (CAD plasma plus 25% NHS) for both phagocytosis and C3 fragment deposition across all experiments.
TNT003 prevents phagocytosis of hRBCs exposed to CAD patient plasma samples and NHS. (A) Representative example of the phagocytosis assay run by using a single patient sample. CellTracker Green–labeled hRBCs sensitized in plasma and subsequently exposed to 25% NHS were incubated in the presence of retinoic acid–treated THP-1 cells to induce phagocytosis. hRBCs sensitized in the presence of normal human plasma and subsequently exposed to NHS showed little C3 fragment deposition on the cell surface (red bars, right y-axis) and were minimally taken up by THP-1 cells (black bars, left y-axis), similar to what was observed with CA-sensitized hRBCs not exposed to NHS. In contrast, CA-sensitized hRBCs exposed to NHS exhibited significant C3 fragment deposition on their cell surface and were readily phagocytosed by THP-1 cells. NHS exposure in the presence of an IC IgG2 mAb or an anti-C5 mAb (both 100 μg/mL) did not prevent C3 fragment deposition or RBC phagocytosis. In contrast, TNT003 (100 μg/mL) prevented both C3 fragment deposition and hRBC phagocytosis. (B) Bar graph depicting data generated from 10 different experiments using 5 separate CAD patient samples (2 experiments per patient sample). Data are normalized to control (CAD plasma plus 25% NHS) for both phagocytosis and C3 fragment deposition across all experiments.
We next tested the ability of TNT003 to prevent CA-mediated complement-dependent uptake of hRBCs. When exposure of CA-presensitized hRBCs to NHS occurred in the presence of 100 μg/mL TNT003, both C3 fragment deposition and phagocytosis were reduced to control levels, similar to what was observed in 10 mM EDTA (Figure 3A-B). In contrast, neither an anti-C5 mAb nor the IC showed inhibition of C3 fragment deposition or phagocytosis. Finally, we checked whether TNT003-C1 complex was present on the hRBC surface. We found that in the presence of CAs, TNT003 can be detected on the hRBCs via flow cytometry, likely bound to the C1 complex which, in turn, is bound to the CA. However, upon warming (as would occur in vivo as the RBCs circulate to the liver) CA-bound TNT003-C1 complexes dissociate from the hRBCs (data not shown).
TNT003 inhibition of CA-mediated hemolysis and anaphylatoxin production
Because the CP is highly dependent on free calcium and magnesium, we exposed CA-presensitized hRBCs to NHS in the presence or absence of 10 mM EDTA to assess whether samples were able to induce direct cellular lysis of hRBCs. We found that CAD samples induced some lysis of hRBCs, even in the presence of 10 mM EDTA, suggesting that in vitro sensitization of hRBCs in CAD plasma induced some complement-independent lysis.18 However, of the 40 CAD samples tested, only plasma from patient 5 (P5), the sample found to contain the highest titers of CAs at both 4°C and 24°C (supplemental Table 1), exhibited significant EDTA-dependent hemolysis. In a concentration-dependent manner, TNT003 completely inhibited complement-dependent lysis of P5-sensitized hRBCs with an IC50 of 5.1 µg/mL (34 nM; Figure 4A).
TNT003 prevents CA-mediated hRBC lysis and anaphylatoxin generation. (A) hRBCs exposed to CAD plasma sample P5 followed by 25% NHS exhibited complement-dependent hemolysis that was inhibited by TNT003, but not IC, in a concentration-dependent manner (IC50 = 5.1 μg/mL; 34 nM). Supernatant from these experiments was assayed for C4a (B; IC50 = 5.2 μg/mL; 35 nM), C3a (C; IC50 = 5.2 μg/mL; 35 nM), and C5a (D; IC50 = 4.9 μg/mL; 33 nM) by plate-based ELISA. Data are normalized to control (P5 plasma plus 25% NHS) and depict the average of 3 experiments.
TNT003 prevents CA-mediated hRBC lysis and anaphylatoxin generation. (A) hRBCs exposed to CAD plasma sample P5 followed by 25% NHS exhibited complement-dependent hemolysis that was inhibited by TNT003, but not IC, in a concentration-dependent manner (IC50 = 5.1 μg/mL; 34 nM). Supernatant from these experiments was assayed for C4a (B; IC50 = 5.2 μg/mL; 35 nM), C3a (C; IC50 = 5.2 μg/mL; 35 nM), and C5a (D; IC50 = 4.9 μg/mL; 33 nM) by plate-based ELISA. Data are normalized to control (P5 plasma plus 25% NHS) and depict the average of 3 experiments.
By using the supernatant from hemolysis experiments performed with P5 plasma, we addressed whether TNT003 was capable of preventing the production of the anaphylatoxins. We found that TNT003 inhibited the generation of C4a, C3a, and C5a driven by P5 plasma in a concentration-dependent manner (Figure 4B-D).
CAD patients are hypocomplementemic for upstream CP substrates
The results from our hemolysis assays suggest that although circulating plasma levels of CAs in CAD patients are capable of inducing C3 fragment deposition on hRBCs, CA titers are rarely high enough to drive terminal complement pathway activation. We hypothesized that if the CP terminates following C3 cleavage in CAD patients, then levels of upstream CP components such as C4 and C2 should be reduced in CAD plasma due to consumption. We found that CAD patients had significantly decreased plasma levels of both C4 and C2 compared with healthy donors (Figure 5A-B). We also compared the concentrations of C5 and found that unlike C4 and C2, C5 levels are comparable between CAD and healthy plasma (Figure 5C). Finally, we found that C1s levels in CAD plasma were significantly lower in CAD samples compared with healthy samples, supporting the specific role of the CP in mediating complement deposition in CAD (Figure 5D).
CAD patient plasma samples are hypocomplementemic for upstream CP components. (A) C4, (B) C2, (C) C5, and (D) C1s concentrations were determined in CAD (solid bars) and healthy (open bars) plasma samples by using plate-based ELISAs. Plasma concentrations of complement components were natural log transformed to yield normal distributions for statistical analysis. C4 was significantly reduced in CAD samples compared with healthy samples (e4.24 ± 0.24 vs e5.98 ± 0.35; n = 28 and 12, respectively). C2 also was reduced in CAD vs healthy samples (e3.40 ± 0.07 vs e3.94 ± 0.13; n = 37 and 12, respectively). By contrast, there was no difference in the C5 plasma concentrations between CAD and healthy samples (e4.50 ± 0.03 vs e4.37 ± 0.06; n = 37 and 12, respectively). Finally, C1s levels in CAD plasma samples were significantly reduced compared with healthy samples (e3.91 ± 0.07 vs e4.24 ± 0.12; n = 40 and 13, respectively). *P < .05; ***P < .001.
CAD patient plasma samples are hypocomplementemic for upstream CP components. (A) C4, (B) C2, (C) C5, and (D) C1s concentrations were determined in CAD (solid bars) and healthy (open bars) plasma samples by using plate-based ELISAs. Plasma concentrations of complement components were natural log transformed to yield normal distributions for statistical analysis. C4 was significantly reduced in CAD samples compared with healthy samples (e4.24 ± 0.24 vs e5.98 ± 0.35; n = 28 and 12, respectively). C2 also was reduced in CAD vs healthy samples (e3.40 ± 0.07 vs e3.94 ± 0.13; n = 37 and 12, respectively). By contrast, there was no difference in the C5 plasma concentrations between CAD and healthy samples (e4.50 ± 0.03 vs e4.37 ± 0.06; n = 37 and 12, respectively). Finally, C1s levels in CAD plasma samples were significantly reduced compared with healthy samples (e3.91 ± 0.07 vs e4.24 ± 0.12; n = 40 and 13, respectively). *P < .05; ***P < .001.
Discussion
CAD is a form of AIHA in which autoantibodies bind to RBCs at low temperatures to activate the CP on the cell surface, leading to RBC loss and anemia. Although the disease is often described as indolent, half of all patients become transfusion-dependent at some point during the course of the disease.6,7 Although many healthy individuals have CAs in their circulation, autoantibody thermal amplitude as well as titer are low, and the vast majority do not exhibit clinical manifestations of the disease.10,17,19 In CAD patients, the high titers of CAs are often attributed to a lymphoproliferative B-cell disorder, identified in approximately 75% of chronic CAD patients.5 In addition to titer, the thermal amplitude of the antibody is a critical parameter that defines the severity of the disease. Antibodies with high thermal amplitudes remain bound to the RBCs for longer periods of time during circulation, thereby increasing the likelihood of complement activation. Additionally, because of the enzymatic nature of the cascade, complement activation is more efficient at higher temperatures. Thus, for patients with high titer and high thermal amplitude CAs, the disease can be debilitating and life-altering to the extent that patients undertake extraordinary measures to ensure avoidance of cold or even moderate temperatures.
Anemia in CAD patients is believed to be the result of CA activation of the CP on the RBCs.9,13 Indeed, our data show a strong correlation between CA titers and complement deposition (Figure 2). We found that although the majority of CAD plasma samples induced complement deposition on RBCs, 12 (30%) did not. Upon further analysis, 10 of these 12 had CA titers ≤256 (supplemental Table 1). Because CA titer is known to fluctuate in CAD patients and has been shown to track with hemolysis,11 our results suggest that these samples were obtained during a relatively quiescent phase of the disease for these patients.
In the majority of CAD patients, the autoantibody is an IgM,5,10 a multivalent immunoglobulin capable of activating C1 complex, the trigger of the CP. This binding event induces autocleavage of the serine protease C1r, which cleaves the zymogen form of C1s. Activated C1s then cleaves soluble C4 and C2 to form the membrane-bound C3 convertase, responsible for the cleavage of C3 and the deposition of the C3b opsonin on the RBC surface. Factor I cleaves C3b into iC3b which retains opsonic activity, but further conversion to C3d attenuates it.9 Recognition of C3b/iC3b-coated cells by complement receptors on phagocytes leads to the removal of these cells from the circulation via EVH (Figure 6).6,20,21 We show that TNT003 inhibits CA-mediated C3 fragment deposition on RBCs, concomitantly preventing their engulfment by macrophage-like THP-1 cells (Figure 3). Finally, we show that TNT003 also prevents the production of the anaphylatoxins C4a, C3a, and C5a driven by CAs (Figure 4B-D). Although inhibition of the production of anaphylatoxins may dampen inflammatory responses to infections, their production may result in the exacerbation of autoimmune pathology. In a murine passive transfer model of AIHA, C5a activation of Kupffer cells was required for the development of full-blown AIHA.22 The authors propose a mechanism by which C5a induces the upregulation of FcγR expression on the surface of Kupffer cells leading to RBC removal via EVH. Although the authors did not check for the presence of complement receptors (CRs), C5aR-mediated upregulation of the phagocytic mediator CR3 (CD11b) on leukocytes has been reported.23,24 Thus, by preventing CA-mediated production of anaphylatoxins, TNT003 may also prevent CR-dependent uptake of C3b/iC3b-opsonized RBCs.
CA activation of the classical complement pathway on RBCs results in anemia in CAD patients. (1) CA-bound RBCs fix C1, the CP-activating complex, on the RBC surface, which triggers the classical complement cascade. (2) Activation of the CP results in complement opsonin deposition (C3b) on the RBC surface. (3) In general, CP activity terminates after C3 cleavage, and C3b/iC3b-opsonized RBCs then travel to the liver where they are phagocytosed by liver-resident macrophages, a process termed extravascular hemolysis. (4) Under exceptional circumstances such as immunologic stress that induces production of complement proteins, activation of the terminal complement cascade can occur, resulting in the formation of the membrane attack complex and direct cellular lysis known as intravascular hemolysis. By inhibiting classical complement pathway activation at the level of the C1 complex, an upstream CP inhibitor such as TNT003 can prevent both extra- and intravascular hemolysis.
CA activation of the classical complement pathway on RBCs results in anemia in CAD patients. (1) CA-bound RBCs fix C1, the CP-activating complex, on the RBC surface, which triggers the classical complement cascade. (2) Activation of the CP results in complement opsonin deposition (C3b) on the RBC surface. (3) In general, CP activity terminates after C3 cleavage, and C3b/iC3b-opsonized RBCs then travel to the liver where they are phagocytosed by liver-resident macrophages, a process termed extravascular hemolysis. (4) Under exceptional circumstances such as immunologic stress that induces production of complement proteins, activation of the terminal complement cascade can occur, resulting in the formation of the membrane attack complex and direct cellular lysis known as intravascular hemolysis. By inhibiting classical complement pathway activation at the level of the C1 complex, an upstream CP inhibitor such as TNT003 can prevent both extra- and intravascular hemolysis.
IVH in CAD patients is a rare phenomenon.6 The membrane-bound complement regulatory proteins CD55 (decay accelerating factor) and CD59 are likely responsible for attenuating complement activation on the RBC surface and preventing MAC formation and subsequent IVH. Indeed, in order to induce CAD sample–mediated complement-dependent hemolysis, previous studies used RBCs from patients with paroxysmal nocturnal hemoglobinuria (PNH) that lack surface expression of both CD55 and CD59.11,25 Of the 40 CAD samples we tested, only sample P5 was able to directly induce MAC-driven lysis of RBCs (Figure 4A). Thus, IVH in CAD is rare but can occur following exposure to extreme cold or upon immunologic stress that induces production of complement proteins that lead to the activation of the terminal complement cascade.16 Our analyses of the complement proteins in plasma samples from 40 CAD patients show that the early CP components C1s, C4, and C2 are found at significantly decreased levels compared with healthy samples, consistent with findings reported in previous studies.8 C5 levels, however, were comparable between healthy and CAD patient samples. Taken together, these results suggest that CA-driven CP activity generally terminates following C3 cleavage.
These results support the idea that RBC destruction in CAD patients occurs primarily as a result of EVH driven by upstream CP opsonins. Nevertheless, TNT003, by virtue of blocking at the level of the C1 complex, prevents both C3b deposition as well as the formation of the MAC. Thus, use of a proximal CP inhibitor would prevent both the EVH responsible for the chronic low-grade anemia characteristic of the quiescent phase of the disease, and though demonstrated with only one patient sample here, likely the episodic IVH that occurs during acute hemolytic crises.
CAD is a rare disease for which there is no approved therapy. The off-label use of rituximab, an anti-CD20 mAb, has been shown to be effective for achieving partial responses in a subset of CAD patients, although most patients ultimately relapse.26-28 In some cases, fludarabine, a chemotherapeutic agent used to treat hematologic malignancies, has been used in combination with rituximab and has shown promise in providing complete responses in some patients.29 However, CAD patients tend to be elderly, and avoiding the use of a chemotherapeutic agent in such a patient population is preferred.30
Because complement-mediated loss of RBCs is the primary driver of anemia in CAD, an alternate approach to treatment could be the use of complement inhibitors. Although not likely to control the underlying lymphoproliferative disorder, complement inhibition would modify the disease by preventing CA-driven hemolysis and anemia. Complement inhibitors have been proven to be safe and efficacious for the treatment of several hematologic disorders. Eculizumab (Soliris), an anti-C5 mAb, is used for the treatment of PNH and atypical hemolytic uremic syndrome.31-34 Both diseases are driven by uncontrolled AP activity on the surface of RBCs resulting in hemolytic anemia. C1 inhibitor (C1-INH) (CINRYZE; Berinert), an endogenous plasma protein responsible for regulating complement activity, is currently used for the treatment of hereditary angioedema.35-37 Case reports document the successful use of both eculizumab and C1-INH for treating CAD15 and warm AIHA,38 respectively, underscoring the role of complement driving AIHA pathology. However, because eculizumab blocks only the terminal complement cascade, the use of eculizumab for CAD would be limited to the most severe cases of CAD in which IVH is a chronic issue. As demonstrated in Figure 3A, C5 inhibition will not prevent upstream complement opsonin deposition and, in fact, may exacerbate the EVH occurring in CAD patients. Indeed, RBCs from PNH patients treated with eculizumab accumulate C3 opsonins on their cell surface. As a result, these RBCs, which in the absence of eculizumab would have undergone IVH, now potentially undergo EVH as evidenced by their sequestration in the liver and spleen of eculizumab-treated PNH patients.39,40 C1-INH, unlike eculizumab, inhibits complement activation upstream of opsonin deposition. However, endogenous levels of C1-INH in plasma are ∼200 μg/mL. Thus, in the lone case report of the successful use of C1-INH for warm AIHA, the authors infused 13 000 U of C1-INH over a period of 50 hours to prevent loss of transfused RBC concentrates.38 Although it was effective at preventing hemolysis, the dosing regimen required to achieve therapeutically meaningful concentrations above already high endogenous plasma C1-INH levels may not be practical for chronic diseases such as CAD or warm AIHA.
The results from this study provide evidence that a C1s inhibitor can prevent complement activation driven by CAs in CAD patients. Unlike eculizumab, which inhibits the terminal complement cascade driven by all complement pathways, or C1-INH, which inhibits both CP and LP, targeting pathway-specific proteins such as C1s provides the advantage of selectively inhibiting only the hyperactive pathway, leaving the other arms of complement to mediate immune surveillance. Although the data presented here have been generated with CAD patient plasma samples, a CP inhibitor could prove useful in other hematologic diseases such as warm AIHA, as well as indications across other therapeutic areas in which CP activity drives disease pathology. The data here lend support for the development of a humanized form of TNT003 for the treatment of CAD and other CP-mediated diseases.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr JoAnn Moulds and LifeShare Blood Centers in Shreveport, LA, for determining thermal amplitudes and CA titers of CAD patient plasma samples. The authors also thank Dr John P. Atkinson, Dr Steven R. Sloan, and members of True North Therapeutics, Inc., for valuable suggestions and discussion. The authors are particularly grateful to all the CAD patients who donated plasma samples for this study, and to Betty Usdan, the coordinator of the online Cold Agglutinin Disease forum (http://www.coldagglutinindisease.org).
Authorship
Contribution: J.S., E.L.R., and S.P. designed the research; J.S., E.L.R., A.S., and S.H. conducted the research and analyzed the data; N.E.S. and G.C.P. provided valuable suggestions and guidance; and J.S. and S.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors are employed by and are shareholders of True North Therapeutics, Inc. N.E.S. is a board member.
Correspondence: Sandip Panicker, True North Therapeutics, Inc., 951 Gateway Blvd, South San Francisco, CA, 94080; e-mail: sandip@truenorthrx.com.