Abstract
Fetal hemoglobin (HbF) modulates the phenotype of sickle cell anemia by inhibiting deoxy sickle hemoglobin (HbS) polymerization. The blood concentration of HbF, or the number of cells with detectable HbF (F-cells), does not measure the amount of HbF/F-cell. Even patients with high HbF can have severe disease because HbF is unevenly distributed among F-cells, and some cells might have insufficient concentrations to inhibit HbS polymerization. With mean HbF levels of 5%, 10%, 20%, and 30%, the distribution of HbF/F-cell can greatly vary, even if the mean is constant. For example, with 20% HbF, as few as 1% and as many as 24% of cells can have polymer-inhibiting, or protective, levels of HbF of ∼10 pg; with lower HbF, few or no protected cells can be present. Only when the total HbF concentration is near 30% is it possible for the number of protected cells to approach 70%. Rather than the total number of F-cells or the concentration of HbF in the hemolysate, HbF/F-cell and the proportion of F-cells that have enough HbF to thwart HbS polymerization is the most critical predictor of the likelihood of severe sickle cell disease.
Background
Fetal hemoglobin (HbF, ∝2γ2) can inhibit the deoxygenation-induced polymerization of sickle hemoglobin (HbS, α2βS2) that drives the pathophysiology of sickle cell disease. This effect of HbF is a result of a reduction of mean cell HbS concentration, a prime determinant of polymerization tendency, and because neither HbF nor its mixed hybrid tetramer (∝2βSγ) can enter the deoxyHbS polymer phase.1 From ∼85% of total hemoglobin at birth, HbF normally falls to <1% at 1 year of age, with a reciprocal increase in adult hemoglobin (HbA). The switch from HbF to HbS in sickle cell anemia (homozygosity for the HbS gene) is delayed, and stable levels of HbF are not reached until age 5 to 10 years.2 In most adults with sickle cell anemia, HbF levels are increased; however, the magnitude of this increase is very variable. HbF production is restricted to a small number of erythroid precursors; their progeny in the blood are called F-cells. Both HbF concentration and its distribution among erythrocytes are heritable. HbF gene expression is regulated by elements linked to the β-globin gene complex that are associated with the β-globin gene haplotype, and by trans-acting elements associated with the BCL11A gene on chromosome 2p16 and the HBS1L-MYB intergenic region on chromosome 6q23.3 The genetic basis of the distribution of HbF among F-cells has not been studied. Hemoglobin switching, the process in which HbF is replaced as the principal hemoglobin by HbA in normal individuals or HbS in sickle cell anemia, has been recently reviewed.4
The total level of HbF in the blood is usually measured by high-performance liquid chromatography. Flow cytometry, the standard clinical method of enumerating F-cells, can detect cells with about 6 pg of HbF. F-cells contain both HbF and HbA (HbS in the case of sickle cell anemia). A high correlation (R2 = 0.97) is present between the number of F-cells and the percent HbF in the hemolysate.5,6 The origins, genetics, and physiology of F-cells have been studied.7,8 Single-cell analyses can detect HbF cells with ∼3 to 4 pg of HbF, but such methods are not used clinically.7 HbF/F-cell remains stable as nucleated red cells transit to reticulocytes that mature to erythrocytes, whereas HbA concentration increases, suggesting that the concentration of HbF/F-cell changes during maturation.9 Quantitative methods for measuring the amount of HbF in each F-cell (HbF/F-cell) and plotting the distribution of HbF among F-cells are not available. HbF/F-cell can be calculated, but this falsely assumes that each F-cell contains the same amount of HbF.10
In 10 normal individuals, mature F-cells, measured by a sensitive method, contained 4 to 5 pg of HbF.7,11 In 46 African Americans with sickle cell anemia, F-cells ranged between 2% and 80% of erythrocytes, and the average HbF/F-cell was 6.4 ± 1.6 pg.7 Patients with sickle cell anemia have individually characteristic distributions of HbF/F-cell regardless of their total HbF level.12
HbF in sickle cell disease
Haplotype of the β-globin gene cluster and HbF
The β-globin gene cluster in sickle cell anemia is found on 4 major haplotypes called Bantu, Benin, Senegal, and Arab-Indian (AI). Mean HbF concentrations differ among these haplotypes. A wide range of HbF concentrations within each haplotype group suggests that elements linked to the β-globin gene cluster are not the dominant determinants of heterogeneity of HbF concentration and F-cell numbers. The AI and Senegal haplotypes of the β-globin gene cluster are associated with the highest HbF levels, and individuals with these haplotypes can have milder disease. Nevertheless, they are anemic and often symptomatic.
HbS and hereditary persistence of HbF
Compound heterozygosity for HbS and gene deletion hereditary persistence of HbF (HPFH) is a condition where patients have symptoms of neither sickle cell disease nor hemolytic anemia, and the typical HbF level is 30%.13 HbF is distributed among all erythrocytes of HbS-HPFH so that with a normal mean corpuscular hemoglobin concentration, each cell should contain ∼10 pg of HbF. Studies suggest that deoxyHbS polymerization is prevented at physiologic venous and capillary O2 saturations of 40% to 70% when HbF/F-cell is 9 to 12 pg.10 HbS polymer is not present in the erythrocytes of HbS-HPFH, either experimentally or after calculating the HbS polymer fraction at 70% O2 saturation.14 HbS polymer is present at 70% O2 saturation in sickle cell anemia, and the polymer fraction rises steeply as O2 saturation falls.14 These data suggest that the number of F-cells with polymer-inhibiting concentrations of HbF is a more important determinant of the features of sickle cell anemia than the total number of F-cells or the concentration of HbF in the hemolysate.
HbF and clinical complications
Adult patients with sickle cell anemia who have the AI haplotype have HbF levels nearly 4 times higher than African American patients and twice as high as Saudi Arabs with the Benin haplotype.15 Even with an HbF of 15% to 20%, painful episodes, acute chest syndrome, and osteonecrosis were nearly as common in adults with the AI haplotype as in African Americans and Saudi Arabs with the Benin haplotype.16,17 Compound heterozygotes with HbS-δβ thalassemia, a genotype caused by large deletions in the β-globin gene cluster, have 15% to 25% HbF. They have anemia and vasoocclusive complications, albeit less severe than in sickle cell anemia. Some African Americans with sickle cell anemia have HbF levels similar to individuals with the AI haplotype.18 Like AI haplotype adult patients, their disease is not benign. In contrast to HbS-HPFH, HbF is unevenly distributed among erythrocytes in all of these patient groups.
HbF and hydroxyurea
Hydroxyurea reduces the morbidity and mortality of sickle cell anemia, an effect mediated primarily, although perhaps not exclusively, by its induction of HbF. The HbF response to hydroxyurea is variable: some patients do not respond to treatment; among responders, some achieve very high HbF levels and others do not.5 Hydroxyurea does not cure sickle cell anemia. Patients are better and probably live longer, but they are rarely asymptomatic, especially when this drug is started in adults after HbF levels stabilize.19,20 HbF is heterocellularly distributed when high levels are successfully induced with hydroxyurea. The calculated mean HbF/F-cell in hydroxyurea-treated sickle cell anemia is 8 pg.
A glass half full?
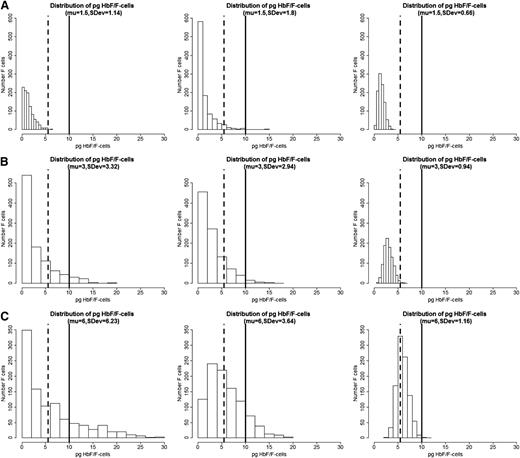
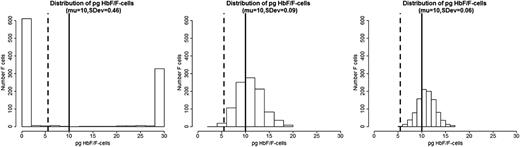
We propose that the distribution of HbF concentrations among F-cells is the most critical element in the pathophysiology of sickle cell anemia. If all F-cells had 10 pg of HbF then, as exemplified by HbS-HPFH, there would be no disease. Total HbF or F-cell percentages are often not a good predictor of disease severity in an individual with sickle cell anemia because they provide no information on the number of F-cells that have levels of HbF sufficient to protect against polymer-induced damage. In the absence of available published data on the distribution of concentrations of HbF/F-cell or a high-throughput method of obtaining such data, we modeled hypothetical scenarios displayed as a series of histograms generated for individuals with blood HbF concentrations of 5% (Figure 1A), 10% (Figure 1B), and 20% (Figure 1C).21 These HbF levels are representative of the Bantu, Benin, and AI haplotypes of sickle cell anemia, respectively, where HbF is distributed heterogeneously among F-cells. In each individual horizontal panel, 3 hypothetical distributions of F-cells (y-axis) are shown that would produce the same mean levels of HbF (x-axis). The data were generated using a mathematical model in which the HbF/F-cell was modeled as a β distribution defined in the range 0 to 30. The β distribution is defined by the mean and the variance parameters, and different combinations of these parameters produce distributions with very different shapes. In the 3 scenarios in Figure 1, we fixed means = 1.5, 3, and 6, and then for each fixed mean, we varied the variance to generate β distributions of different concentrations of HbF/F-cell. We then generated 1000 points from each of these β distributions to represent hypothetical samples of F-cells. The lower limit of HbF detectable by flow cytometry, or ∼6 pg, is represented by the dashed vertical line. This concentration of HbF is insufficient to thwart HbS polymerization where O2 saturation is low, even though these cells are recognizable as F-cells. The level of HbF that inhibits deoxyHbS polymerization, or ∼10 pg, is shown by the solid vertical line. The numbers of these cells cannot be estimated by calculating the mean HbF/F-cell. As shown by these simulations, the number of protected cells must be very low when HbF levels are 5% or 10%, reflecting the severity of disease in patients with these HbF levels. It is nearly impossible to have a clinically important number of protected F-cells when HbF is 5% and no more than 15% of cells can be protected when HbF is 10%. When the HbS levels reaches 20%, it is possible for nearly 25% of F-cells to have polymer-inhibiting HbF levels, although the concentration of HbF/F-cell can be much lower even when the total number of F-cells is high. This is consistent with the clinical observation that nearly all patients with natural or therapy-induced HbF levels of 20% still have hemolytic anemia, and many of these patients are ill. The amount of HbS polymer in their cells is sufficient to injure the cell membrane and lead to hemolysis and sickle vasoocclusion. We then modeled a HbF concentration of 30%, a level rarely seen in adults, even with hydroxyurea treatment. Only with this concentration of HbF can the percent of protected cells reach 70% (Figure 2). Based on the clinical practice of lowering HbS concentration to <30% of total hemoglobin when transfusing a patient for stroke prevention or to limit complications from acute chest syndrome, this level of protected cells might be considered a therapeutic goal for induction of HbF. Clinical observations in Saudi Arabs with sickle cell anemia suggest that the disease is milder in children that have HbF concentrations near 30% but that symptoms become common in adults when HbF levels fall to 15% to 20%.17 Table 1 shows the percent of F-cells that have protective HbF concentrations for the 4 HbF concentrations modeled in Figures 1 and 2 and quantifies the remarkable breadth of the distributions of cells with sufficient HbF to prevent deoxyHbS polymerization that is possible with the same HbF level. The ratio of protected cells to all F-cells is shown in Table 1 and illustrates that with the same HbF level, the number of F-cells does not equate with the number of protected cells.
Hypothetical distribution of HbF/F-cell and the percent F-cells in 3 patients with HbF levels of 5%, 10%, and 20%. (A) HbF 5%. (B) HbF 10%. (C) HbF 20%. Plots were generated by simulating a sample of 1000 cells in each plot using a β distribution defined in the range 0 to 30, with mean HbF content per cell of 5% (μ = 1.5 pg), 10% (μ = 3 pg), and 20% (μ = 6 pg). With a fixed mean, the standard deviation (SDev) was changed to show how the distribution of HbF per cell can greatly vary even if the mean is the same (see text for additional details). The simulations were conducted using the R package.21 Vertical dashed lines show the approximate lower level of HbF/F cells needed to detect F-cells by fluorescence-activated cell sorter, and the solid vertical line shows the approximate lower level of HbF needed to protect the cell from deoxyHbS polymerization at physiologic O2 saturations.
Hypothetical distribution of HbF/F-cell and the percent F-cells in 3 patients with HbF levels of 5%, 10%, and 20%. (A) HbF 5%. (B) HbF 10%. (C) HbF 20%. Plots were generated by simulating a sample of 1000 cells in each plot using a β distribution defined in the range 0 to 30, with mean HbF content per cell of 5% (μ = 1.5 pg), 10% (μ = 3 pg), and 20% (μ = 6 pg). With a fixed mean, the standard deviation (SDev) was changed to show how the distribution of HbF per cell can greatly vary even if the mean is the same (see text for additional details). The simulations were conducted using the R package.21 Vertical dashed lines show the approximate lower level of HbF/F cells needed to detect F-cells by fluorescence-activated cell sorter, and the solid vertical line shows the approximate lower level of HbF needed to protect the cell from deoxyHbS polymerization at physiologic O2 saturations.
Hypothetical distribution of HbF/F-cell and the percent F-cells in a patient with 30% HbF. Data were generated as in Figure 1, using a β distribution with fixed mean = 10 and decreasing standard deviation. The left panel shows an extreme example of U-shaped distribution with a mean of 10 pg but very large variance. In this situation, the F-cells are either totally protected (100% HbF) or totally unprotected (0% HbF), with very few cells with intermediate concentrations of HbF/F-cell.
Hypothetical distribution of HbF/F-cell and the percent F-cells in a patient with 30% HbF. Data were generated as in Figure 1, using a β distribution with fixed mean = 10 and decreasing standard deviation. The left panel shows an extreme example of U-shaped distribution with a mean of 10 pg but very large variance. In this situation, the F-cells are either totally protected (100% HbF) or totally unprotected (0% HbF), with very few cells with intermediate concentrations of HbF/F-cell.
HbF as a predictor of phenotypes
Survival was improved in sickle cell anemia patients with HbF >8.6% compared with patients with lower HbF levels.22 These observations are consonant with our simulations that suggest that very few protected F-cells are present when HbF levels are ∼5% but more protected cells are possible when HbF reaches levels of 10%. In epidemiologic studies of hundreds of patients, HbF concentration is associated with a reduced likelihood of having certain disease subphenotypes. For the individual patient, HbF concentration has little prognostic value because of the highly variable distribution of HbF among F-cells. In cross-sectional studies, higher HbF levels were associated with a reduced rate of acute painful episodes, fewer leg ulcers, less osteonecrosis, less frequent acute chest syndromes, and reduced disease severity. However, HbF level had a weak or no clear association with priapism, urine albumin excretion, stroke and silent cerebral infarction, systemic blood pressure, and tricuspid regurgitant velocity.23 This dichotomy in the disease-modifying effects of HbF might be a consequence of intravascular hemolysis of erythrocytes without protective HbF levels. Lysis of these cells release heme products into the circulation that scavenge nitric oxide and permit the development of endothelial damage and other consequences of reduced nitric oxide bioavailability.24 It is also possible that the pathophysiology of some hemolysis-associated complications is less dependent on HbS polymerization, and thus less likely to be influenced by HbF, and more dependent on blood flow, oxidant injury, or inflammation, important mediators of disease pathobiology. Different concentrations of HbF were postulated to protect against different complications of disease, although 1 study suggested that any increment in HbF was associated with longevity.22,25 Only the calculated mean value of HbF/F-cell was reported and only in some of these studies.10 We propose that HbF/F-cell and the proportion of F-cells that have sufficient HbF to interfere with deoxyHbS polymerization is the more critical predictor of some disease subphenotypes.
Therapeutic implications
Induction of high levels of HbF is the most promising approach to the pharmacologic treatment of sickle cell anemia because it targets the proximal pathophysiologic trigger of disease. Hydroxyurea can induce HbF in most patients and is clinically beneficial in many. However, even some patients who respond to hydroxyurea with HbF near 20% can have sudden life-threatening complications. Most patients have persistent, albeit reduced, vasoocclusive events and hemolytic complications after starting treatment after the first few years of life. Starting treatment earlier seems to retard the fall in HbF, but the gradual fall in HbF after age 10 suggests that these patients will also develop increasing symptoms because even with 20% HbF, many F-cells will continue to be poorly protected from polymer-induced damage.
This has prompted the search for other HbF induction therapies. BCL11A is a major repressor of γ-globin gene expression. Its inactivation abrogated the complications of sickle cell disease in transgenic sickle mice. These animals had a pancellular distribution of HbF in their erythrocytes, akin to that seen in HbS-HPFH, and the expression of γ-globin genes was 28.3% of non–α-globin chains compared with 1.3% in control animals.26 Whether or not targeting BCL11A or its pathway in humans will result in a pancellular or heterocellular distribution of HbF among erythrocytes is unknown. If the distribution is pancellular-like in HbS-HPFH and if total HbF levels of 30% can be achieved, then a pharmacologic cure of most disease complications should be realized. If the distribution is heterocellular, like in hydroxyurea-treated patients, combination treatment with drugs that effect the kinetics of erythropoiesis that might broaden the distribution of F-cells, coupled with an agent whose primary mechanism of action is to increase the transcription of the γ-globin genes, might be the most fruitful approach to HbF induction therapy and more efficacious than single agent treatment.
Authorship
Contribution: M.H.S. developed the ideas and wrote the manuscript; D.H.K.C. wrote and edited the manuscript; G.J.D. wrote and edited the manuscript; P.S. wrote and edited the manuscript and provided calculations; and A.A. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin H. Steinberg, 72 E. Concord St, Boston, MA 02118; e-mail: mhsteinb@bu.edu.