Key Points
Antibodies causing FNAIT have decreased Fc fucosylation, unlike in refractory thrombocytopenia.
Decreased Fc fucose increases affinity to FcγRIIIa/b, enhances platelet phagocytosis, and correlates with increased disease severity.
Abstract
Immunoglobulin G (IgG) formed during pregnancy against human platelet antigens (HPAs) of the fetus mediates fetal or neonatal alloimmune thrombocytopenia (FNAIT). Because antibody titer or isotype does not strictly correlate with disease severity, we investigated by mass spectrometry variations in the glycosylation at Asn297 in the IgG Fc because the composition of this glycan can be highly variable, affecting binding to phagocyte IgG-Fc receptors (FcγR). We found markedly decreased levels of core fucosylation of anti-HPA-1a–specific IgG1 from FNAIT patients (n = 48), but not in total serum IgG1. Antibodies with a low amount of fucose displayed higher binding affinity to FcγRIIIa and FcγRIIIb, but not to FcγRIIa, compared with antibodies with a high amount of Fc fucose. Consequently, these antibodies with a low amount of Fc fucose showed enhanced phagocytosis of platelets using FcγRIIIb+ polymorphonuclear cells or FcγRIIIa+ monocytes as effector cells, but not with FcγRIIIa– monocytes. In addition, the degree of anti-HPA-1a fucosylation correlated positively with the neonatal platelet counts in FNAIT, and negatively to the clinical disease severity. In contrast to the FNAIT patients, no changes in core fucosylation were observed for anti-HLA antibodies in refractory thrombocytopenia (post platelet transfusion), indicating that the level of fucosylation may be antigen dependent and/or related to the immune milieu defined by pregnancy.
Introduction
Fetal or neonatal alloimmune thrombocytopenia (FNAIT) is a potentially life-threatening disease where the fetal platelets are targeted by maternal anti-platelet immunoglobulin G (IgG) alloantibodies crossing the placenta. This leads to IgG-Fc receptor (FcγR)–mediated uptake by phagocytes in the fetal spleen and liver, finally resulting in thrombocytopenia.1 Clinical outcome may differ from asymptomatic to petechiae or intracerebral hemorrhage. The strength of the interaction between IgG and FcγR depends on several factors, including the IgG subclass formed during the immune response, its relative affinity to FcγRs, the expression levels of FcγR allotypes, FcγR copy number variation, cytokines (influencing the expression of FcγR), and also the IgG-Fc glycosylation pattern.2
IgG antibodies are glycoproteins containing a branched sugar moiety attached to the Asn297 residue in the Fc part. This glycan is essential for the maintenance of a functional structure and for binding of IgG with FcγR.3-5 In addition, the Asn297-linked glycans are substituted with variable amounts of galactose and sialic acid and may additionally carry a bisecting N-acetylglucosamine (GlcNAc) and core fucose. Variation in this composition influences antibody affinity to FcγR and thus antibody effector activity. IgG galactosylation has been found to be decreased in several inflammatory diseases (primary osteoarthritis, rheumatoid arthritis, and tuberculosis),6,7 as well as in ovarian cancer.8 Interestingly, increase in galactosylation correlates with remission of arthritis in pregnant rheumatoid arthritis patients.7,9,10 The degree of IgG galactosylation has been shown to depend on age and gender; IgG galactosylation is higher in females at a young age and decreases for both males and females with increasing age.11,12 In addition, an increase for total IgG galactosylation has been observed in pregnancy.7,9,10,13 Recently, an anti-inflammatory activity of immune complexes containing highly Fc-galactosylated mouse IgG1 was reported to enhance the association of FcγRIIb with dectin-1.14
Changes related to age, gender, and pregnancy have also been described for IgG sialylation. Sialylation slightly reduces the affinity of IgG to FcγRs but enhances the binding to dendritic cell-specific intercellular adhesion molecule-3–grabbing nonintegrin (DC-SIGN), thereby leading to an anti-inflammatory response attributable to increased expression of the inhibitory FcγRIIb.15-17
Importantly, a lack of core fucose has been demonstrated to result in a stronger binding affinity to FcγRIIIa and FcγRIIIb because of glycan-glycan interactions between Asn162 found only in FcγRIII and Asn297 in IgG1.5,18,19 Curiously, although nonfucosylated antibodies clearly enhance the binding to FcγRIIIa and FcγRIIIb, this only results in enhanced antibody-dependent cellular cytotoxicity on mononuclear cells through FcγRIIIa,3,20-24 but not through the glycosylphosphatidylinositol (GPI)–linked FcγRIIIb on polymorphonuclear cells (PMNs).18,25 The therapeutic anticancer potential of antibodies with decreased fucosylation, however, has been well recognized, and their power is currently being harnessed in clinical trials.18,20-24,26-30
In whites, the main antibodies causing FNAIT are the anti–human platelet antigen (HPA) 1a antibodies, found in ∼85% of the cases and in ∼1:1500 pregnancies.31-33 The HPA-1a epitope resides in the GPIIIa protein of the GPIIb/IIIa complex and is missing in individuals with a single nucleotide polymorphism resulting in an L to P change at position 33 in the mature protein.31 In a previous study, we reported a reduced fucosylation, an increased galactosylation, and sialylation of various anti-platelet IgG1 for a small series of FNAIT sera (including 2 samples with anti-HPA-1a antibodies).32 We now expand this to a cohort of 48 FNAIT anti-HPA-1a samples. Furthermore, we compare the glycosylation of IgG formed during immune responses against platelets in pregnancy with that of IgG formed against HLA after platelet transfusion or pregnancies. Our main findings show that Fc fucosylation is decreased in FNAIT, but not in refractory thrombocytopenia (RT), based on Fc analysis of anti-HPA-1a antibodies and anti-HLA class I antibodies, respectively, indicating that Fc fucosylation is regulated. Decreased Fc fucosylation increased the IgG affinity to FcγRIIIa and FcγRIIIb and also increased platelet phagocytosis by both monocytes and neutrophils, a key process in the pathogenesis of FNAIT.
Methods
Patient samples
Anti-HPA-1a platelet alloantibodies were diagnosed at Sanquin (Amsterdam, The Netherlands; n = 39), at University Hospital North Norway (Tromso, Norway; n = 5), and at National Health Service Blood and Transplant (Bristol, United Kingdom; n = 4), from maternal FNAIT sera using a monoclonal antibody immobilization of platelet antigens assay, performed as described by Kiefel et al.33 The HLA class I antibodies were all diagnosed at Sanquin (n = 13) and detected in serum from RT patients (refractory for platelet transfusions), using the Lifescreen Luminex HLA class I antibody screening method.34 Patient characteristics for all samples included in the study are listed in supplemental Table 1 (see the Blood Web site). Samples were obtained with informed consent from the patients in accordance with the Declaration of Helsinki.
Purification of anti-platelet antibodies from sera
HPA-1a–specific alloantibodies were purified in a similar way as described previously,32 but now instead of eluting from platelets, we increased the specificity by eluting the antibodies with formic acid from antigen-coated plates (PAK12; Immucor GTI Diagnostics, Waukesha, WI). The same plates were used for purification of anti-HLA class I antibodies. Further details are described in the supplemental Methods.
Mass spectrometric IgG-Fc glycosylation analysis
Nano liquid chromatography–tandem mass spectrometry was performed as described in the supplemental Methods.
Production of recombinant anti-TNP IgG1 antibodies with low and high amounts of Fc fucose
The variable regions of the heavy and light chains (VH, VL) of the mouse IgG1 anti-2,4,6-trinitrophenol (TNP) hapten antibodies were cloned onto human IgG1 or κ backbone, respectively, as described previously,35 and produced in the HEK-293F FreeStyle cell line expression system (Life Technologies, Paisley, United Kingdom), but now in the presence or absence of 2-deoxy-2-fluoro-l-fucose (2F; Carbosynth, Compton, Berkshire, United Kingdom) to control the level of fucosylation.36 Antibodies were purified on a protein A (WT IgG1) HiTrap HP column (GE Healthcare Life Sciences, Little Chalfont, United Kingdom) using the Acta Prime Plus system (GE Healthcare Life Sciences) and dialyzed against phosphate-buffered saline (PBS) overnight. IgG-Fc glycosylation was determined by mass spectrometry.
Surface plasmon resonance (SPR)
SPR measurements were performed with the Biacore 3000 system (Biacore AB, Breda, The Netherlands) at 25°C. Anti-histidine antibody (GE Healthcare, The Netherlands), 25 μg/mL in sodium acetate buffer pH 4.5 (GE Healthcare), was coupled covalently to a CM5 chip (GE Healthcare) following activation with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (Sigma-Aldrich, Zwijndrecht, The Netherlands) 0.4 M in water and N-hydroxysuccinimide (Sigma-Aldrich) 0.1 M. To measure binding of monoclonal IgG to FcγRs, 20 ng of human recombinant polyHisTag FcγRIIa or 10 ng of human recombinant polyHisTag FcγRIIIa (Novoprotein, Summit, NJ) or 5 ng human recombinant polyHisTag FcγRIIIb (Sino Biologicals Inc., Beijing, China), diluted in 0.01 M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% v/v Surfactant P20 buffer (GE Healthcare), was injected, followed by the injection of anti-TNP IgG at different concentrations. Further details are described in the supplemental Methods.
Cell isolations, TNP haptenization, labeling, and antibody opsonization
Human platelets, PMNs, and monocytes were isolated freshly, and platelets were haptenized with TNP, as described in the supplemental Methods.
Hereafter, platelets were labeled with pHrodo by resuspending a platelet pellet in 0.23 mM pHrodo succinimidyl ester (100 μL/108 platelets) (Molecular Probes; Invitrogen, Eugene, OR), in 100 ng/mL Prostaglandin E1, for 45 minutes in the dark at room temperature. Then, the platelets were washed twice and resuspended in PBS/EDTA/Prostaglandin E1 at 108 platelets per mL.
The haptenized and pHrodo-labeled platelets were opsonized by resuspending a pellet of platelets with 100 µL of 10 µg/mL anti-TNP antibody low/high fucose or isotype antibody for every 108 platelets and subsequent incubation for 30 minutes at room temperature. Hereafter, platelets were washed twice with PBS/bovine serum albumin and resuspended at 108/mL in Normal Human Serum (NHS).
Similarly, unhaptenized and pHrodo-labeled platelets were opsonized by resuspending a pellet of platelets with 100 µL FNAIT/anti-HPA-1a serum diluted to 35 arbitrary units (AU) of anti-HPA-1a antibody (using a human anti-HPA-1a standard, 100 AU, National Institute for Biological Standards and Control code: 03/152; National Institute for Biological Standards and Control, Hertfordshire, United Kingdom), for every 108 platelets. NHS was used as isotype control. Hereafter, the platelets were incubated for 30 minutes at room temperature, washed twice with PBS, and resuspended at 108/mL in NHS.
Platelet phagocytosis using anti-TNP antibodies or FNAIT anti-HPA-1a sera
Phagocytosis was carried out using freshly isolated human PMNs or CD16+/CD16– (FcγRIIIa+/ FcγRIIIa–) sorted peripheral monocytes (effector cells). Equal volumes of 2.0 × 106/mL effector cells and 1.0 × 108/mL opsonized platelets were mixed in a total volume of 100 µL in 1.4-mL U-bottom tubes (Micronic, Lelystad, The Netherlands) for 20 minutes in a shaking incubator at 37°C. After the reaction, the cells were kept on ice and washed in cold PBS. Samples were measured with a flow cytometer (LSRII; BD Bioscience, San Jose, CA) and analyzed for colored events using FacsDIVA software (BD Bioscience). Phagocytosis data were eventually compared with the degree of Fc fucosylation as determined by mass spectrometry, as described previously.
Results
Fc glycosylation of anti-HPA-1a antibodies formed in FNAIT
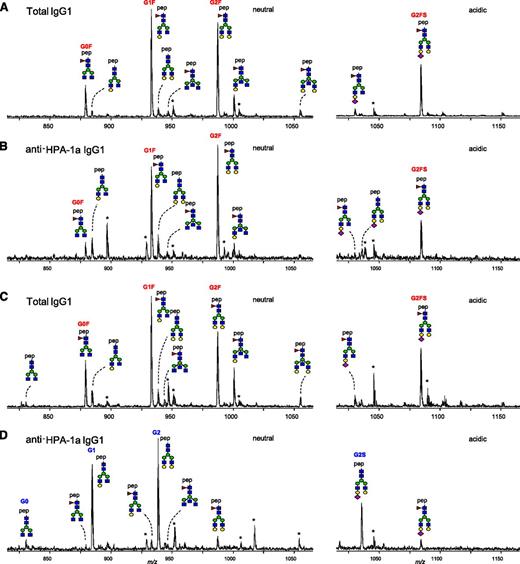
Total IgG and anti-HPA-1a antibodies were purified from 48 maternal anti-HPA-1a FNAIT sera by affinity absorption and elution on HPA-1a–containing immunosorbent plates, subjected to tryptic digestion, and the resulting IgG1 Asn297-Fc glycopeptides analyzed by mass spectrometry. No IgG-Fc glycopeptides were detected when NHS (without anti-HPA-1a antibodies) were subjected to the anti-HPA-1a purification procedure (data not shown). Likewise, no IgG glycopeptides were detected when anti-HPA-1a FNAIT sera were applied to immunosorbent matrices containing irrelevant antigens. Together, these experiments demonstrate the specificity of the anti-HPA-1a IgG1 purification procedure. A total of 14 different IgG1-Fc glycopeptides were monitored (Table 1). For some patients, only small differences in glycosylation profiles of total IgG1 and anti-HPA-1a IgG1 were observed (example of full glycoprofiles in Figure 1A-B), whereas marked differences were observed for others (example in Figure 1C-D): For total IgG1, the Fc glycosylation profiles were always dominated by fucosylated glycan structures (G0F, G1F, G2F, and G2FS; Figure 1 A,C); whereas the major glycoforms registered for anti-HPA-1a IgG1, often (Figure 1D), but not always (Figure 1B), lacked core fucose (G1, G2, and G2S).
Theoretical mass-to-charge ratios (m/z) of human IgG1-Fc glycopeptides detected by nano-liquid chromatography–electrospray ionization-mass spectrometry
| Glycan species* . | Structural scheme† . | Theoretical glycopeptide m/z . |
|---|---|---|
| G0 | 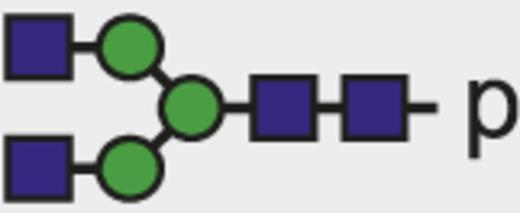 | 830.00 |
| G0F | 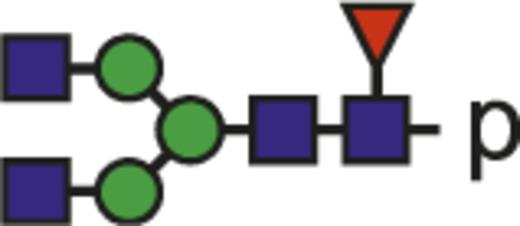 | 878.69 |
| G1 | 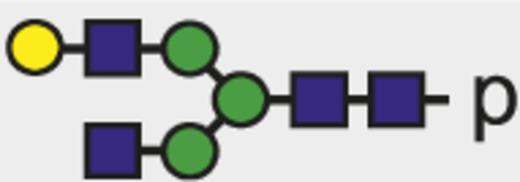 | 884.02 |
| G1F | 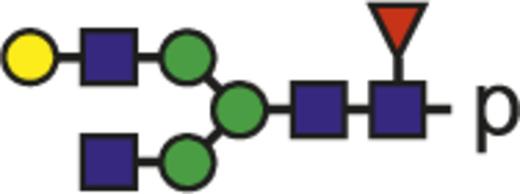 | 932.71 |
| G2 | 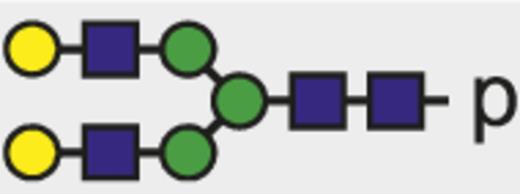 | 938.04 |
| G0FN | 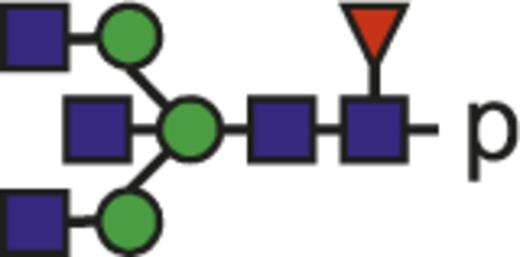 | 946.38 |
| G2F |  | 986.72 |
| G1FN | 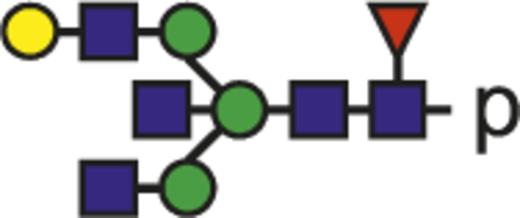 | 1000.40 |
| G2FN | 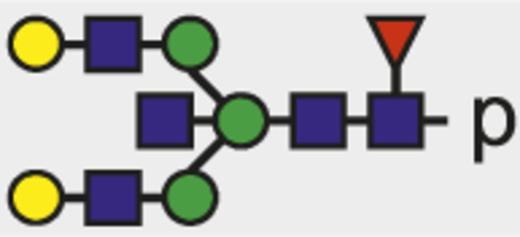 | 1054.42 |
| G1FS | 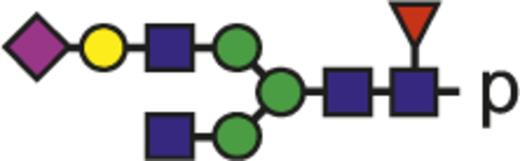 | 1029.74 |
| G2S | 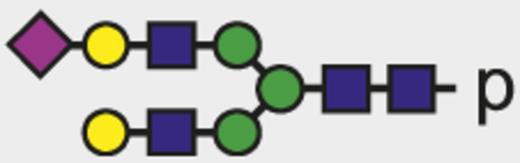 | 1035.07 |
| G2FS | 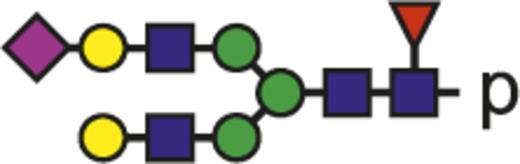 | 1083.75 |
| G1FNS |  | 1097.43 |
| G2FNS | 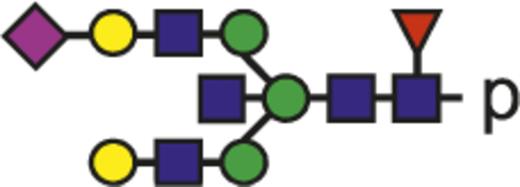 | 1151.45 |
| Glycan species* . | Structural scheme† . | Theoretical glycopeptide m/z . |
|---|---|---|
| G0 |  | 830.00 |
| G0F |  | 878.69 |
| G1 |  | 884.02 |
| G1F |  | 932.71 |
| G2 |  | 938.04 |
| G0FN |  | 946.38 |
| G2F |  | 986.72 |
| G1FN |  | 1000.40 |
| G2FN |  | 1054.42 |
| G1FS |  | 1029.74 |
| G2S |  | 1035.07 |
| G2FS |  | 1083.75 |
| G1FNS |  | 1097.43 |
| G2FNS |  | 1151.45 |
The observed tryptic IgG1-Fc glycopeptides have a common peptide moiety (p) of E293EQYNSTYR301 (Swiss-Prot entry number P01857). The m/z values of the triple protonated species are given.
Glycan structural features are given in terms of number of galactoses (G0, G1, G2), fucose (F), bisecting N-acetylglucosamine (N), and N-acetylneuraminic acid (sialic acid) (S).
Blue square indicates N-acetylglucosamine; green circle, mannose; yellow circle, galactose; purple diamond, N-acetylneuraminic acid (sialic acid); and red triangle, fucose.
Mass spectrometric analysis of Fc glycopeptides of total IgG1 and anti-HPA-1a IgG1 from 2 pregnant women. One person showed high degrees of fucosylation for total IgG1 (97%; A) and anti-HPA-1a IgG1 (86%; B). The other also showed a high degree of fucosylation for total IgG1 (86%; C), yet a low degree of fucosylation for anti-HPA-1a IgG1 (9%; D). Major fucosylated glycoforms are labeled in red, and nonfucosylated glycoforms are labeled in blue or are unlabeled. Blue square indicates N-acetylglucosamine; red triangle, fucose; green circle, mannose; yellow circle, galactose; purple diamond, N-acetylneuraminic acid; pep, peptide moiety; and asterisk, contaminant. Triple-protonated glycopeptide signals were observed throughout. For the assignment of the glycopeptides signals, see Table 1. The level of galactosylation, sialylation, bisection (bisecting N-acetylglucosamine), and fucosylation were calculated according to the following formulas: Galactosylation = (G1F + G1FN + G1FS + G1FNS + G1) × 0.5 + G2F + G2FN + G2FS + G2FNS + G2 + G2S. Agalactosylated structures = G0F + G0FN + G0. Digalactosylated structures = G2F + G2FN + G2FS + G2FNS + G2 + G2S. Sialylation = G1FS + G2FS + G1FNS + G2FNS + G2S. Bisection = G0FN + G1FN + G2FN + G1FNS + G2FNS. Fucosylation = G0F + G1F + G2F + G0FN + G1FN + G2FN + G1FS + G2FS.
Mass spectrometric analysis of Fc glycopeptides of total IgG1 and anti-HPA-1a IgG1 from 2 pregnant women. One person showed high degrees of fucosylation for total IgG1 (97%; A) and anti-HPA-1a IgG1 (86%; B). The other also showed a high degree of fucosylation for total IgG1 (86%; C), yet a low degree of fucosylation for anti-HPA-1a IgG1 (9%; D). Major fucosylated glycoforms are labeled in red, and nonfucosylated glycoforms are labeled in blue or are unlabeled. Blue square indicates N-acetylglucosamine; red triangle, fucose; green circle, mannose; yellow circle, galactose; purple diamond, N-acetylneuraminic acid; pep, peptide moiety; and asterisk, contaminant. Triple-protonated glycopeptide signals were observed throughout. For the assignment of the glycopeptides signals, see Table 1. The level of galactosylation, sialylation, bisection (bisecting N-acetylglucosamine), and fucosylation were calculated according to the following formulas: Galactosylation = (G1F + G1FN + G1FS + G1FNS + G1) × 0.5 + G2F + G2FN + G2FS + G2FNS + G2 + G2S. Agalactosylated structures = G0F + G0FN + G0. Digalactosylated structures = G2F + G2FN + G2FS + G2FNS + G2 + G2S. Sialylation = G1FS + G2FS + G1FNS + G2FNS + G2S. Bisection = G0FN + G1FN + G2FN + G1FNS + G2FNS. Fucosylation = G0F + G1F + G2F + G0FN + G1FN + G2FN + G1FS + G2FS.
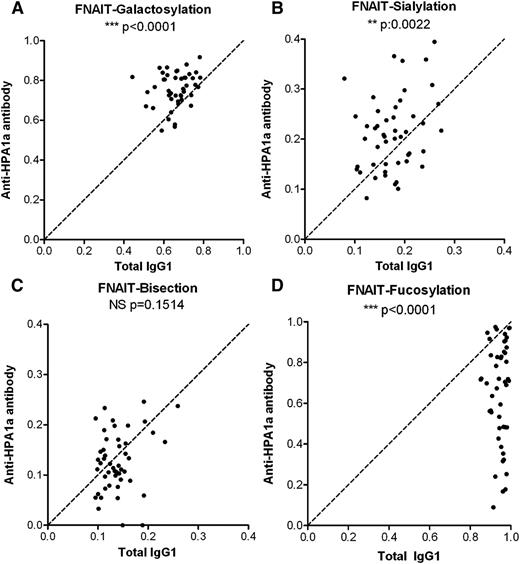
Systematic analysis of the Fc glycosylation with regard to galactosylation, sialylation, presence of bisecting GlcNAc (bisection), and fucosylation, comparing the glycosylation of total IgG1 with that of anti-HPA-1a IgG1 in a pairwise manner, revealed a slight but significant increase in the levels of Fc galactosylation (Figure 2A). Similar, but less pronounced, increases were observed for Fc sialylation (Figure 2B). As expected, the level of sialylation correlated significantly with the level of galactosylation for both total IgG and specific HPA-1a antibodies (supplemental Figure 1), as the galactosylated structures form the substrate for the sialyltransferase (see Table 1; Figure 1). No significant differences were observed for the bisecting GlcNAc content of anti-HPA-1a antibodies compared with total serum IgG1 (Figure 2C). However, although total IgG1 contained normal levels of core fucose at Asn297, the majority of anti-HPA-1a antibody samples showed an overrepresentation of IgG1 without fucose (Figure 2D).
Anti-HPA-1a antibodies in FNAIT display a pronounced lowering of Fc fucosylation. Relative expression levels of major IgG-Fc Asn297 glycoforms for both total IgG1 (x-axis) and antigen-specific IgG1 (y-axis) for 48 FNAIT anti-HPA-1a serum samples (A-D). Serum populations were analyzed for Fc galactosylation (A), sialylation (B), bisection (C), and fucosylation (D). The statistical outcome between 2-tailed paired Student t test analysis of total IgG1 vs specific antibodies is listed in each panel. The diagonal, dotted line represents the equal ratio between total IgG1 and the specific antibody.
Anti-HPA-1a antibodies in FNAIT display a pronounced lowering of Fc fucosylation. Relative expression levels of major IgG-Fc Asn297 glycoforms for both total IgG1 (x-axis) and antigen-specific IgG1 (y-axis) for 48 FNAIT anti-HPA-1a serum samples (A-D). Serum populations were analyzed for Fc galactosylation (A), sialylation (B), bisection (C), and fucosylation (D). The statistical outcome between 2-tailed paired Student t test analysis of total IgG1 vs specific antibodies is listed in each panel. The diagonal, dotted line represents the equal ratio between total IgG1 and the specific antibody.
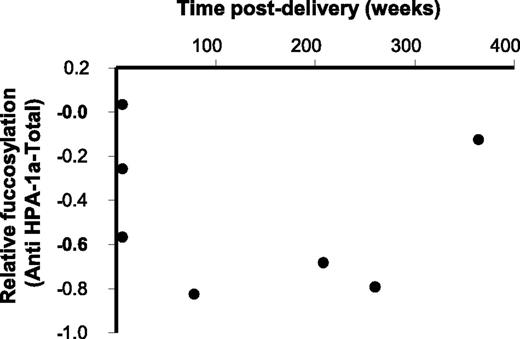
Maternal IgG samples were taken within a time range of weeks to years after delivery. However, samples that were taken a prolonged period after delivery (N = 7) still demonstrated a lowered degree of anti-HPA-1a fucosylation (specific IgG1 fucosylation minus total IgG1 fucosylation; Figure 3).
FNAIT anti-HPA-1a antibodies with decreased fucosylation are present years after delivery. The degree of anti-HPA-1a Fc fucosylation (specific IgG1 fucosylation minus total IgG1 fucosylation) is plotted against the time after delivery, for 7 anti-HPA-1a FNAIT samples (period of 6 weeks to 7 years postdelivery).
FNAIT anti-HPA-1a antibodies with decreased fucosylation are present years after delivery. The degree of anti-HPA-1a Fc fucosylation (specific IgG1 fucosylation minus total IgG1 fucosylation) is plotted against the time after delivery, for 7 anti-HPA-1a FNAIT samples (period of 6 weeks to 7 years postdelivery).
We hypothesized that this skewing in the IgG response against platelets in FNAIT patients may also affect glycosylation features other than fucosylation. We therefore investigated whether the degree of anti-HPA-1a fucosylation correlates with the degree of bisection, galactosylation, and sialylation. We observed a weak but significant positive correlation between anti-HPA-1a fucosylation and bisection (R2 = 0.112) and a negative correlation between anti-HPA-1a fucosylation and galactosylation (R2 = 0.200), whereas there was no significant correlation between anti-HPA-1a fucosylation and sialylation (supplemental Figure 2).
Fc glycosylation of anti-HLA antibodies
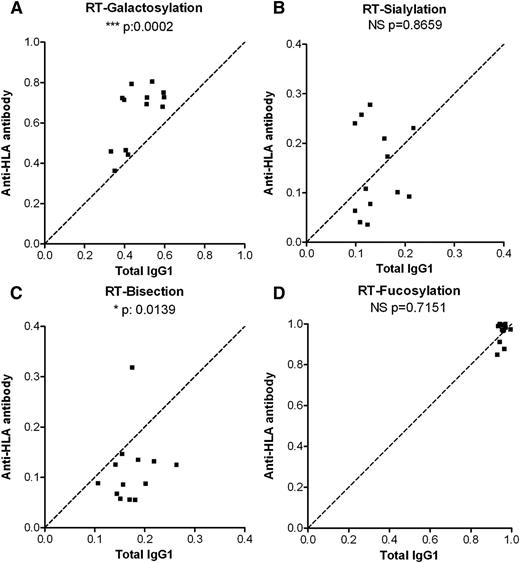
To investigate whether the skewing of glycosylation seen in anti-platelet responses during pregnancies was attributable to a general anti-platelet response or to an anti-HPA-1a–related response in pregnancy, we also investigated the IgG responses formed against platelets in sera of 13 RT patients (lack of adequate posttransfusion platelet-count increments, in which anti-HLA antibodies are frequently implicated). Similar to the anti-platelet responses in FNAIT, we found the anti-HLA Fc galactosylation in RT to be increased compared with total serum IgG1 levels (Figure 4A). Unlike the FNAIT samples, no specific increases were observed for Fc sialylation (Figure 4B), and the bisecting GlcNAc content of the anti-HLA antibodies was significantly decreased (Figure 4C). Also, in contrast to the Fc fucosylation of anti-HPA-1a antibodies, the anti-HLA Fc fucosylation did not show any changes compared with total serum IgG1 (Figure 4D). In addition, we investigated an FNAIT anti-HPA-1a sample, which also contained anti-HLA antibodies. The anti-HPA-1a antibodies displayed a markedly lowered degree of fucosylation (43% as compared with 94% for total IgG1), whereas the anti-HLA antibodies from the same sample were not skewed in fucosylation (87%; supplemental Figure 3).
Anti-HLA antibodies in RT do not display a pronounced lowering of Fc fucosylation. Relative expression levels of major IgG-Fc Asn297 glycoforms for both total IgG1 (x-axis) and antigen-specific IgG1 (y-axis) for 13 RT anti-HLA class I serum samples (A-D). Serum populations were analyzed for Fc galactosylation (A), sialylation (B), bisection (C), and fucosylation (D). The statistical outcome between 2-tailed paired Student t test analysis of total IgG1 vs specific antibodies is listed in each panel. The diagonal, dotted line represents the equal ratio between total IgG1 and the specific antibody.
Anti-HLA antibodies in RT do not display a pronounced lowering of Fc fucosylation. Relative expression levels of major IgG-Fc Asn297 glycoforms for both total IgG1 (x-axis) and antigen-specific IgG1 (y-axis) for 13 RT anti-HLA class I serum samples (A-D). Serum populations were analyzed for Fc galactosylation (A), sialylation (B), bisection (C), and fucosylation (D). The statistical outcome between 2-tailed paired Student t test analysis of total IgG1 vs specific antibodies is listed in each panel. The diagonal, dotted line represents the equal ratio between total IgG1 and the specific antibody.
IgG with low Fc fucose has increased binding affinity to FcγRIIIa and FcγRIIIb
To test the biological influence of the lowered Fc fucosylation of IgG antibodies in the clearance of platelets, we produced TNP-specific antibodies in HEK-293F FreeStyle cells in the absence or presence of 2F, which has recently been described to deplete the level of guanosine diphosphate fucose, the substrate of fucosyltransferases, and thereby prevent fucose incorporation into proteins.36 In agreement with those results, addition of 100, 200, and 400 µM resulted in a dramatic reduction of core fucose incorporation into IgG, dropping from 76% fucosylation to 14%, 8%, and 6%, respectively (supplemental Figure 4). For the following experiments, the 76% fucosylated sample was used as anti-TNP high fucose, and the 6% fucosylated sample was used as anti-TNP low fucose. In accordance with the unique presence of Asn162 in the human FcγRIII family, required for glycan-glycan interaction of IgG and FcγR,5 we observed that the lack of core fucosylation dramatically increased the binding affinity to FcγRIIIa and FcγRIIIb but not FcγRIIa (Table 2).
Decreased Kd values for anti-TNP low-fucose antibodies compared with anti-TNP high-fucose antibodies with respect to FcγRIIIa and FcγRIIIb, but not for FcγRIIa
| . | FcγRIIIa (Kd, µM) ± SD . | FcγRIIIb (Kd, µM) ± SD . | FcγRIIa (Kd, µM) ± SD . |
|---|---|---|---|
| Anti-TNP high fucose | 0.12 ± 0.01 | 0.58 ± 0.11 | 0.22 ± 0.11 |
| Anti-TNP low fucose | 0.05 ± 0.02 | 0.08 ± 0.00 | 0.20 ± 0.08 |
| Paired Student t test anti-TNP high fucose vs anti-TNP low fucose | 0.001, *** | 0.008, ** | 0.2252, NS |
| . | FcγRIIIa (Kd, µM) ± SD . | FcγRIIIb (Kd, µM) ± SD . | FcγRIIa (Kd, µM) ± SD . |
|---|---|---|---|
| Anti-TNP high fucose | 0.12 ± 0.01 | 0.58 ± 0.11 | 0.22 ± 0.11 |
| Anti-TNP low fucose | 0.05 ± 0.02 | 0.08 ± 0.00 | 0.20 ± 0.08 |
| Paired Student t test anti-TNP high fucose vs anti-TNP low fucose | 0.001, *** | 0.008, ** | 0.2252, NS |
SPR analyses of anti-TNP antibodies with high fucose vs anti-TNP antibodies with low fucose, interacting with FcγRIIIa (n = 3), FcγRIIIb (n = 3), and FcγRIIa (n = 4). Kd values (µM): the constant rate of dissociation of antibody from the receptors at equilibrium state, with standard deviation (SD) are shown. One-tailed paired-test outcome is depicted. **P ≤ .01; ***P ≤ 001. NS, nonsignificant.
Lack of IgG core fucosylation increases platelet phagocytosis
We next TNP-haptenized platelets, labeled them with pHrodo (only fluorescent in acid environment), and opsonized them with the anti-TNP antibody. For both PMNs and CD16+ monocytes, expressing FcγRIIIb or FcγRIIIa, respectively, fucosylated antibodies resulted in low platelet ingestion; whereas low-fucosylated antibodies showed a significantly increased phagocytic response with both effector cell types (Figure 5A-B). However, CD16– monocytes showed no preference for low- or high-fucosylated antibodies for platelet phagocytosis, in accordance with their lack of FcγRIIIa expression (Figure 5C).
Absence of IgG-Fc core fucose enhances platelet phagocytosis through FcγRIII on PMNs and monocytes. Platelets were haptenized with TNP; labeled for phagocytosis with pHrodo; opsonized with either isotype antibody, anti-TNP low- or high-fucose antibodies; subjected to phagocytosis by either neutrophils (PMNs; A), FcγRIIIa+ monocytes (B), or FcγRIIIa– monocytes (C) as effector cells; and expressed as anti-TNP–specific phagocytosis (Mean Fluorescence Intensity (MFI) anti-TNP − MFI isotype IgG). Anti-TNP IgG1 antibodies with low fucose displayed increased phagocytosis compared with highly fucosylated anti-TNP antibodies using PMNs or FcγRIIIa+ monocytes (A-B, respectively) as effector cells, but not with FcγRIIIa– monocytes (C). Platelets were labeled with pHrodo, opsonized with 35 AU of anti-HPA-1a antibodies from maternal FNAIT sera, and subjected to phagocytosis by neutrophils (D). Phagocytosis was expressed as anti-HPA-1a–specific phagocytosis (MFI anti-HPA-1a from maternal FNAIT sera − MFI isotype IgG from NHS). Statistical analyses: 1-tailed unpaired Student t test (A-C) and Pearson correlation (D). *P ≤ .05; **P ≤ .01. NS, nonsignificant.
Absence of IgG-Fc core fucose enhances platelet phagocytosis through FcγRIII on PMNs and monocytes. Platelets were haptenized with TNP; labeled for phagocytosis with pHrodo; opsonized with either isotype antibody, anti-TNP low- or high-fucose antibodies; subjected to phagocytosis by either neutrophils (PMNs; A), FcγRIIIa+ monocytes (B), or FcγRIIIa– monocytes (C) as effector cells; and expressed as anti-TNP–specific phagocytosis (Mean Fluorescence Intensity (MFI) anti-TNP − MFI isotype IgG). Anti-TNP IgG1 antibodies with low fucose displayed increased phagocytosis compared with highly fucosylated anti-TNP antibodies using PMNs or FcγRIIIa+ monocytes (A-B, respectively) as effector cells, but not with FcγRIIIa– monocytes (C). Platelets were labeled with pHrodo, opsonized with 35 AU of anti-HPA-1a antibodies from maternal FNAIT sera, and subjected to phagocytosis by neutrophils (D). Phagocytosis was expressed as anti-HPA-1a–specific phagocytosis (MFI anti-HPA-1a from maternal FNAIT sera − MFI isotype IgG from NHS). Statistical analyses: 1-tailed unpaired Student t test (A-C) and Pearson correlation (D). *P ≤ .05; **P ≤ .01. NS, nonsignificant.
Phagocytosis was also performed using anti-HPA-1a antibodies from 7 FNAIT sera. Platelets were labeled with pHrodo, opsonized with 35 AU of anti-HPA-1a antibody derived from maternal FNAIT sera, and subjected to phagocytosis by PMNs. The degree of phagocytosis correlated significantly with the degree of anti-HPA-1a Fc fucosylation (P = .023, R2 = 0.590; Figure 5D).
A lowered anti-HPA-1a Fc fucosylation correlates with decreased neonatal platelet counts and increased disease severity in FNAIT patients
We then investigated if core fucosylation of the HPA-1a antibodies affected the disease outcome clinically. A significant correlation between neonatal platelet counts, obtained directly after delivery, and the degree of anti-HPA-1a fucosylation was found in the FNAIT patients, with lowered anti-HPA-1a Fc fucosylation corresponding to low neonatal platelet counts (Figure 6A; P = .043, ρ = 0.320). We also found a significant correlation between the degree of anti-HPA-1a fucosylation and the clinical disease severity directly after delivery (ranging from asymptomatic to mild symptoms such as petechiae, to moderate symptoms including organ bleeding, to severe symptoms such as intracranial hemorrhages; Figure 6B; P = .020, ρ = –0.377). A lowered degree of anti-HPA-1a fucosylation corresponded to more severe clinical disease severity, with all asymptomatic patients bearing a high level of IgG-Fc core fucosylation (on average 85%; Figure 6B).
Degree of fucosylation correlates with neonatal platelet counts in FNAIT and clinical severity. Degree of fucosylation was compared with neonatal platelet counts in FNAIT directly after delivery (A), as well as with the clinical disease severity score (B). The scores are as follows: 0 indicates no symptoms; 1, mild symptoms (petechiae); 2, moderate symptoms (other bleeding, including organ bleeding); and 3, severe symptoms (intracranial hemorrhages) (B). Statistical analyses were performed using Spearman rank correlation, with a positive correlation in panel A (ρ = 0.320) and a negative correlation in panel B (ρ = −0.377). *P ≤ .05.
Degree of fucosylation correlates with neonatal platelet counts in FNAIT and clinical severity. Degree of fucosylation was compared with neonatal platelet counts in FNAIT directly after delivery (A), as well as with the clinical disease severity score (B). The scores are as follows: 0 indicates no symptoms; 1, mild symptoms (petechiae); 2, moderate symptoms (other bleeding, including organ bleeding); and 3, severe symptoms (intracranial hemorrhages) (B). Statistical analyses were performed using Spearman rank correlation, with a positive correlation in panel A (ρ = 0.320) and a negative correlation in panel B (ρ = −0.377). *P ≤ .05.
Discussion
In this study, we analyzed a large FNAIT serum cohort consisting of 48 serum samples, all containing anti-platelet alloantibodies formed during pregnancy and directed against the most common immunogenic alloantigen on platelets, the HPA-1a epitope of glycoprotein IIb/IIIa. The glycosylation patterns of anti-HPA-1a IgG1 antibodies were compared with those of total IgG1 antibodies within the same individual and to anti-platelet antibodies formed after platelet transfusion in RT patients with anti-HLA antibodies. Antigen-specific antibodies were affinity purified using surface-immobilized antigen, and the tryptic Fc glycopeptides of the pathogenic IgG1 as well as of total IgG1 were analyzed by mass spectrometry. The Fc-glycosylation patterns of both FNAIT and RT cohorts of anti-platelet or anti-HLA antibodies displayed a slight, but significant, increase in the level of galactosylation. Only in the FNAIT group did we find anti-GPIIIa antibodies with a slight, but significant, increase in the level of sialylation, and only in the RT patient group did we find a slight decrease in the level of bisecting GlcNAc of the anti-HLA antibodies. The main difference between the patient groups was observed for core fucosylation, with the majority of the FNAIT patients with anti-HPA-1a–specific antibodies showing a markedly lowered IgG1-Fc fucosylation in pregnancy (levels down to 10% fucosylation), a very atypical form of IgG-Fc glycosylation (normal values being >90%).13,37 To our knowledge, a lowered fucosylation has only been found for HIV-specific antibodies in elite controllers of HIV infection, but unlike in our study, these also displayed agalactosylated and asialylated glycans.38
We further observed normal high levels of core fucosylation for anti-HLA class I antibodies (RT patients), of which 12 of the 13 patients were women who we cannot exclude to have been immunized already during a previous pregnancy. Our previous study also featured patients (N = 2) with anti-HPA-1a antibodies (normally observed in FNAIT patients) formed after transfusion, and they also did not display a skewing in core fucosylation.32 Because other glycosylation patterns were affected as well, we hypothesize that this kind of IgG response must be governed by the very settings under which these antibodies are formed: either the T helper 2–dominant features of pregnancies,39 the anti-platelet response, the anti-HPA-1a response, or a combination of these factors. We also investigated an FNAIT anti-HPA-1a sample, which also contained anti-HLA antibodies, with lowered Fc fucosylation for the anti-HPA-1a antibodies, but not for the anti-HLA antibodies. Together with the data on normal fucosylation of anti-HLA antibodies from the RT patients, this further lends support to the hypothesis that other factors besides pregnancy are involved in this type of IgG fucosylation.
Among the 48 FNAIT samples analyzed, 7 samples were taken in a period ranging from 6 weeks to 7 years after delivery; whereas all the other samples were taken within 2 days after delivery. Surprisingly, the lower levels of IgG fucose (specific values of relative abundance minus total values) seemed to persist for a prolonged period after delivery, indicating that the type of IgG glycosylation acquired during the onset of the immune response is a subject of imprinting or attributable to long-lived plasma cells.
The increased levels of specific galactosylation of antigen-specific IgG1 compared with total IgG1 in the cohorts with anti-HPA-1a antibodies developed after pregnancies and anti-HLA antibodies in platelet-transfused patients appear to be, to our knowledge, the first disease settings in which this phenomenon is described. A high degree of galactosylation has been found to negatively affect IgG half-life in mice,40 suggesting that galactosylation may also influence the transport of IgG from mother to child because both half-life and transport of IgG are mainly mediated by the neonatal Fc receptor FcRn.41 However, no such skewing for IgG transport across the human placenta was found in our previous study,42 in accordance with the fact that the Fc glycans are not involved in binding to FcRn.43 The increased levels of galactosylation and sialylation observed for the anti-HPA-1a antibodies in FNAIT might also enable a downregulation of the immune response, opposing the stimulatory effect caused by a decreased anti-HPA-1a fucosylation.
Various B-cell stimuli have been shown to modulate Fc glycosylation. Both the Toll-like receptor 9 ligand cytosine guanine dinucleotide oligodeoxynucleotide and interleukin 21 have been shown to increase Fc galactosylation and reduce bisecting GlcNAc levels. In contrast, all-trans retinoic acid decreases galactosylation and sialylation levels.44 Recently, T-cell independent B-cell activation has also been shown to induce immunosuppressive sialylated IgG antibodies in mouse models.45 In the present study, we observed increased levels of galactosylation and lowered levels of fucosylation. This may perhaps be regulated via a higher expression of β4-galactosyltransferase and a downregulation of fucosyltransferase 8 (FUT8) in the anti-HPA-1a–producing plasma cells in the FNAIT patients. For HIV-specific antibodies, which also displayed decreased fucosylation, FUT8 expression was found to be decreased in controllers and treated progressors compared with untreated progressors, whereas the expression of the fucosidase FUCA2 was found to be increased in controllers and untreated subjects.38 Recently, the transcription factor hepatocyte nuclear factor (HNF) 1a and its downstream target HNF4a have been identified as transcriptional regulators of key fucosyltransferases and fucose biosynthesis genes.46 However, the exact stimuli and the regulatory pathways have yet to be elucidated. Also, the degree of anti-HPA-1a fucosylation demonstrated a low but statistically significant positive correlation with the level of bisecting GlcNAc, as well as a negative correlation with the level of galactosylation. This indicates that the glycosylation of the IgG antibodies is dictated by the specific immune reaction, forming a differential spectrum of Fc-glycosylated antibodies that depends on the nature of the immune response.
FcγRIIIa is expressed either with valine or phenylalanine at position 158, with the V variant showing a stronger affinity for IgG1 and IgG3.47,48 The expression of the higher-affinity form (V158) is associated with higher incidence of inosine 5′-triphosphate, suggesting that this receptor may be highly relevant for the clearance of platelets.49 In FNAIT, the decreased Fc fucosylation of anti-platelet alloantibodies most likely contributes to enhanced platelet phagocytosis via increased Fc receptor interaction, as we confirmed by biosensor analysis and by phagocytosis of platelets. The increased affinity was seen only for FcγRIIIa, expressed on a subset of monocytes, macrophages, and natural killer cells; for FcγRIIIb, expressed on granulocytes; but not for other FcγR receptor types because these do not express the glycan found at position 162, only found in both FcγRIII types.5 The glycan attached to Asn162 in FcγRIII has been shown to interact directly with the IgG-Fc glycan, which is critically influenced by the presence or absence of the core fucose. In the latter case, this also allows for binding of the Fc-protein moiety to FcγRIII, which does not take place when fucose is present.5 In accordance, both neutrophils and FcγRIII+ (∼5% of peripheral) monocytes showed strong phagocytosis only with low-fucosylated IgG; whereas FcγRIII– monocytes, the majority of monocytes in peripheral blood, showed no preference for platelets opsonized with either high- or low-fucosylated antibodies. In addition, we found the degree of FNAIT anti-HPA-1a Fc fucosylation to correlate significantly with the degree of platelet phagocytosis, with sera containing less core fucosylation in their anti-HPA-1a antibodies giving rise to stronger phagocytosis. In line with this, we also found the degree of anti-HPA-1a Fc fucosylation to correlate positively with neonatal platelet counts directly after delivery.
The association of the functional single nucleotide polymorphism within FcγRIIIa with the higher incidence of inosine 5′-triphosphate fits with data demonstrating that the spleen is the most important site for IgG-opsonized platelet clearance by residing splenic macrophages and/or monocytes (that uniformly express FcγRIIIa). This suggests that fucosylation of anti-platelet antibodies plays an important role. In support of this, we found anti-HPA-1a Fc-fucosylation levels to correlate negatively with the clinical disease severity in FNAIT. Although our FNAIT cohort is limited by the fact that most asymptomatic anti-HPA-1a patients remain undiagnosed, our study did include a few patients who were asymptomatic directly after delivery. All of these asymptomatic patients demonstrated high levels of anti-HPA-1a Fc fucosylation. Fucosylation status of anti-platelet antibodies may therefore prove to be a useful diagnostic marker to evaluate disease severity. However, and perhaps somewhat remarkably, the function of the anti-HLA antibodies from RT patients, all with normal fucosylation, seems less affected by their fucosylation because they apparently are fully capable of causing potent platelet destruction. However, other factors, such as titer, should also be taken into account, but also the FcγR most strongly involved in the destruction. For anti-HPA-1a antibodies, this appears to be FcγRIIIa, the function of which is highly sensitive to core fucosylation; however, for anti-HLA class I antibodies, this may be a different FcγR not sensitive to changes in core fucosylation.
In conclusion, the Fc glycosylation of anti-platelet antibodies in FNAIT and RT is associated with a slightly increased level of Fc galactosylation that predisposes the IgG for further modification by the addition of sialic acid. In FNAIT, an additional IgG modification frequently appears in the form of a very pronounced decrease of Fc fucosylation, resulting in increased binding affinity to FcγRIIIa and FcγRIIIb, as well as increased platelet phagocytosis by PMNs or FcγRIIIa+ monocytes. This is also reflected by the positive correlation between the degree of anti-HPA-1a Fc fucosylation and the neonatal platelet counts in FNAIT. In addition, the extent of reduced Fc fucosylation of anti-HPA-1a correlated significantly with more severe bleeding tendency. In contrast, we do not see this decreased fucosylation phenomenon for anti-HLA antibodies in RT, strongly suggesting that IgG glycosylation, and therefore its functional potential, is under tight regulation in humans.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Sanquin (PPOC-09- 025) (R.K.), the Landsteiner Foundation for Blood Transfusion (grant 0721) (H.K.E. and R.V.), and the European Union’s Seventh Framework Programme (FP7-Health-F5-2011) under Grant Agreement No. 278535 (HighGlycan) (M.W.).
Authorship
Contribution: R.K. coordinated all sample gathering, analyzed clinical data, and performed phagocytosis experiments; L.P., M.d.H., A.V., D.J., B.K., D.B., B.S., M.K.K., and T.E.M. assisted with gathering patient material for this study; H.K.E. and R.V. produced recombinant antibodies; I.K. performed SPR experiments and coanalyzed them together with T.R.; C.A.M.K. prepared samples and conducted the mass spectrometry; M.W. and A.M.D. processed the raw mass spectrometry data; R.K., M.W., and G.V. made the figures and tables; R.K. performed statistical analyses; G.V. conceived and supervised the study; M.W. and C.E.v.d.S. cosupervised the study; R.K., G.V., C.E.v.d.S., and M.W. wrote the paper, which was critically revised and approved by all authors; and all authors contributed to analysis and interpretation of data.
Conflict-of-interest disclosure: B.S. is CEO, M.K.K. is COO, and both are stock owners of Prophylix Pharma AS. The remaining authors declare no competing financial interests.
Correspondence: Dr Gestur Vidarsson, Department of Experimental Immunohematology, Sanquin Research and Landsteiner Laboratory, Plesmanlaan 125, 1066 CX, Amsterdam, The Netherlands; e-mail: g.vidarsson@sanquin.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal