In this issue of Blood, Ivanciu et al, Welsh et al, Tomaiuolo et al, and Stalker et al, in a series of studies from the University of Pennsylvania, use intravital microscopy to evaluate how thrombi form following vascular injury and reveal unexpected interactions and physical forces that dictate clot initiation and architecture.1-4
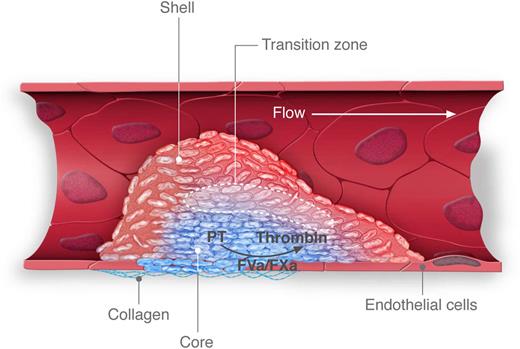
Thrombus formation following penetrating injury. In this depiction of thrombus formation induced by laser or sharpened glass probe, injury results in the exposure of phosphatidylserine on the endothelial cell surface and initiates assembly of the prothrombinase complex. A core of tightly packed platelets (blue) forms at the injury site and restricts the transport of agonists, including thrombin. A shell of loosely adherent, unactivated platelets (red) forms around the core. The core expands over time as agonists diffuse out of the core into the shell, creating a transition zone (dotted lines) in which activation of loosely adherent platelets occurs. Professional illustration by Luk Cox, Somersault1824.
Thrombus formation following penetrating injury. In this depiction of thrombus formation induced by laser or sharpened glass probe, injury results in the exposure of phosphatidylserine on the endothelial cell surface and initiates assembly of the prothrombinase complex. A core of tightly packed platelets (blue) forms at the injury site and restricts the transport of agonists, including thrombin. A shell of loosely adherent, unactivated platelets (red) forms around the core. The core expands over time as agonists diffuse out of the core into the shell, creating a transition zone (dotted lines) in which activation of loosely adherent platelets occurs. Professional illustration by Luk Cox, Somersault1824.
In 1882, Dr Giulio Bizzozero described the use of intravital microscopy to detect the accumulation of platelets following needle-induced injury of the mesenteric artery in rabbits and guinea pigs.5 Bizzozero’s paper was not only the first to define the platelet in name (“Blutplättchen” in German) but was also the first to functionally define platelets as the primary cell type contributing to thrombus formation. Over the past decade, the study of thrombus formation using intravital microscopy has been transformed by the use of high-speed, near-simultaneous acquisition to image multiple fluorescent probes during thrombus formation in vivo.6,7 Real-time imaging of platelets, leukocytes, endothelial cells, tissue factor, fibrin, phosphatidylserine, and plasma proteins in living mice has revolutionized our understanding of how vascular injury rapidly evokes clot formation. These in vivo approaches have served as an important counterpoint to in vitro studies performed on isolated platelets, sometimes confirming in vitro findings, but often not. Studies from the University of Pennsylvania laboratories of Drs Rodney Camire and Skip Brass now provide exceptional examples of how intravital experiments can contradict long-standing dogma derived from ex vivo evaluation of isolated platelets and how a systems approach assessing the clot as a functional unit can reveal information not attainable by studying isolated platelets.
In the first article, Ivanciu et al debunk the long-held view that activated platelets provide the primary surface for assembly of the prothrombinase complex.1 They infused site-specifically labeled factors Va and Xa into hemophilia B mice to evaluate the localization of the prothrombinase complex during thrombus formation. The observation that factor Va corrects the thrombotic defect in the hemophilia B mice proves that it localizes to areas of clotting in this model.8 The authors found that the prothrombinase complex assembled largely on damaged endothelium and not on activated platelets (see figure). Preventing platelet accumulation at the injury site had little effect on prothrombinase formation. Consistent with previous reports, the investigators showed that inhibition of platelet accumulation did not affect fibrin generation following vascular injury.9 To verify endothelial localization, the authors infused labeled factor Xa into mice that express green fluorescent protein (GFP) under the endothelial cell-specific Tie2 promoter. These studies confirmed that factor Xa associates with endothelial cell surfaces. Further studies showed that factor Xa localizes to areas of phosphatidylserine exposure on injured endothelium. This work defines a pathway whereby injury-dependent phosphatidylserine exposure on endothelium leads to FVa and FXa binding with subsequent fibrin generation and shows that, although platelets are able to support fibrin formation in vitro, the endothelium provides the surface for assembly of the prothrombinase complex in this in vivo model.
Beyond showing how thrombi are initiated, new studies from investigators at the University of Pennsylvania reveal how platelet-rich thrombi grow and achieve their characteristic architecture. Previous work from this group demonstrated that platelet-rich thrombi consists of 2 zones: a core of tightly packed, activated platelets and a shell of loosely packed, unactivated platelets.10 In their new study, Welsh et al showed that the transport of diffusible agonists was slower in the core of the thrombus and faster in the shell.2 As an example, they showed that thrombin diffuses very little from the thrombus core, which is juxtaposed to the damaged endothelium. This arrangement sets up a transition zone in which thrombin (and other agonists) at the edge of the core activates resting platelets in the adjacent edge of the shell region (see figure). In this manner, the core grows over time. These studies demonstrate that the degree of platelet activation required to elicit granule release does not occur at the periphery of the outer shell, which is exposed to flowing blood, but rather at its core. An important correlate of this observation is that platelets accumulating in thrombi release their contents not into flowing blood but rather into the body of the thrombus.
What are the consequences of releasing soluble agonists into the thrombus rather than into flowing blood? Tomaiuolo et al use a computational approach to show that the effect is profound.3 They modeled the movement of soluble agonists in a thrombus and showed that convection had very little influence on the movement of agonists released into a thrombus. Instead, the movement of these agonists was controlled by diffusion within the body of the thrombus. Modeling suggested that intrathrombus plasma velocity was ∼1000-fold slower than bulk velocity in the vessel and that this “solute haven” is achieved in surprisingly small thrombi (16 platelets). The rate of diffusion is critically regulated by the packing density of platelets, which can be measured by the size of the gaps between platelets.
If packing density is an important determinant of agonist diffusion in a thrombus, then modulation of packing density should have substantial effects on thrombus growth and architecture. This prediction is supported by computation studies and is validated in a mouse model in which packing density is impaired. Stalker et al studied thrombus formation and solute transport in the diYF mouse4 in which tyrosines in the β tail of αIIbβ3 are mutated to phenylalanine, resulting in a defect in outside-in signaling.11 Although platelets from these mice exhibited normal fibrinogen binding and granule release, they had a defect in clot retraction that led to lower packing densities. This defect increased intrathrombus transport rates and decreased thrombin and platelet activity within the thrombus core because thrombin leached out of thrombi more quickly in diYF mice compared with wild-type controls. These results confirm that gap size, which is increased in diYF mice, is a critical determinant of solute transport and, in turn, agonist distribution and platelet activation within the thrombus.
This series of articles raises important questions for the evaluation of existing antiplatelet agents and the design of novel antithrombotics. Might indications for shell-targeted antiplatelet agents (eg, aspirin, clopidogrel) differ from those for core-targeted agents (eg, hirudin, vorapaxar)? Might reagents targeting platelet packing density rather than platelet activation demonstrate better risk-benefit ratios in clinical use?
In commenting on previous attempts to define blood platelets in his famous 1882 publication, Giulio Bizzozero noted that, “It is astonishing that none of the previous investigators made use of the observation of circulating blood in living animals.”5 One hundred thirty years later, the strategy of looking closely at platelets in vivo and considering how they act as a unit, rather studying them in isolation, continues to provide information beyond that obtained by the evaluation of individual platelets. This approach will serve to improve our understanding of how thrombi form, expand, and ultimately resolve.
Conflict-of-interest disclosure: The author declares no competing financial interests.