Key Points
In a mouse model, BCR-ABL1+ leukemia stem cells are more dependent on selectins and their ligands for homing and engraftment than normal HSCs.
Blockade of selectin-ligand interactions might prevent leukemic engraftment and relapse in autografted patients.
Abstract
We investigated adhesion pathways that contribute to engraftment of breakpoint cluster region-Abelson murine leukemia viral oncogene homolog 1 (BCR-ABL1)–induced chronic myelogenous leukemia (CML)-like myeloproliferative neoplasia in a mouse retroviral transduction/transplantation model. Compared with normal stem/progenitor cells, BCR-ABL1+ progenitors had similar expression of very late antigen-4 (VLA4), VLA5, leukocyte functional antigen-1, and CXCR4 but lower expression of P-selectin glycoprotein ligand-1 (PSGL-1) and of L-selectin. Whereas vascular cell adhesion molecule-1 and P-selectin were not required, deficiency of E-selectin in the recipient bone marrow endothelium significantly reduced engraftment by BCR-ABL1–expressing stem cells following intravenous injection, with leukemogenesis restored by direct intrafemoral injection. BCR-ABL1–expressing cells deficient for PSGL-1 or the selectin ligand-synthesizing enzymes core-2 β1,6-N-acetylglucosaminyltransferase or fucosyltransferases IV/VII were impaired for engraftment, and destruction of selectin ligands on leukemic progenitors by neuraminidase reduced engraftment. BCR-ABL1–expressing L-selectin–deficient progenitors were also defective in homing and engraftment, with leukemogenesis rescued by coexpression of chimeric E/L-selectin. Antibody to L-selectin decreased the engraftment of BCR-ABL1–transduced stem cells. These results establish that BCR-ABL1+ leukemic stem cells rely to a greater extent on selectins and their ligands for homing and engraftment than do normal stem cells. Selectin blockade is a novel strategy to exploit differences between normal and leukemic stem cells that may be beneficial in autologous transplantation for CML and perhaps other leukemias.
Introduction
The treatment of chronic myelogenous leukemia (CML) was revolutionized by imatinib mesylate, an inhibitor of the breakpoint cluster region-Abelson murine leukemia viral oncogene homolog 1 (BCR-ABL1) tyrosine kinase.1 However, acquired resistance to imatinib and other tyrosine kinase inhibitors (TKIs) is frequent in advanced CML, and the inability of these drugs to kill quiescent Ph+ stem cells2 suggests that TKI therapy alone may not eradicate the disease in most patients. Allogeneic hematopoietic stem cell (HSC) transplantation remains the only proven curative treatment of CML but is limited by graft-versus-host disease and donor availability. Autologous transplantation (autografting), high-dose chemoradiotherapy followed by infusion of the patient’s own stem cells, avoids graft-versus-host disease and has been used extensively for treatment of CML, lymphoma, and plasma cell myeloma.3 In patients with CML in the chronic phase, autologous transplantation (autografting) with polyclonal Ph– stem/progenitor cells4,5 can induce cytogenetic remissions and prolong survival relative to historical controls,6,7 although a benefit has yet to be confirmed in randomized trials. However, autografted CML patients eventually suffer recurrence of Ph+ leukemia, perhaps because residual malignant stem cells re-engraft and contribute to relapse.8 Attempts to purge autografts of contaminating Ph+ progenitors with antisense oligodeoxynucleotides against BCR-ABL19 and c-MYB10 or by in vitro culture11 have had limited success. An alternative strategy would be to selectively block the homing and engraftment of BCR-ABL1–expressing stem cells without interfering with repopulation by nonmalignant HSCs.
Homing and engraftment of normal HSCs is a multistep process.12,13 The β1 integrins very late antigen-4 (VLA4; α4β1) and VLA5 (α5β1) bind to vascular cell adhesion molecule-1 (VCAM-1) and fibronectin on bone marrow (BM) endothelium and extracellular matrix, respectively, and antibodies against VLA4, VLA5, or VCAM-114-17 or genetic deletion of hematopoietic β1 or α4 integrins18,19 impairs HSC homing and engraftment. The β2 integrin leukocyte functional antigen-1 (LFA-1) and its BM receptor intercellular adhesion molecule-1 ordinarily play a minor role in HSC homing but make a major contribution when the VLA4 pathway is compromised.20,21 E- and P-selectins are constitutively expressed on BM endothelium and cooperate with VLA4 to mediate HSC rolling16 and homing.17,21 By contrast, L-selectin is required for lymphocyte recirculation but has no apparent role in HSC homing or engraftment.16,22 CXCL12 stromal-derived factor 1 (SDF-1) functions as the predominant HSC chemoattractant through its receptor CXCR4,23 cooperates with β1 and β2 integrins to mediate HSC homing,20,24 and is required for stable engraftment.25 Finally, CD44 has been implicated in HSC homing through antibody blocking studies,22,26 although HSC lacking CD44 home and engraft normally.27
Relative to normal HSCs, malignant stem/progenitor cells from patients with CML exhibit multiple adhesive abnormalities. Primitive Ph+ hematopoietic progenitors have decreased adhesion to BM stroma as a consequence of defective β1 integrin function, despite normal expression of VLA4 and VLA5.28 BCR-ABL1–expressing hematopoietic cell lines29 and primary CML progenitors30 express CXCR4 but demonstrate impaired chemotaxis toward SDF-1 and reduced SDF-1–mediated, integrin-dependent adhesion. Finally, CML progenitors have reduced expression of L-selectin due to shedding and downregulation of SELL gene transcription,31 but the functional consequences are unknown.
Whereas CML stem/progenitor cells have functional defects in adhesion pathways required for homing and engraftment of normal HSCs but can nonetheless engraft and contribute to relapse following autografting, we hypothesized they must rely on alternative adhesion receptors. We used a well-characterized retroviral model of CML-like myeloproliferative neoplasia (MPN)32 along with donor and recipient mice with mutations in adhesion molecules to analyze these pathways. Our previous results identified a requirement for CD44 in homing and engraftment of BCR-ABL1–expressing leukemic stem cells.33 Here we extend these studies to define complementary roles for recipient E-selectin and selectin ligands on leukemic cells in engraftment of CML-like MPN and demonstrate a novel function for L-selectin on CML stem cells in homing and engraftment. Our findings suggest strategies to prevent re-engraftment of Ph+ leukemic stem cells in CML patients treated by autologous transplantation.
Materials and methods
Mouse strains
All mutant mouse strains were in a mixed B6;129 background, backcrossed into B6 for varying numbers of generations at the time of transplantation. Selplg−/− mice (∼5 generations backcrossed to B6) were obtained from Dr Rodger McEver.34 Selplg−/− mice were intercrossed with Cd44−/− mice (Jackson Laboratory)35 to generate Selplg−/−Cd44−/− mice, which were ∼4 generations backcrossed to B6. Sell−/− mice (>7 generations backcrossed to B6) were obtained from Dr Tanya Mayadas, Gcnt1−/− mice (∼4 generations backcrossed to B6) were from Dr James Marth,36 and Fut4−/−/Fut7−/− mice (∼6 generations backcrossed to B6) were from Dr John Lowe. For recipient mice, Vcam1flox/flox/TIE2Cre+ mice (∼4 generations backcrossed to B6) were the kind gift of Dr Pandelakis Koni,37 whereas Selp−/−, Sele−/−, and Selp−/−Sele−/− mice (all >7 generations backcrossed to B6) were obtained from Dr Richard Hynes. B6×129S F2/J (Jackson Laboratory) mice were used as control donors and recipients, as described previously.33 All experiments were approved by the Institutional Animal Care and Use Committees of Tufts Medical Center and Massachusetts General Hospital.
BM transplantation
BM was harvested from 5-fluorouracil–treated donor mice, transduced with p210 BCR-ABL1 MSCV-IRES/GFP retrovirus as described,32 and 3 × 105 cells were injected intravenously (i.v.) into lethally irradiated (900 cGy for Balb/c, 1050 cGy for B6129 F2) recipients. Transduction efficiency of primary BM progenitors was equivalent between wild-type (WT) and the various mutant donors. Intrafemoral BM injections were performed as described38 by injecting 3 × 105 cells in a volume of 30 μL Hanks balanced salt solution via a 0.5-mL U-100 insulin syringe. For antibody blocking experiments, 2 to 3 × 106 transduced donor BM cells from WT Balb/c mice were incubated with 10 μg of anti-mouse CD62L (clone MEL-14) or isotype-matched control antibody in 1 mL Hanks balanced salt solution for 30 minutes, washed, and injected into irradiated syngeneic recipients.
Southern blot analysis
Genomic DNA from leukemic tissues was digested with BglII, transferred to a nylon membrane, and hybridized with a radioactively labeled probe from green fluorescent protein (GFP) or the human E-selectin cDNA to detect distinct retroviral integration events and, subsequently, with an ABL1 probe to determine the total proviral content of each sample.
Neuraminidase treatment of BM
Following retroviral transduction, BM was plated at 2 × 107 cells/mL in Dulbecco’s modified Eagle medium containing stem cell factor, interleukin-3, and interleukin-6. Varying concentrations of Vibrio cholerae neuraminidase (NA; Roche) were added, and cells were incubated at 37°C for 30 minutes with occasional shaking, as described previously.39 For control experiments, NA was heat-inactivated at 94°C for 30 minutes. Cells were collected on ice to prevent resynthesis of selectin ligands and immediately injected i.v. into irradiated recipient mice. To quantify selectin ligands on hematopoietic progenitors, we prepared concentrated conditioned medium from 293T cells transfected with an expression plasmid for an E-selectin/IgM fusion protein40 and stained cells with a 1:300 dilution of this reagent and an anti-human IgM-phycoerythrin (PE) conjugate (1:100; BD Pharmingen). Selectin ligands were detected in phosphate-buffered saline containing 1.3 mM CaCl2 and 1 mM MgSO4, whereas phosphate-buffered saline with 5 mM EGTA was used as a negative control.
Competitive homing analysis
BM from 5-fluorouracil–treated WT or Sell−/− donors was transduced with BCR-ABL1 + GFP or BCR-ABL1 + nerve growth factor receptor (NGFR) retrovirus, respectively, and transplanted into lethally irradiated B6×129 F2 recipients. Three weeks later, leukemic mice were euthanized, BM/splenocytes were isolated, and an equal mixture of cells of the 2 donors was prepared. The input ratio of GFP+ to NGFR+ cells in the c-Kit+Lin– fraction of the mixture was determined by flow cytometry using PE-conjugated antibody to CD271/human NGFR (clone C40-1457; BD Pharmingen). One to 2 × 108 cells from this mixture were injected into each of 3 irradiated WT recipients. Two hours after injection, the recipients were euthanized, and BM was harvested and analyzed as above for the proportions of c-Kit+Lin– cells expressing GFP or NGFR. The homing index was calculated as the ratio of [c-Kit+ Lin–NGFR-PE+]tissue/[c-Kit+ Lin–GFP+]tissue to [c-Kit+ Lin–NGFR-PE+]input/[c-Kit+ Lin–GFP+]input.
Results
BCR-ABL1+ stem/progenitor cells have normal expression of β1 and β2 integrins but decreased expression of P-selectin glycoprotein ligand-1 and L-selectin
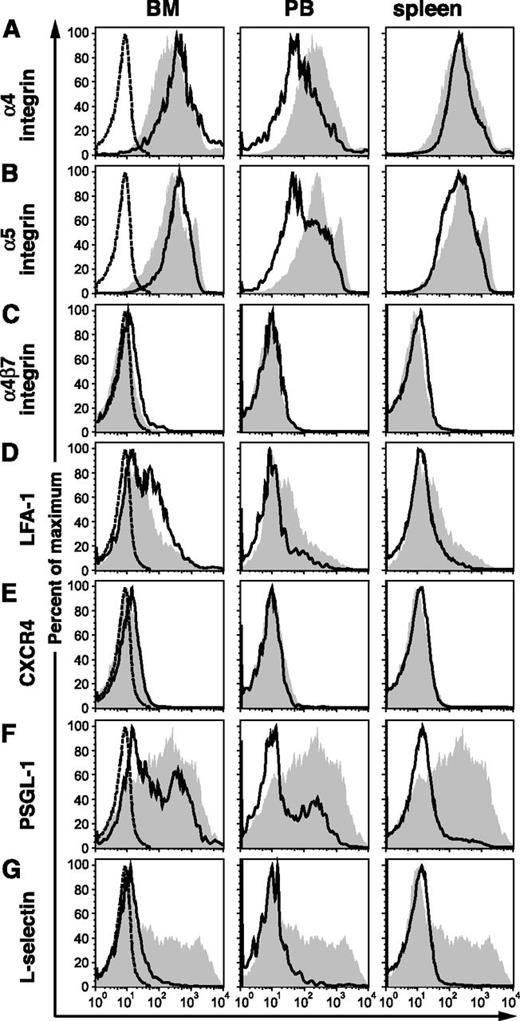
The expression level of adhesion molecules on BCR-ABL1+ (GFP+) c-Kit+Lin– progenitors from BM, peripheral blood (PB), and spleen of leukemic mice was compared with that of normal c-Kit+Lin– BM cells (supplemental Figure 1 on the Blood Web site). Our previous studies demonstrated that BCR-ABL1 increases the expression of functional selectin ligands on murine hematopoietic stem/progenitor cells.33 Here, we found normal expression of α4, α5, α4β7, and αLβ2 (LFA-1) integrins on leukemic progenitors from BM and spleen but lower levels of β1 and β2 integrins on progenitors circulating in PB (Figure 1A-D). CXCR4, the receptor for SDF-1/CXCL12, was expressed at low but comparable levels on both normal and leukemic progenitors (Figure 1E). Interestingly, we observed that the majority of leukemic stem/progenitor cells from PB and spleen and a subset of those from BM had decreased expression of P-selectin glycoprotein ligand-1 (PSGL-1), a scaffold protein that is a major source of selectin carbohydrate ligands on hematopoietic cells (Figure 1F). Finally, we found lower expression of L-selectin on BCR-ABL1–expressing progenitors from all tissues (Figure 1G). These results demonstrate that, similar to human Ph+ progenitors,28 there is no quantitative defect of β1 or β2 integrin expression in leukemic BCR-ABL1+ BM progenitors from mice, whereas the downregulation of L-selectin expression by BCR-ABL1 in human CML31 is reproduced in this mouse model system.
The expression ofL-selectin and PSGL-1 are decreased on c-Kit+Lin–BCR-ABL1+stem/progenitor cells. Flow cytometric analysis of the normalized expression level of adhesion molecules (x-axis) on (left) BM, (center) PB, and (right) spleen cells from a mouse with BCR-ABL1–induced CML-like disease (black lines), gated on c-Kit+Lin–GFP+ cells, vs normal c-Kit+Lin– BM cells (gray): (A) α4-integrin, (B) α5-integrin, (C) α4β7-integrin, (D) LFA-1 (αLβ2), (E) CXCR4, (F) PSGL-1, and (G) L-selectin. Staining with PE-conjugated isotype antibody is indicated by the dotted histograms in the BM samples. Data are representative of 3 independent experiments on mice euthanized 3 weeks after transplantation.
The expression ofL-selectin and PSGL-1 are decreased on c-Kit+Lin–BCR-ABL1+stem/progenitor cells. Flow cytometric analysis of the normalized expression level of adhesion molecules (x-axis) on (left) BM, (center) PB, and (right) spleen cells from a mouse with BCR-ABL1–induced CML-like disease (black lines), gated on c-Kit+Lin–GFP+ cells, vs normal c-Kit+Lin– BM cells (gray): (A) α4-integrin, (B) α5-integrin, (C) α4β7-integrin, (D) LFA-1 (αLβ2), (E) CXCR4, (F) PSGL-1, and (G) L-selectin. Staining with PE-conjugated isotype antibody is indicated by the dotted histograms in the BM samples. Data are representative of 3 independent experiments on mice euthanized 3 weeks after transplantation.
Recipient VCAM-1 and P-selectin are not required for engraftment of BCR-ABL1+ leukemia-initiating cells
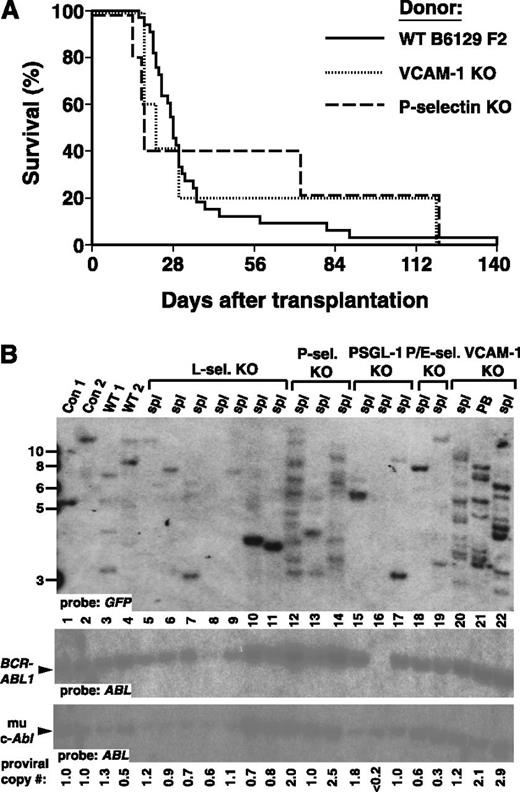
As a genetic approach to test the role of different adhesion pathways in homing and engraftment of BCR-ABL1+ leukemia-initiating cells, we used mice with targeted mutations in adhesion molecules as donors or recipients in the retroviral BM transduction/transplantation model of CML.32 Retroviral transduction of the BCR-ABL1 gene into mouse BM cells, followed by transplantation into irradiated syngeneic mice, induces CML-like MPN in all recipients within 8 weeks after transplantation. The disease is polyclonal and originates from transduced hematopoietic stem/progenitor cells with multilineage repopulating activity.32 To determine the role of the VLA4 pathway in engraftment of CML-like leukemia, we used recipient mice lacking VCAM-1, the principal BM receptor for the β1 integrin VLA4. Whereas germ-line mutation of VCAM-1 causes embryonic lethality due to placental and cardiac defects, we used mice with a conditional allele of Vcam-1 that express Cre recombinase in endothelial cells from the Tyrosine kinase with Ig and EGF homology domains-2 (TIE2) promoter.37 The resulting Vcam1flox/flox/TIE2Cre+ mice lack VCAM-1 expression in the vascular endothelium of BM and lymphoid tissue37 but have normal myelopoiesis, consistent with the lack of requirement for β1 integrins for postnatal hematopoiesis.41 We also assessed the contribution of BM P-selectin to engraftment of CML-like leukemia by using recipients that were homozygous for a germ-line null mutation in the Selp gene. As both Vcam1 and Selp mutant strains were in a mixed B6;129 genetic background, B6×129 F2 hybrid mice were used as WT controls for donor and recipient.
The efficiency of induction of CML-like MPN in this mixed genetic background is lower than for syngeneic Balb/c mice,32 although B6 and 129 share the same H-2 haplotype. Nonetheless, the majority (88%) of WT recipients of BCR-ABL1–transduced WT BM died of fatal CML-like leukemia within 2 months after transplantation (Figure 2A). Interestingly, CML-like leukemia developed earlier in the majority of Vcam1 and Selp mutant recipients (median survival of 18-22 vs 27 days for WT recipients), although the overall difference in survival was not significantly different between the 3 cohorts. The CML-like disease in all 3 groups was characterized by leukocytosis, hepatosplenomegaly, and infiltration of the lung parenchyma with maturing myeloid cells with accompanying hemorrhages.32 Although there were no significant differences in PB leukocyte counts or splenomegaly between the cohorts, both mutant recipients exhibited increased pulmonary infiltration and hemorrhage (data not shown), accounting for the shorter disease latency.
Recipient P-selectin and VCAM-1 are not required for engraftment of BCR-ABL1–expressing leukemic stem cells. (A) Kaplan-Meier survival curve for WT (B6129 F2, solid line, n = 33), VCAM-1 KO (Vcam1flox/flox/TIE2Cre) mutant (dotted line, n = 5), or P-selectin KO (Selp−/−) mutant (dashed line, n = 5) recipients of BCR-ABL1–transduced B6129 F2 BM. Here and in subsequent figures, the curve for the WT donor/recipient combination represents cumulative results from all transplants in this study. All mice that died prior to day 56 developed CML-like MPN. The difference in overall survival between the 3 cohorts was not significant (P = .81 for WT vs VCAM-1 KO and P = .96 for WT vs P-selectin KO; Mantel-Cox tests). (B) Increased clonality of CML-like disease in Vcam1 and Selp mutant recipients. Genomic DNA from spleen (spl) or PB of leukemic mice was analyzed by (upper) Southern blot with a GFP probe to detect distinct proviral integration events and (lower) subsequently with an ABL1 probe to allow determination of the total proviral content of each sample,32 shown at the bottom. Con 1 and Con 2 are control DNAs from cell lines that each contain a single BCR-ABL1 provirus, whereas WT 1 and WT 2 are spleen DNA samples of WT recipients with BCR-ABL1–induced CML-like disease. Lanes 12 to 14 show the clonality of disease in P-selectin mutant recipients, whereas lanes 20 to 22 are representative leukemia samples from VCAM-1 mutant recipients.
Recipient P-selectin and VCAM-1 are not required for engraftment of BCR-ABL1–expressing leukemic stem cells. (A) Kaplan-Meier survival curve for WT (B6129 F2, solid line, n = 33), VCAM-1 KO (Vcam1flox/flox/TIE2Cre) mutant (dotted line, n = 5), or P-selectin KO (Selp−/−) mutant (dashed line, n = 5) recipients of BCR-ABL1–transduced B6129 F2 BM. Here and in subsequent figures, the curve for the WT donor/recipient combination represents cumulative results from all transplants in this study. All mice that died prior to day 56 developed CML-like MPN. The difference in overall survival between the 3 cohorts was not significant (P = .81 for WT vs VCAM-1 KO and P = .96 for WT vs P-selectin KO; Mantel-Cox tests). (B) Increased clonality of CML-like disease in Vcam1 and Selp mutant recipients. Genomic DNA from spleen (spl) or PB of leukemic mice was analyzed by (upper) Southern blot with a GFP probe to detect distinct proviral integration events and (lower) subsequently with an ABL1 probe to allow determination of the total proviral content of each sample,32 shown at the bottom. Con 1 and Con 2 are control DNAs from cell lines that each contain a single BCR-ABL1 provirus, whereas WT 1 and WT 2 are spleen DNA samples of WT recipients with BCR-ABL1–induced CML-like disease. Lanes 12 to 14 show the clonality of disease in P-selectin mutant recipients, whereas lanes 20 to 22 are representative leukemia samples from VCAM-1 mutant recipients.
We assessed the efficiency of engraftment of BCR-ABL1–transduced leukemia-initiating cells by quantifying the number of proviral clones in genomic DNA from leukemic cells using Southern blotting (Figure 2B and supplemental Table 1). In WT recipients, the CML-like disease was oligo- to polyclonal as previously described,32 with an average of 4.5 ± 1.2 independent clones (Figure 2B, lanes 3 and 4). Paradoxically, the CML-like leukemia that developed in Vcam1 and Selp mutant recipients showed an increase in the number of proviral clones, with 10.7 ± 1.5 and 9.3 ± 3.1 clones, respectively (P = .003 and P = .010, respectively, vs WT; Student t test). These results demonstrate that engraftment of BCR-ABL1+ stem/progenitor cells is independent of the VLA4/VCAM-1 pathway and of recipient P-selectin.
Recipient E-selectin contributes to homing and engraftment of BCR-ABL1+ leukemic stem cells
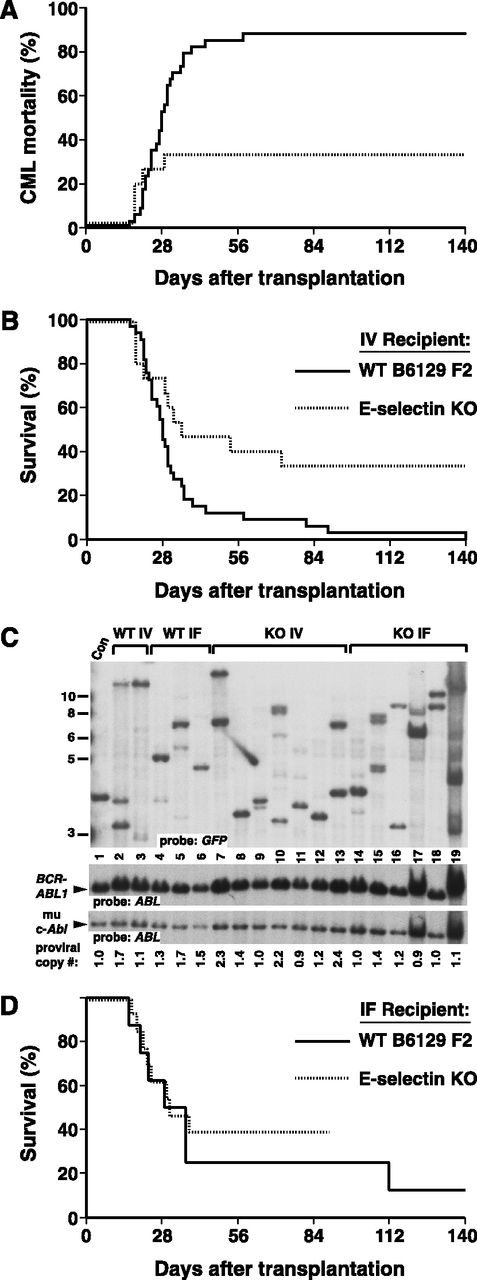
Strikingly different results were obtained when recipients with homozygous germ-line mutations in the Sele gene, lacking expression of E-selectin, were tested (Figure 3). Following i.v. injection of BCR-ABL1–transduced WT BM, engraftment of CML-like leukemia in E-selectin knockout (KO) recipients was inefficient, with only a third of recipients developing MPN (Figure 3A). Another third of the recipients succumbed to other BCR-ABL1–induced hematologic malignancies such as lymphoid leukemia or histiocytic sarcoma32 or had delayed graft failure, whereas the others engrafted with BCR-ABL1– hematopoiesis. The overall survival (Figure 3B) was significantly prolonged relative to WT recipients (P = .021, Mantel-Cox test). These results suggest that E-selectin expression on the BM endothelium of the recipient is critical for efficient engraftment of BCR-ABL1+ leukemia-initiating cells. Consistent with this, the clonality of the leukemia in those few recipients that engrafted with CML-like disease was significantly reduced (P = .0025, Student t test), with most recipients repopulated with only 1 or 2 BCR-ABL1+ stem cells (Figure 3C and supplemental Table 1).
Recipient E-selectin is required for efficient engraftment of BCR-ABL1+stem cells. (A-B) Kaplan-Meier curves for (A) mortality due to CML-like leukemia and (B) overall survival of B6129 F2 control (solid line, n = 33) or E-selectin KO (dotted line, n = 15) recipients of BCR-ABL1–transduced BM from WT B6129F2 donors, administered via an i.v. route. The difference in overall survival between the 2 cohorts was significant (P = .021, Mantel-Cox test). (C) Engraftment of E-selectin mutant recipients, assessed by Southern blotting of leukemic cell DNA as in Figure 2B. Note that E-selectin KO i.v. leukemia samples in lanes 7, 10, and 13 had proviral copy number ≥2, indicating engraftment by leukemia-initiating cells with multiple proviral integrations per cell. (D) Intrafemoral transplantation increases engraftment of E-selectin mutant recipients. Kaplan-Meier survival curve for B6129 F2 control (solid line, n = 8) or E-selectin KO (dotted line, n = 13) recipients of BCR-ABL1–transduced BM from WT B6129 F2 donors, administered by direct i.f. injection. All mice that succumbed within 2 months of transplantation developed CML-like leukemia. There was no significant difference in overall survival between the 2 groups (P = .62, Mantel-Cox test).
Recipient E-selectin is required for efficient engraftment of BCR-ABL1+stem cells. (A-B) Kaplan-Meier curves for (A) mortality due to CML-like leukemia and (B) overall survival of B6129 F2 control (solid line, n = 33) or E-selectin KO (dotted line, n = 15) recipients of BCR-ABL1–transduced BM from WT B6129F2 donors, administered via an i.v. route. The difference in overall survival between the 2 cohorts was significant (P = .021, Mantel-Cox test). (C) Engraftment of E-selectin mutant recipients, assessed by Southern blotting of leukemic cell DNA as in Figure 2B. Note that E-selectin KO i.v. leukemia samples in lanes 7, 10, and 13 had proviral copy number ≥2, indicating engraftment by leukemia-initiating cells with multiple proviral integrations per cell. (D) Intrafemoral transplantation increases engraftment of E-selectin mutant recipients. Kaplan-Meier survival curve for B6129 F2 control (solid line, n = 8) or E-selectin KO (dotted line, n = 13) recipients of BCR-ABL1–transduced BM from WT B6129 F2 donors, administered by direct i.f. injection. All mice that succumbed within 2 months of transplantation developed CML-like leukemia. There was no significant difference in overall survival between the 2 groups (P = .62, Mantel-Cox test).
To confirm that the defect in E-selectin KO recipients was related to BM homing, we injected the same number of WT BCR-ABL1–transduced stem/progenitor cells directly into the femoral BM cavity38 of WT or E-selectin–deficient recipients (Figure 3D). In WT recipients, intrafemoral (i.f.) transplantation induced CML-like MPN in around 75% of recipients within 6 weeks, reflecting the technical difficulty of this procedure. A similar frequency of CML-like leukemia was induced in E-selectin KO recipients by i.f. transplantation (Figure 3D), demonstrating that delivery of BCR-ABL1–expressing stem cells directly to the BM can bypass the contribution of E-selectin to engraftment. Importantly, the clonality of the leukemia induced in E-selectin KO recipients by i.f. transplantation was significantly increased (Figure 3C, lanes 14-19) to 4.3 ± 2.3 clones per recipient vs 1.9 ± 1.2 clones for cells transplanted i.v. (P = .0282, Student t test). Whereas deficiency of E-selectin alone does not affect BM homing and repopulation by normal HSCs,42 our results identify this adhesion pathway as being of major importance for engraftment of leukemic stem cells expressing BCR-ABL1.
Selectin ligands on BCR-ABL1–expressing leukemia-initiating cells contribute to engraftment
Given the prominent role of recipient BM E-selectin for engraftment of BCR-ABL1–induced CML-like disease, we next explored the role of donor selectin ligands, which are increased on BCR-ABL1+ leukemic stem cells.33 Selectin counter-receptors are carbohydrates with α2,3-sialylation and α1,3-fucosylation on capping structures such as sialyl Lewis x (sLex), which bind to the lectin domains of selectins.43 PSGL-1, the sole P-selectin ligand on human HSCs,44 binds to P-selectin through an N-terminal region that contains sLex and several sulfated tyrosine residues but can also bind to E-selectin through distinct sites. Leukocytes from PSGL-1–deficient (Selplg−/−) mice lack interaction with P-selectin and display moderate defects in rolling on E-selectin in vivo,34 whereas PSGL-1–deficient hematopoietic progenitors have reduced E-selectin–dependent BM homing.42
To test whether selectin ligands contributed by PSGL-1 mediate engraftment of CML-like leukemia, we transduced Selplg−/− donor BM with BCR-ABL1 retrovirus and transplanted the cells by i.v. injection into WT recipients. There was a significant decrease in the incidence of CML-like leukemia in recipients of PSGL-1–deficient BM (Figure 4A; P = .0393, Mantel-Cox test), with approximately two-thirds of recipients developing MPN. Two recipients died after long latency to BCR-ABL1+ T-cell leukemia/lymphoma, which probably results from transduction and subsequent engraftment of a BM T-lymphoid progenitor32 (Figure 4B). In those mice that developed CML-like MPN, there was a decrease in the number of engrafting proviral clones (Figure 2B and supplemental Table 1) to 1.7 ± 0.6 clones per recipient (P = .008 vs WT donors, Student t test). A specialized glycoform of CD44 is another major source of hematopoietic cell E/L-selectin ligands,39 whereas our previous studies showed that CD44 is increased on BCR-ABL1+ leukemic stem cells and contributes to their homing and engraftment.33 Indeed, BCR-ABL1–expressing stem cells lacking both PSGL-1 and CD44 had a more profound engraftment defect, with only approximately one-quarter of recipients developing MPN (Figure 4A), resulting in a significant increase in survival compared with WT donors (P = .010, Mantel-Cox test; Figure 4B) and a decrease in the number of engrafted proviral clones (WT 2.67 ± 1.41 vs PSGL-1/CD44 double KO 1.20 ± 0.45; P = .046, Student t test; Figure 4C and supplemental Table 1). By contrast, when WT and Cd44−/−Selplg−/− HSCs were transduced with GFP retrovirus without BCR-ABL1 and myeloid engraftment was assessed 2 months after transplant, there was low-level but equivalent GFP+ BM engraftment (12.8 ± 4.5% vs 9.0 ± 2.0%; P = .484, Student t test) by the 2 donor HSC populations (supplemental Figure 2A), with similar numbers of engrafting proviral clones in evaluable recipients (supplemental Figure 2B). These results demonstrate that both PSGL-1 and CD44 contribute to the engraftment of BCR-ABL1–expressing leukemic stem cells, most likely through their expression of selectin ligands, but are less important for normal stem cell engraftment. As P-selectin has no role in this process (Figure 2A), the relevant BM counter-receptor may be E-selectin.
Selectin ligands on BCR-ABL1–expressing leukemic stem cells contribute to engraftment. (A-B) Kaplan-Meier curves for (A) mortality due to CML-like leukemia and (B) overall survival of B6129 F2 WT recipients of BCR-ABL1–transduced BM from B6129 F2 WT control (solid line, n = 33), PSGL-1 KO (Selplg−/−; dotted line, n = 8), and PSGL-1/CD44 double KO (Selplg−/−Cd44−/−; dashed line, n = 14) donors. Only 5 of 8 recipients of PSGL-1–deficient BM developed CML-like leukemia. Two mice succumbed at 127 days after transplant to BCR-ABL1–induced T-cell acute lymphoblastic leukemia, whereas another died of delayed graft failure (see text). The overall survival of PSGL-1 KO recipients was not significantly different from recipients of BCR-ABL1–transduced WT BM. (C) Deficiency of PSGL-1 and CD44 in donor BM leads to decreased engraftment of BCR-ABL1–induced CML-like leukemia. Southern blot of genomic DNA from BM of recipients of BCR-ABL1-transduced WT (lanes 2-11) and PSGL-1/CD44 double KO (lanes 12-22) donor BM, hybridized with a GFP probe. Recipients in lanes 6 and 15 to 21 did not develop clinical disease, whereas the recipient in lane 22 succumbed to histiocytic sarcoma. *Background bands. (D-E) Kaplan-Meier curves for (D) mortality due to CML-like leukemia and (E) overall survival for WT recipients of WT (solid line, n = 33), Core2GlcNAcT-I KO (Gcnt1−/−; dashed line, n = 6), and FucT-IV/FucT-VII double KO (Fut4−/−/Fut7−/−; dotted line, n = 6) BCR-ABL1–transduced BM. The difference in survival between recipients of WT and FucT-IV/FucT-VII double KO BM was of borderline significance (P = .13, Mantel-Cox test), whereas that of Core2GlcNAcT-I KO recipients was not. One recipient in the FucT-IV/FucT-VII double KO cohort had circulating BCR-ABL1–expressing myeloid cells early after transplant, but these leukocytes disappeared, and the animal ultimately engrafted with BCR-ABL1– cells (data not shown). (F) Donor Core-2 and Fuc-T IV/VII deficiency leads to a reduction of clonality of CML-like leukemia. Genomic DNA from the indicated tissues was analyzed by Southern blot as in Figure 2B. Lanes 5 to 8 and 9 to 12, respectively, contain the samples of WT recipients of BCR-ABL1–transduced Core-2 KO or Fuc-T IV/VII double KO BM. Note the reduction of clonality in lanes 5 to 12 to 1 or 2 clones. Proviral copy number is indicated below.
Selectin ligands on BCR-ABL1–expressing leukemic stem cells contribute to engraftment. (A-B) Kaplan-Meier curves for (A) mortality due to CML-like leukemia and (B) overall survival of B6129 F2 WT recipients of BCR-ABL1–transduced BM from B6129 F2 WT control (solid line, n = 33), PSGL-1 KO (Selplg−/−; dotted line, n = 8), and PSGL-1/CD44 double KO (Selplg−/−Cd44−/−; dashed line, n = 14) donors. Only 5 of 8 recipients of PSGL-1–deficient BM developed CML-like leukemia. Two mice succumbed at 127 days after transplant to BCR-ABL1–induced T-cell acute lymphoblastic leukemia, whereas another died of delayed graft failure (see text). The overall survival of PSGL-1 KO recipients was not significantly different from recipients of BCR-ABL1–transduced WT BM. (C) Deficiency of PSGL-1 and CD44 in donor BM leads to decreased engraftment of BCR-ABL1–induced CML-like leukemia. Southern blot of genomic DNA from BM of recipients of BCR-ABL1-transduced WT (lanes 2-11) and PSGL-1/CD44 double KO (lanes 12-22) donor BM, hybridized with a GFP probe. Recipients in lanes 6 and 15 to 21 did not develop clinical disease, whereas the recipient in lane 22 succumbed to histiocytic sarcoma. *Background bands. (D-E) Kaplan-Meier curves for (D) mortality due to CML-like leukemia and (E) overall survival for WT recipients of WT (solid line, n = 33), Core2GlcNAcT-I KO (Gcnt1−/−; dashed line, n = 6), and FucT-IV/FucT-VII double KO (Fut4−/−/Fut7−/−; dotted line, n = 6) BCR-ABL1–transduced BM. The difference in survival between recipients of WT and FucT-IV/FucT-VII double KO BM was of borderline significance (P = .13, Mantel-Cox test), whereas that of Core2GlcNAcT-I KO recipients was not. One recipient in the FucT-IV/FucT-VII double KO cohort had circulating BCR-ABL1–expressing myeloid cells early after transplant, but these leukocytes disappeared, and the animal ultimately engrafted with BCR-ABL1– cells (data not shown). (F) Donor Core-2 and Fuc-T IV/VII deficiency leads to a reduction of clonality of CML-like leukemia. Genomic DNA from the indicated tissues was analyzed by Southern blot as in Figure 2B. Lanes 5 to 8 and 9 to 12, respectively, contain the samples of WT recipients of BCR-ABL1–transduced Core-2 KO or Fuc-T IV/VII double KO BM. Note the reduction of clonality in lanes 5 to 12 to 1 or 2 clones. Proviral copy number is indicated below.
As an alternative approach to study selectin counter-receptors on leukemia-initiating cells, we used mice lacking enzymes involved in the biosynthesis of these ligands. Virtually all selectin ligands on mature myeloid cells are synthesized from serine-threonine–linked branched oligosaccharide precursors known as core 2 O-glycans, the products of core-2 β1,6-N-acetylglucosaminyltransferase (core2 GlcNAcT-I). In subsequent steps, α-1,3-fucosylation of α-2,3-sialyated O-glycans, catalyzed by the 2 principal α-1,3-fucosyltransferases (FucTs) expressed in mammalian leukocytes (FucT-IV and FucT-VII), is required for maturation of selectin ligands.43 BCR-ABL1–expressing stem cells from core2 GlcNAcT-I–deficient (Gcnt1−/−) donors36 induced CML-like MPN in the majority of WT recipients (Figure 4D), accompanied by a slight prolongation in survival that was not statistically significant (Figure 4E). There was, however, a significant reduction in the efficiency of engraftment of mutant leukemic stem cells (Figure 4F, lanes 5-8), manifested by a decrease in the average number of proviral clones in recipients of core2 GlcNAcT-I–deficient BM to 2.0 ± 0.8 (P = .008 vs WT donors, Student t test; supplemental Table 1). The modest effect of core2 GlcNAcT-I deficiency on leukemic stem cell engraftment was perhaps to be anticipated, given that leukocytes from these mice completely lack P-selectin ligands but have less severe defects in E-selectin counter-receptor activity.36,45 Qualitatively similar results were obtained when mice lacking both FucT-IV and FucT-VII (Fut4−/−Fut7−/−)46 were used as BM donors (Figure 4D-E), with an engraftment defect (Figure 4F, lanes 9-12) and a reduction in disease clonality (1.8 ± 1.0 clones; P = .006 vs WT donors, Student t test; supplemental Table 1).
Collectively, these results argue that fucosylated selectin ligands expressed on PSGL-1 and other counter-receptors contribute to the engraftment of BCR-ABL1+ leukemia-initiating cells in CML. However, it is notable that the engraftment defect of FucT-IV/FucT-VII–deficient leukemic cells was less profound than that of WT BCR-ABL1–expressing cells in E-selectin–deficient recipients, given that neutrophils from the former mice are virtually devoid of selectin-binding activity.46 This raises the possibility that some E-selectin ligands on CML stem cells might be synthesized by other FucT family members or be independent of α-1,3-fucosyltransferase activity altogether. To address this, we treated untransduced and BCR-ABL1–transduced WT BM with NA, which removes essential terminal sialic acid residues from O-, N-, and lipid-linked glycans with potential selectin-binding activity.43 Whereas preliminary experiments indicated that extensive NA treatment was deleterious to engraftment of both normal and BCR-ABL1–transduced BM, we tested a series of NA concentrations for the extent of removal of selectin ligands on Lin– BM progenitors, using an E-selectin/IgM fusion protein as a staining reagent.40 Approximately 28% of the Lin– BM progenitors expressed detectable E-selectin ligands, and treatment with 12.5 to 25 mU NA for 30 minutes reduced the intensity of staining approximately sixfold (mean fluorescence intensity, 594 vs 101; supplemental Figure 3A).
When BCR-ABL1–transduced BM was treated with NA prior to transplantation into WT recipients, there was modest prolongation in survival that did not reach statistical significance (Figure 5A), but we observed a significant reduction in the engraftment of BCR-ABL1+ leukemic stem cells (2.0 ± 0.7 clones for 12.5 mU/mL NA treatment vs 4.4 ± 2.1 clones for cells incubated without NA; P = .040, Student t test), whereas exposure of cells to heat-inactivated NA did not affect engraftment (Figure 5B). To assess the ability of NA-treated normal and leukemic stem cells to competitively engraft recipients, we transduced separate aliquots of BM with parental or BCR-ABL1 retroviruses expressing either GFP or monomeric red fluorescent protein (mRFP),47 treated the mRFP-expressing cells with NA, and transplanted a mixture of untreated GFP+ and NA-treated mRFP+ progenitors into WT recipients. As expected, all recipients of BCR-ABL1–tranduced BM died of CML-like MPN by 22 days after transplantation, and treatment with 12.5 mU/mL NA significantly decreased BM myeloid engraftment by mRFP+ donor cells (P = .0063, Student t test; Figure 5C, left; supplemental Figure 3B) but did not affect engraftment of normal HSCs (Figure 5C, right). At higher NA concentrations, engraftment of both leukemic and normal stem cells was diminished. The fact that the modest reduction in selectin ligands by low concentrations of NA (supplemental Figure 3A) nonetheless significantly impaired engraftment of BCR-ABL1+ leukemic stem cells emphasizes that these progenitors are more sensitive than normal HSCs on the expression of one or more sialyated glycoproteins or glycolipids. This molecule could be a selectin ligand that is independent of sLex and FucT-IV/VII, as suggested by other studies.48,49
Neuraminidase treatment of donor BM leads to impaired engraftment of BCR-ABL1+leukemia-initiating cells. (A) Kaplan-Meier curve for survival of Balb/c recipients of BCR-ABL1–transduced BM incubated without NA (0 NA, solid line, n = 5), treated with NA at 6.25 (dotted line, n = 6) or 12.5 (dashed line, n = 6) mU/mL, or with heat-inactivated (HI) NA (dotted-dashed line, n = 5) prior to transplantation into WT recipients. All recipients died of CML-like leukemia. (B) NA treatment leads to decreased engraftment of BCR-ABL1–induced CML-like leukemia. Southern blot of genomic DNA from BM of recipients of BCR-ABL1–transduced BM incubated without NA (lanes 2-6), with 6.25 mU/mL NA (lanes 7-10), with 12.5 mU/mL NA (lanes 11-16), or with heat-inactivated NA (lanes 16-19). *Background band. (C) NA treatment selectively reduces leukemic stem cell engraftment. (Left) Ratio of mRFP+ to GFP+ BM myeloid (Mac-1+) cells from recipients of NA-treated BM transduced with BCR-ABL1 retrovirus, assessed at the time of morbidity or death. (Right) mRFP+ to GFP+ ratio of BM myeloid cells from recipients of BM transduced with parental retrovirus, assessed at 65 days after transplantation. The reduction in the mRFP+:GFP+ ratio at 12.5 mU/mL NA was significant for recipients of BCR-ABL1–expressing cells (P = .0063, Student t test) but not for normal HSCs.
Neuraminidase treatment of donor BM leads to impaired engraftment of BCR-ABL1+leukemia-initiating cells. (A) Kaplan-Meier curve for survival of Balb/c recipients of BCR-ABL1–transduced BM incubated without NA (0 NA, solid line, n = 5), treated with NA at 6.25 (dotted line, n = 6) or 12.5 (dashed line, n = 6) mU/mL, or with heat-inactivated (HI) NA (dotted-dashed line, n = 5) prior to transplantation into WT recipients. All recipients died of CML-like leukemia. (B) NA treatment leads to decreased engraftment of BCR-ABL1–induced CML-like leukemia. Southern blot of genomic DNA from BM of recipients of BCR-ABL1–transduced BM incubated without NA (lanes 2-6), with 6.25 mU/mL NA (lanes 7-10), with 12.5 mU/mL NA (lanes 11-16), or with heat-inactivated NA (lanes 16-19). *Background band. (C) NA treatment selectively reduces leukemic stem cell engraftment. (Left) Ratio of mRFP+ to GFP+ BM myeloid (Mac-1+) cells from recipients of NA-treated BM transduced with BCR-ABL1 retrovirus, assessed at the time of morbidity or death. (Right) mRFP+ to GFP+ ratio of BM myeloid cells from recipients of BM transduced with parental retrovirus, assessed at 65 days after transplantation. The reduction in the mRFP+:GFP+ ratio at 12.5 mU/mL NA was significant for recipients of BCR-ABL1–expressing cells (P = .0063, Student t test) but not for normal HSCs.
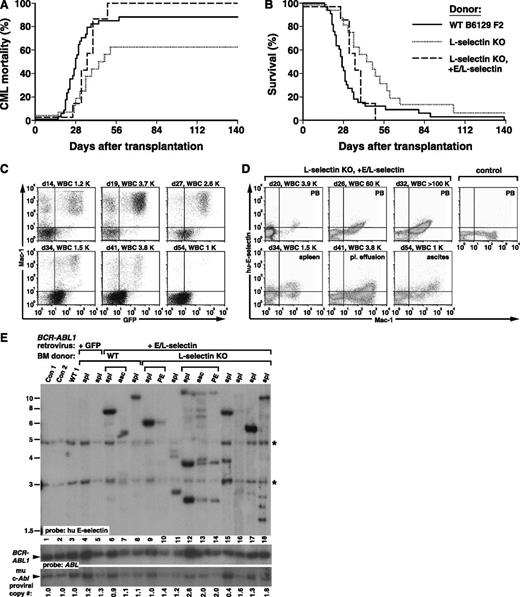
Novel role for L-selectin for homing and engraftment of BCR-ABL1+ leukemic stem cells
To determine the contribution of L-selectin to engraftment and pathogenesis of CML, we transduced BM from donor mice with targeted disruption of the Sell gene, who exhibit normal hematopoiesis but defective leukocyte homing to lymphoid tissues and sites of inflammation.50 BCR-ABL1–expressing L-selectin–deficient progenitors were defective for induction of CML-like leukemia after i.v. injection into WT recipients (Figure 6A). Only 63% (10/16) of recipients developed CML-like disease, with a significant prolongation in overall survival (P = .05 vs WT donor cells, Mantel-Cox test; Figure 6B). Although it is possible that impaired neutrophil infiltration of tissues contributed to the increased survival of this cohort, there was also clear evidence of a defect in engraftment by L-selectin–deficient leukemia-initiating cells. Most of the recipients that did not develop overt MPN had evidence of delayed graft failure, with anemia or pancytopenia developing after disappearance of circulating BCR-ABL1–expressing cells (Figure 6C), which likely reflects engraftment by leukemic progenitors with short-term but not long-term repopulating activity. In agreement, the clonality of the CML-like disease was significantly reduced in recipients of transduced L-selectin KO BM that developed leukemia (Figure 2B, lanes 5-11 and supplemental Table 1), with an average of only 1.2 ± 0.4 proviral clones per recipient (P < .0001 vs WT donors, Student t test).
L-selectin contributes to engraftment of BCR-ABL1+leukemic stem cells. (A) Kaplan-Meier–style curves for (A) mortality due to CML-like leukemia and (B) overall survival of WT B6129 F2 recipients of BCR-ABL1–transduced BM from WT (solid line, n = 33) and L-selectin KO (Sele−/−; dotted line, n = 16) donors transduced with BCR-ABL1 retrovirus and of L-selectin KO BM transduced with retrovirus coexpressing BCR-ABL1 and chimeric E/L-selectin (dashed line, n = 7). The difference in survival between recipients of WT and L-selectin KO BM was significant (P = .05, Mantel-Cox test), whereas there was no significant difference between WT and L-selectin KO + E/L-selectin (P = .45). Interestingly, the majority of mice in the E/L-selectin rescue cohort had evidence of malignant ascites and/or pleural effusions in addition to organomegaly, which might reflect an increased tendency of leukocytes expressing the stabilized selectin to traffic to serosal surfaces.50 (C) Serial flow cytometric analysis of a representative B6129 F2 WT recipient of BCR-ABL1–transduced L-selectin KO BM on days 14, 19, 27, 34, 41, and 54 after transplantation, with the PB leukocyte count indicated. (D) Flow cytometric analysis of a representative B6129 F2 WT recipient of L-selectin KO BM transduced with retrovirus coexpressing BCR-ABL1 and human E/L-selectin chimera, where human E-selectin expression is detected with monoclonal antibody CL-37 (y-axis). (Upper) Serial analysis of PB, showing rapid accumulation of circulating myeloid cells expressing human E-selectin. PB from an untransplanted WT mouse is shown in the last panel. (Lower) Analysis of leukocytes from spleen, pleural effusion, and ascites at necropsy. (E) Restoration of polyclonal engraftment by coexpression of E/L-selectin. Genomic DNA from the indicated tissues (asc, ascites; PE, pleural effusion) was analyzed by Southern blot using a probe from the human E-selectin gene. Brackets indicate different tissues from the same mouse. The BM donor was WT in lanes 4 to 8 and L-selectin KO in lanes 9 to 18. The BCR-ABL1 retrovirus used for transduction coexpressed GFP in lanes 3 to 5 and human E/L-selectin in lanes 6 to 18. Note the increased clonality in most recipients in lanes 9 to 18. Proviral copy number is indicated below. *Bands from the endogenous murine E-selectin gene.
L-selectin contributes to engraftment of BCR-ABL1+leukemic stem cells. (A) Kaplan-Meier–style curves for (A) mortality due to CML-like leukemia and (B) overall survival of WT B6129 F2 recipients of BCR-ABL1–transduced BM from WT (solid line, n = 33) and L-selectin KO (Sele−/−; dotted line, n = 16) donors transduced with BCR-ABL1 retrovirus and of L-selectin KO BM transduced with retrovirus coexpressing BCR-ABL1 and chimeric E/L-selectin (dashed line, n = 7). The difference in survival between recipients of WT and L-selectin KO BM was significant (P = .05, Mantel-Cox test), whereas there was no significant difference between WT and L-selectin KO + E/L-selectin (P = .45). Interestingly, the majority of mice in the E/L-selectin rescue cohort had evidence of malignant ascites and/or pleural effusions in addition to organomegaly, which might reflect an increased tendency of leukocytes expressing the stabilized selectin to traffic to serosal surfaces.50 (C) Serial flow cytometric analysis of a representative B6129 F2 WT recipient of BCR-ABL1–transduced L-selectin KO BM on days 14, 19, 27, 34, 41, and 54 after transplantation, with the PB leukocyte count indicated. (D) Flow cytometric analysis of a representative B6129 F2 WT recipient of L-selectin KO BM transduced with retrovirus coexpressing BCR-ABL1 and human E/L-selectin chimera, where human E-selectin expression is detected with monoclonal antibody CL-37 (y-axis). (Upper) Serial analysis of PB, showing rapid accumulation of circulating myeloid cells expressing human E-selectin. PB from an untransplanted WT mouse is shown in the last panel. (Lower) Analysis of leukocytes from spleen, pleural effusion, and ascites at necropsy. (E) Restoration of polyclonal engraftment by coexpression of E/L-selectin. Genomic DNA from the indicated tissues (asc, ascites; PE, pleural effusion) was analyzed by Southern blot using a probe from the human E-selectin gene. Brackets indicate different tissues from the same mouse. The BM donor was WT in lanes 4 to 8 and L-selectin KO in lanes 9 to 18. The BCR-ABL1 retrovirus used for transduction coexpressed GFP in lanes 3 to 5 and human E/L-selectin in lanes 6 to 18. Note the increased clonality in most recipients in lanes 9 to 18. Proviral copy number is indicated below. *Bands from the endogenous murine E-selectin gene.
To confirm a requirement for L-selectin in leukemic engraftment, we used a retroviral vector to coexpress a chimeric E/L-selectin fusion protein, consisting of the extracellular domain of E-selectin fused to the transmembrane and intracellular domains of L-selectin,51,52 together with BCR-ABL1. Coexpression of E/L-selectin rescued the leukemogenic defect of L-selectin–deficient BM, with 100% of recipient mice developing CML-like leukemia with the same latency as recipients of WT BM (Figure 6A-B), whereas coexpression of E/L-selectin with BCR-ABL1 in WT BM did not significantly alter the CML-like disease (data not shown). As the chimeric E/L-selectin is resistant to shedding induced by BCR-ABL1,53 malignant myeloid cells from these leukemic mice expressed human E/L-selectin on their cell surface (Figure 6D). Importantly, the engraftment defect of the L-selectin–deficient leukemic stem cells was also rescued by coexpression of the stabilized E/L-selectin chimera (Figure 6E), with an increase in the clonality of the leukemia from 1.2 ± 0.4 proviral clones per recipient of L-selectin KO BM to 4.6 ± 3.3 clones expressing E/L-selectin (P = .028, Student t test). Although the lectin-binding specificities of E- and L-selectin are not identical, we infer that the leukemogenesis defect of L-selectin–deficient BCR-ABL1–expressing stem cells is a consequence of lack of binding to a ligand or ligands expressed on the BM endothelium of the recipient that is recognized by both E- and L-selectin.
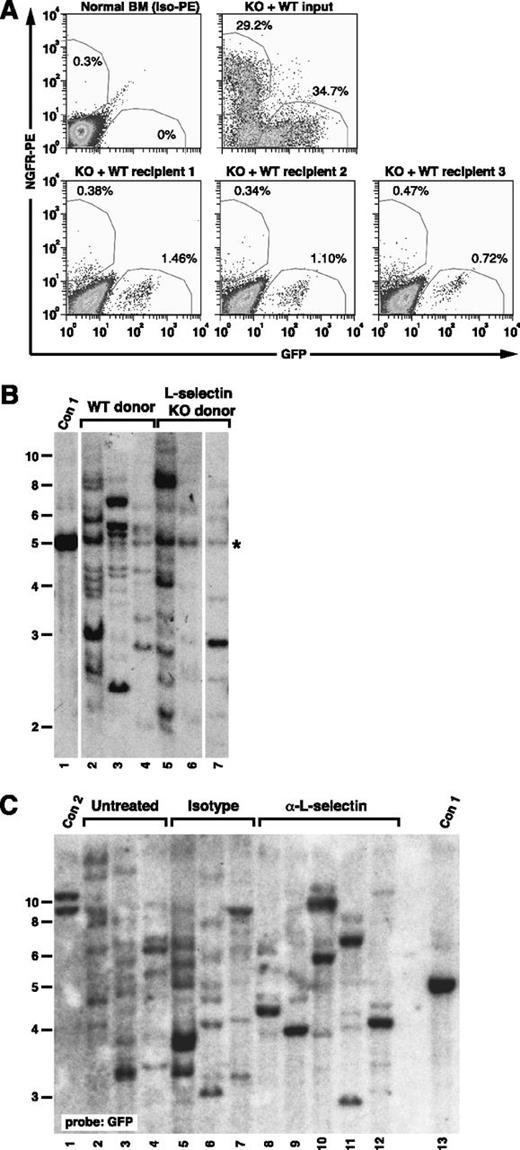
To provide direct evidence for a role for L-selectin in engraftment of leukemic stem cells, we carried out a competitive homing experiment. We observed a marked decrease in the recovery of BCR-ABL1–expressing Sell−/− progenitors (NGFR+c-Kit+Lin–) relative to BCR-ABL1–expressing WT progenitors (GFP+c-Kit+Lin–) from recipient BM 2 hours after injection (Figure 7A), with a homing index for BCR-ABL1+Sell−/− progenitors of 0.23, indicating a greater than fourfold decrease in BM homing relative to WT progenitors. By contrast, we observed equivalent numbers of proviral clones in BM-derived myeloid cells of recipients of vector-transduced WT (10.3 ± 3.1) and Sell−/− (9.7 ± 3.8) BM (P = .824, Student t test), indicating that, in the absence of BCR-ABL1 expression, transduced Sell−/− stem cells engraft as efficiently as WT HSC (Figure 7B). Finally, L-selectin antibody treatment significantly reduced the engraftment of BCR-ABL1+ leukemic stem cells relative to recipients of untreated or isotype control antibody–treated cells (Figure 7C), with 8.3 ± 0.6 clones in recipients of isotype control antibody–treated BM compared with 3.8 ± 1.3 clones in recipients of anti–L-selectin–treated BM (P = .001, Student t test). These results demonstrate that L-selectin is selectively required on BCR-ABL1–expressing leukemic stem cells for efficient engraftment and subsequent development of CML-like MPN.
L-selectin blockade decreases engraftment of BCR-ABL1+leukemia-initiating cells. (A) L-selectin–deficient leukemic progenitors have a defect in BM homing. Irradiated recipient mice (n = 3) were injected with a mixture of BM cells from mice with BCR-ABL1–induced CML-like disease induced from WT donor cells (expressing GFP) and Sell−/− donor cells (expressing NGFR) and euthanized 2 hours later. (Upper left) Flow cytometric analysis of c-Kit+Lin– normal BM stained with isotype-PE antibody. (Upper right) Input mixture of leukemic WT and Sell−/− progenitors. (Lower) Corresponding c-Kit+Lin– populations isolated from BM of the 3 recipients, stained with PE-conjugated antibody against human NGFR. The percentage of GFP+ and NGFR+ cells is shown adjacent to the respective regions. (B) No defect in engraftment of Sell−/− HSC transduced with empty GFP virus. BM from WT and Sell−/− donors was transduced with empty retrovirus expressing GFP alone and equivalent numbers of cells transplanted into lethally irradiated WT recipients. Ten weeks after transplant, recipient BM was harvested, and the number of engrafting proviral clones determined by Southern blotting of BglII-digested DNA with a GFP probe. Lanes 2 to 4 are recipients of WT BM, whereas lanes 5 to 7 are recipients of Sell−/− BM. The probe also faintly detects a common sequence (*) in mouse genomic DNA. (C) Anti–L-selectin antibody treatment impairs engraftment of BCR-ABL1–transduced progenitors. Southern blot analysis with a GFP probe of genomic DNA of leukemic BM from recipients of BCR-ABL1–transduced WT BM that was untreated (lanes 2-4), isotype control antibody–treated (lanes 5-7), and anti–L-selectin antibody–treated (lanes 8-12). Con1 and Con2 are control DNAs containing 1 and 2 proviral clones, respectively.
L-selectin blockade decreases engraftment of BCR-ABL1+leukemia-initiating cells. (A) L-selectin–deficient leukemic progenitors have a defect in BM homing. Irradiated recipient mice (n = 3) were injected with a mixture of BM cells from mice with BCR-ABL1–induced CML-like disease induced from WT donor cells (expressing GFP) and Sell−/− donor cells (expressing NGFR) and euthanized 2 hours later. (Upper left) Flow cytometric analysis of c-Kit+Lin– normal BM stained with isotype-PE antibody. (Upper right) Input mixture of leukemic WT and Sell−/− progenitors. (Lower) Corresponding c-Kit+Lin– populations isolated from BM of the 3 recipients, stained with PE-conjugated antibody against human NGFR. The percentage of GFP+ and NGFR+ cells is shown adjacent to the respective regions. (B) No defect in engraftment of Sell−/− HSC transduced with empty GFP virus. BM from WT and Sell−/− donors was transduced with empty retrovirus expressing GFP alone and equivalent numbers of cells transplanted into lethally irradiated WT recipients. Ten weeks after transplant, recipient BM was harvested, and the number of engrafting proviral clones determined by Southern blotting of BglII-digested DNA with a GFP probe. Lanes 2 to 4 are recipients of WT BM, whereas lanes 5 to 7 are recipients of Sell−/− BM. The probe also faintly detects a common sequence (*) in mouse genomic DNA. (C) Anti–L-selectin antibody treatment impairs engraftment of BCR-ABL1–transduced progenitors. Southern blot analysis with a GFP probe of genomic DNA of leukemic BM from recipients of BCR-ABL1–transduced WT BM that was untreated (lanes 2-4), isotype control antibody–treated (lanes 5-7), and anti–L-selectin antibody–treated (lanes 8-12). Con1 and Con2 are control DNAs containing 1 and 2 proviral clones, respectively.
Discussion
Our results demonstrate that BCR-ABL1–expressing leukemic stem cells are more dependent on selectins and their ligands for homing and engraftment than normal HSCs. Engraftment of CML-like leukemia was independent of recipient P-selectin or VCAM-1, the latter result anticipated because BCR-ABL1 induces profound defects in β1 integrin function.28 The acceleration of the MPN in Vcam-1 and Selp mutant recipients observed here and by others54 is likely due to increased engraftment of BCR-ABL1+ leukemic stem cells, although we cannot exclude that loss of the negative effect of β1 integrin28 or P-selectin44 binding on HSC proliferation might contribute. The mechanism of increased leukemic engraftment in the absence of VCAM-1 or P-selectin is unknown but might be related to compensatory changes in the level of expression or the spatial distribution of one adhesion molecule when another is missing or to distinct effects of the 2 selectins on HSC proliferation and differentiation.44 By contrast, we found that leukemic stem cells required E-selectin expression in the recipient BM for efficient engraftment. Thus, P- and E-selectin have opposite roles in CML stem cell engraftment, unlike their cooperation in promoting rolling of normal hematopoietic progenitors and mature leukocytes.16 In agreement, when recipient mice with deficiency of both P- and E-selectin were tested, the efficiency of induction of CML-like leukemia was not reduced further relative to E-selectin–deficient recipients (data not shown).
Despite lower levels of L-selectin expression on CML progenitors, this adhesion pathway is nonetheless important for efficient engraftment of these cells following transplantation. Whereas there is no contribution of L-selectin to homing or engraftment of normal HSCs,16,22 this is likely to be highly specific for leukemic stem cells (Figure 7B). It is possible that L-selectin–ligand interactions also contribute to BCR-ABL1 leukemogenesis after engraftment has occurred, through effects on leukemic stem cell survival, expansion, or differentiation. However, the efficiency of induction of CML-like leukemia by L-selectin KO BM was increased significantly after a 24-hour incubation in vitro to allow cell surface expression of the E/L-selectin chimera (data not shown), arguing that L-selectin has a critical engraftment function immediately following injection.
The leukemic stem cell counter-receptors for E-selectin may include PSGL-142 and hematopoietic cell E/L-selectin ligand/CD44.33,39 The intermediate effect of deficiency of FucT-IV and -VII on CML engraftment suggests further that at least some of these counter-receptors may lack the α1,3-fucosylation characteristic of selectin ligands, such as sLex, that predominate on mature leukocytes. The ligand for L-selectin on BM endothelium is unknown. Although L-selectin–mediated rolling of mature leukocytes on inflamed endothelium is dependent on PSGL-1, BM chimera experiments demonstrate that the source of PSGL-1 is leukocytes.45 Leukemogenesis experiments with donor mice lacking combinations of FucT-IV/VII, PSGL-1, CD44, and L-selectin may clarify the identity and inter-relationships of the important selectin ligands on CML stem cells.
Although autologous transplantation in CML has been supplanted by the advent of TKIs, autografting is still a therapeutic option for patients ineligible for allogeneic stem cell transplantation who have advanced TKI-refractory CML or Ph+ B-cell acute lymphoblastic leukemia,55 and blockade of selectin-ligand interactions might be an effective strategy for preventing re-engraftment of malignant Ph+ stem cells. Although anti–E-selectin mAb can partially block rolling of hematopoietic progenitors when injected directly into the BM microvasculature,16 we were unable to inhibit engraftment of BCR-ABL1–expressing stem cells with an anti–E-selectin mAb delivered i.v. (data not shown), possibly due to the high number of antigenic sites in the whole animal. By contrast, we could decrease engraftment of BCR-ABL1+ stem cells with mAb to L-selectin (Figure 7C) or to PSGL-1,33 but neither mAb alone significantly prolonged survival, possibly due to the aggressive nature of the CML-like disease in Balb/c mice.32 Hence, blocking both L-selectin and selectin counter-receptors (CD44 and/or PSGL-1) on leukemic stem cells in the graft, combined with pretreatment of recipients with small molecule selectin inhibitors, currently in clinical trials in sickle cell disease,56 may be an optimal strategy. Further studies will be necessary to move this therapeutic approach into the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Irina Mazo for assistance with genotyping and flow cytometry, Dr John Lowe for FucT mutant mice, Dr Pandelakis Koni for VCAM-1 mutant mice, and Dr John Dick for advice about intrafemoral injections.
This work was supported by National Institutes of Health, National Cancer Institute grants R01 CA90576 (R.A.V.E.), F31 CA136153 (J.B.L.), and K08 CA138916 (to D.S.K.).
Authorship
Contribution: D.S.K. performed the experiments, analyzed the data, and wrote the manuscript; K.L. and J.B.L. assisted with the experiments; and U.H.v.A. and R.A.V.E. supervised the research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.B.L. is Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN.
The current affiliation for R.A.V.E. is Chao Family Comprehensive Cancer Center, University of California at Irvine, Irvine, CA.
Correspondence: Richard A. Van Etten, Division of Hematology/Oncology, and Chao Family Comprehensive Cancer Center, University of California, 839 Medical Sciences Court, Sprague Hall, Rm 124, Irvine, CA 92697; e-mail: vanetten@uci.edu.