In this issue of Blood, Kapur et al show that maternal human platelet-specific antigen 1a (HPA 1a)-specific antibodies causing neonatal alloimmune thrombocytopenia (NAIT) possess oligosaccharides that are deficient in “core fucose” residues and appear to be more effective than fucosylated antibodies in promoting phagocytosis of antibody-coated platelets.1
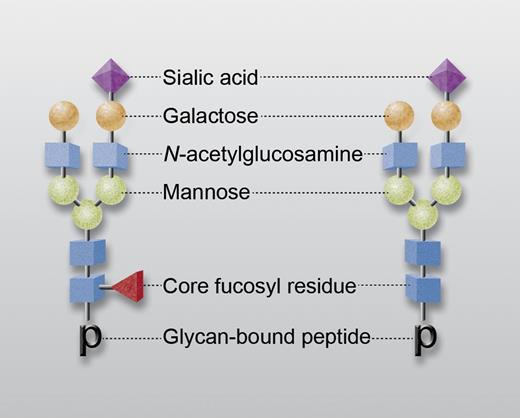
Representative IgG-associated glycans with (left) and without (right) a core fucosyl residue (red triangle). Other saccharides are N-acetylglucosamine (blue), mannose (green), galactose (orange), and sialic acid (purple). “p” designates glycan-bound peptide in tryptic digest subjected to mass spectroscopic analysis. Professional illustration by Alice Y. Chen.
Representative IgG-associated glycans with (left) and without (right) a core fucosyl residue (red triangle). Other saccharides are N-acetylglucosamine (blue), mannose (green), galactose (orange), and sialic acid (purple). “p” designates glycan-bound peptide in tryptic digest subjected to mass spectroscopic analysis. Professional illustration by Alice Y. Chen.
Each immunoglobulin G (IgG) molecule contains 2 oligosaccharide groups linked to asparagine residues at the 297 positions of the Fc domain. Each glycan usually consists of a complex heptasaccharide core containing N-acetylglucosamine (GlcNAc) and mannose to which variable numbers of galactose, fucose, sialic acid, and sometimes bisecting GlcNAc residues are attached (see figure). It is now well established that the character of these glycans can critically influence immunoglobulin function, particularly by modulating affinity for Fcγ receptors.2-4 One of these posttranslational modifications, the addition of a fucose residue in α1,6 linkage to the first GlcNAc of the oligosaccharide core (“core fucosylation”), modulates the affinity of IgG Fc for the FcγRIII receptor expressed on natural killer cells, macrophages, neutrophils, and other cells. IgG molecules lacking a core-fucose residue bind more tightly to FcγRIII and exhibit enhanced cellular immune function, for example, are more effective in antibody-dependent cellular cytotoxicity.5-7 The molecular basis for this effect was recently characterized by Ferrara and coworkers8 ; the potential advantage of using monoclonal antibodies lacking a core-fucose residue in cancer chemotherapy is currently under investigation.3,9 Up to 30% of IgG molecules in normal human serum lack a core-fucose residue, but how core fucosylation is regulated, and the extent to which it influences the severity of antibody-mediated human disease, are poorly understood.
NAIT, a significant cause of morbidity and mortality in newborns, is caused by maternal antibodies specific for an HPA inherited by the fetus from its father.10 The antigen against which these antibodies are most often directed is designated “HPA-1a.” In a woman sensitized to HPA-1a and carrying a fetus at risk for NAIT, a tool capable of predicting NAIT severity could be extremely helpful in optimizing prenatal and perinatal management. Various studies have shown that serologic measurement of antibody potency alone is not sufficient for this purpose.10
In this issue of Blood, Kapur et al describe studies in which HPA-1a antibodies were isolated from serum of 48 women sensitized to HPA-1a who gave birth to an infant with NAIT.1 The isolated immunoglobulins were digested with trypsin and subjected to nano liquid-chromatography tandem mass spectrometry analysis to define the composition of IgG-associated glycans. Total IgG from the same individuals was similarly studied. Fourteen distinct glycan species were identified. Slight but significant increases in sialylation and galactosylation were found in the HPA-1a antibodies relative to total IgG. However, the most striking finding was a marked decrease in core fucosylation, which in some cases was as low as 10% of the value for total IgG. This difference persisted even in HPA-1a antibodies obtained several years after delivery. Similar studies of antibodies specific for class I HLA antigens present in 13 nonpregnant individuals who were refractory to platelet transfusions showed that the HLA antibodies did not differ from total IgG in the extent of core fucosylation. However, core fucosylation of an HLA antibody from one of the women sensitized to HPA-1a was significantly lower (43%) than that of total IgG (94%). In studies involving seven of the NAIT sera, it was found that decreased core fucosylation correlated with more effective phagocytosis of antibody-coated platelets by neutrophils. To evaluate the clinical significance of these findings, perinatal status of infants born to the women studied was evaluated retrospectively. A statistically significant correlation was found between decreased core fucosylation of maternal antibody and increased severity of NAIT. However, the data were widely scattered, making it uncertain whether measuring core fucosylation in a particular maternal antibody would be helpful in prenatal management of an infant at risk for NAIT.
The authors leave open the question of whether the anomalous properties of glycans identified in the HPA-1a antibodies reflects the fact that the original antigenic challenge occurred during pregnancy. It seems counterintuitive that this might be the case, because skewing of glycan synthesis to favor production of HPA antibodies lacking a core fucose could be deleterious to a fetus. On the other hand, production of such antibodies against a pathogen acquired during pregnancy could be a protective adaptation. Whatever the explanation, the interesting and provocative findings described by Kapur et al should stimulate further studies to characterize the effects of pregnancy on the properties of IgG glycans and the importance of IgG glycan variation in antibody-mediated human disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.