Key Points
Fibtem is an early and rapidly available biomarker for predicting progression of moderate to severe postpartum hemorrhage.
Fibtem was predictive of need for blood transfusion and invasive procedures, bleeds >2500 mL, duration of bleed, and time in high dependency.
Abstract
This prospective, observational study investigated the utility of Fibtem A5 and Clauss fibrinogen as predictors of progression of postpartum hemorrhage (PPH). A consecutive cohort of 356 women experiencing 1000 to 1500 mL PPH was recruited. Fibtem and fibrinogen were measured and subsequent transfusions, invasive procedures, and bleed volume recorded. Women progressing to 8 U blood products (red blood cells [RBCs] + fresh frozen plasma [FFP] + platelets) had a median (interquartile range) fibrinogen and Fibtem A5 of 2.1 (1.8-3.4) g/L and 12 (7-17) mm, respectively, compared with 3.9 (3.2-4.5) and 19 (17-23) for those not progressing. On multivariate analysis, Fibtem was an independent predictor for progression to bleeds >2500 mL (95% confidence interval [CI], 0.85 [0.77-0.95]). Receiver operating characteristic area under the curve (95% CI) for progression to RBC transfusion was 0.67 (0.60-0.74) for fibrinogen and 0.61 (0.54-0.68) for Fibtem, and progression to >2500 mL was 0.71 (0.61-0.81) and 0.75 (0.66-0.85) for fibrinogen and Fibtem, respectively. Fibtem A5 <10 mm was associated with more prolonged bleeds (median [95% CI], 127 [44-210] compared with 65 [59-71] minutes; P = .018) and longer stay in the high-dependency unit (23.5 [18.4-28.5] compared with 10.8 [9.7-11.8] hours). Fibtem is a rapidly available early biomarker for progression of PPH.
Introduction
Postpartum hemorrhage (PPH) was the direct cause of death of 130 women in the United Kingdom between 1985 and 20081 and 73 000 women worldwide in 2012.2 The incidence of PPHs >2500 mL is increasing in many countries,3-6 and PPH accounts for 75% of severe childbirth-related morbidity.4 PPH results from a combination of physical causes such as uterine atony or placental adherence or abruption and is often exacerbated by hemostatic impairment. It is recognized that fibrinogen falls to critically low levels earlier than other coagulation factors during PPH and other situations of massive hemorrhage.7,8 The appropriate fibrinogen level during massive hemorrhage is debated, and guidelines offer differing recommendations.9-12 Current evidence derives from trauma-related bleeding, and it may not be appropriate to extrapolate these data to PPH, because the fibrinogen level at term is 4 to 6 g/L compared with 1.5 to 4 g/L in the general population.13,14
Clauss fibrinogen, taken early during PPH, has been suggested as a biomarker to predict progression to transfusion and invasive procedures.15-17 The evidence for this is limited, because the timing of the blood tests in previous studies has varied widely; the clinical status of the women was not fully described, and one study was a post hoc analysis of data collected for other reasons.16 These limitations reduced clinical applicability.
Furthermore, the time to obtain a laboratory fibrinogen (about 60 minutes) is usually too long to inform clinical decisions during acute bleeds, leading to formulaic transfusion policies, endorsed by guidelines,9-12,18 or the use of point-of-care tests (POCTs).19 Fibtem performed on the Rotem machine (Tem International, Munich, Germany) measures fibrin-based clot strength in blood after platelet inhibition. The maximum clot firmness (MCF) measures clot strength and correlates moderately with laboratory fibrinogen during PPH.20 The amplitude after 5 minutes (A5) is related to the MCF but is available sooner. No data are available on whether Fibtem has a similar utility for predicting progression of PPH as Clauss fibrinogen.
In this study, we investigated the utility of Fibtem as a biomarker for progression to severe PPH with the aim of using this information to design a randomized trial to investigate early fibrinogen replacement during PPH.
Methods
Between April 12, 2012, and April 8, 2013, data were collected prospectively from a consecutive single center cohort of women aged >15 years experiencing moderate PPH at the University Hospital of Wales, Cardiff, United Kingdom. Antenatally, women were given an information leaflet and an opportunity to discuss the study. Written, informed consent to collate and analyze data was taken as soon as the woman had recovered from the acute bleed. The study was approved by the South East Wales Research Ethics Committee (11/RPM/5300) and was conducted in accordance with the Declaration of Helsinki.
Entry criteria were either (1) a bleed of 1000 to 1499 mL plus 1 of the following: cesarean section, uterine atony, placental abruption, placenta previa, microvascular oozing or cardiovascular instability (pulse above 100 bpm or systolic blood pressure <100 mm Hg); or (2) any bleed >1500 mL (Figure 1). When women fulfilled the entry criteria, blood samples were taken for full blood count, prothrombin time (PT), activated partial thromboplastin time (aPTT), Clauss fibrinogen, and Fibtem. Blood samples were taken before administration of blood components, fibrinogen concentrate, or tranexamic acid. The treating clinicians did not know the Fibtem results. The treating clinicians were aware of the Clauss fibrinogen result, which became available after about 1 hour. Obstetric management was at the clinicians’ discretion.
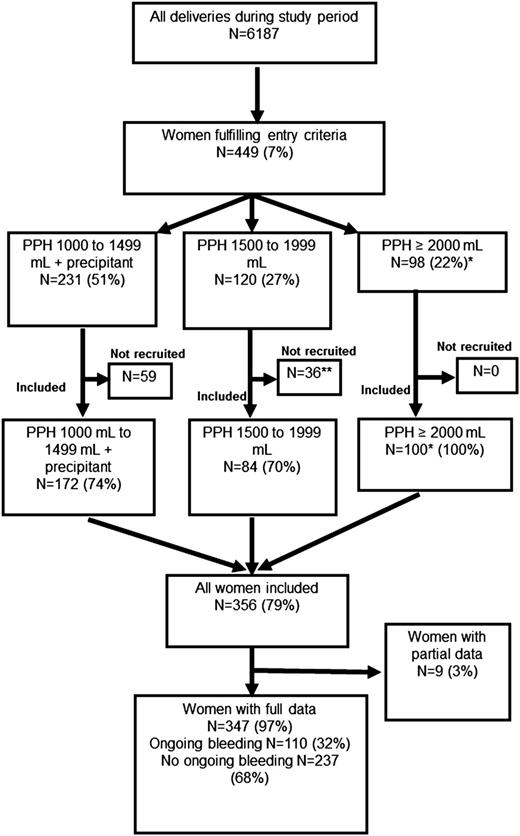
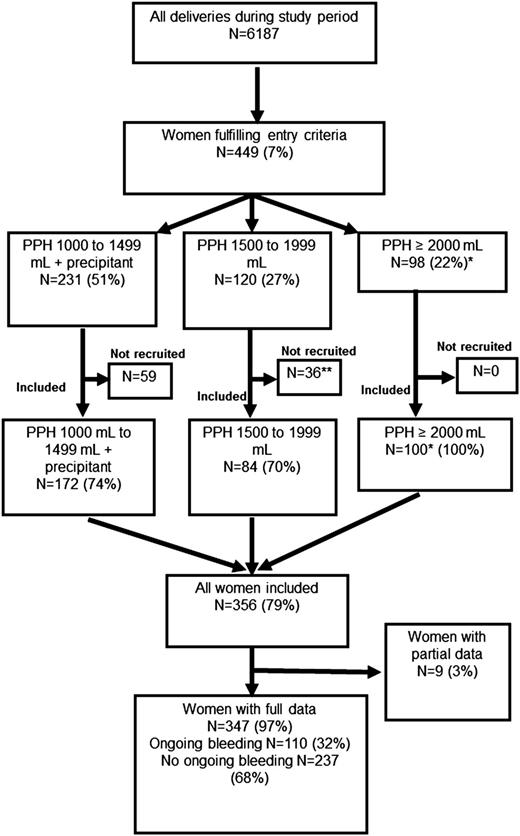
The women recruited into the study are shown compared with the number of women delivered during the study period as reported in the Cardiff Maternity Database. *The number of women experiencing bleeds of ≥2000 mL among all women delivering during the study period, according to data from the Cardiff Maternity Database, was 98. This is fewer than the number of women recorded as experiencing bleeds of this volume enrolled in the study (n = 100). **One woman was recruited but withdrew consent and is not included in the analyses. A precipitant was one of uterine atony, cesarean section, placental abruption, placenta previa, microvascular oozing, or cardiovascular instability (pulse >100 bpm or systolic blood pressure <100 mm Hg).
The women recruited into the study are shown compared with the number of women delivered during the study period as reported in the Cardiff Maternity Database. *The number of women experiencing bleeds of ≥2000 mL among all women delivering during the study period, according to data from the Cardiff Maternity Database, was 98. This is fewer than the number of women recorded as experiencing bleeds of this volume enrolled in the study (n = 100). **One woman was recruited but withdrew consent and is not included in the analyses. A precipitant was one of uterine atony, cesarean section, placental abruption, placenta previa, microvascular oozing, or cardiovascular instability (pulse >100 bpm or systolic blood pressure <100 mm Hg).
Red blood cells (RBCs) were transfused to maintain hemoglobin (Hb) >79 g/L based on POCTs. The time of RBC, fresh frozen plasma (FFP), and cryoprecipitate infusions was recorded along with total measured blood loss. Blood loss was measured prospectively every 15 to 20 minutes using a gravimetric method that involved weighing all blood-soiled material and added to blood in drains. This method was shown to be more accurate than visual estimation of blood loss.21
The prespecified primary outcome was to investigate the utility of Fibtem and/or fibrinogen for predicting progression to PPH >2500 mL or to transfusion of any RBCs by receiver operating characteristic (ROC) curve. Other prespecified outcomes were categorized as follows:
transfusions of <4 or ≥4 U RBCs;
transfusions of <8 or ≥8 U total blood products (RBCs + FFP + platelets);
≤2500 or >2500 mL total blood loss;
an invasive procedure or not (defined as compression sutures, intrauterine balloon, interventional radiology, or hysterectomy);
time of bleed (recognition of abnormal bleeding to hemostasis based on clinical notes); and
length of stay in the high-dependency unit (HDU).
These categories were chosen because they are recognized markers of severity of PPH.4,10,22 Data were collected retrospectively from the routine clinical record.
Anonymized, aggregate data from the Cardiff Maternity Database were used to describe the study population and denominator data. Information on the database is entered by the treating midwife soon after the delivery. Data on study participants including age, weight, height, mode of delivery, primary cause of hemorrhage, use of oxytocics, invasive procedures, fluid resuscitation, and length of stay in the HDU were collected immediately after the PPH and entered into an electronic database. Data collected from the women in the study were checked for quality and accuracy verified. These variables were described for 3 populations: all women who delivered in the study period, women enrolled into the study, and women with ongoing bleeding (defined as >250 mL further bleeding after study entry).
The sample size was based on retrospective data on women with PPH ≥1000 mL showing fibrinogen was predictive of progression (ROC area under the curve [AUC], 0.80; 95% confidence interval [CI], 0.72 to 0.88). Using this diagnostic accuracy as a guide, a sample of 280 women would be required to ensure a standard error of small magnitude of 0.04. It was assumed that around 80% of women would agree to participate and so the study would recruit for 12 months.
Statistical analysis
The cohort and outcomes are reported using descriptive statistics. Means and standard deviations (SDs) were used to summarize normally distributed data (age and body mass index [BMI]). Medians and 25th to 75th centiles were used to summarize skewed data (blood loss at study entry, total bleed volume, PT, and aPTT) and number and percentage used for categorical variables. Associations between explanatory variables (bleed volume at study entry and hematology test results) and progression to transfusion or invasive procedure were analyzed by the Mann-Whitney U statistic and χ2 tests. The utility of fibrinogen and/or Fibtem to predict an outcome was investigated by ROC curves and positive and negative predictive values (PPV and NPV). Time-to-event analysis was determined by Kaplan-Meier plots and the log-rank test.
A multivariate logistic regression model incorporating age, BMI, cause of bleed, Hb on admission, Hb at study entry, PT, aPTT, fibrinogen, and Fibtem A5 was used to investigate whether any of these parameters were independently predictive of study outcomes. PT and aPTT were included as binary variables (defined as within or above the normal range), and bleed precipitants were grouped as a categorical variable (uterine atony, genital tract trauma or surgical, placental abruption, retained or adherent placenta, placenta previa, and no identified cause). Risk factors associated at the univariate level were included in the final multivariate model.
A complete case analysis was performed, and missing data were not imputed. Analyses were performed using IBM SPSS Statistics, version 20 (IBM, Amrock, NY).
Results
Study population
There were 6187 deliveries during the study; 449 (7%) fulfilled the entry criteria, and 356 women (79%) were enrolled (Figure 1). Women who were not enrolled stopped bleeding coincidently with, or very soon after, reaching the entry criteria, and in some of these cases, blood tests were not performed and the women not approached for recruitment. All women with bleeds ≥2000 mL (n = 100) were enrolled. There were 110 women with ongoing bleeding (>250 mL further bleeding after study entry). Full details were unavailable for 9 women; only blood test results and transfusion data had been recorded, leaving 347 women (97%) with full clinical data (Figure 1). Full blood count was available for 349 (97%); PT, aPTT, and fibrinogen for 345 (96%); and Fibtem for 330 (92%).
The characteristics of all women delivered at the center, women enrolled in the study, and women with ongoing bleeding are shown in Table 1. The age and BMI of the women in the 3 groups were comparable, with an average age 29 to 31 years and BMI of 25 to 28 kg/m2. Mode of delivery varied in the 3 groups, with the majority (62%) of all women having a vaginal delivery, whereas in the subgroup of women enrolled in the study, there was a higher proportion of nonelective cesareans (36%). For women enrolled in the study, the most common causes of bleeding were uterine atony (42%) and surgical bleed/genital tract trauma (36%).
The median (25th-75th centiles) blood loss at study entry was 1200 (1000-1500) mL and total blood loss 1500 (1200-2000) mL compared with total blood loss of 350 (200-500) mL for all women delivered during the study period. The incidence of PPH >2500 mL was 37 out of 6187 (6.0/1000). Estimated blood loss and Hb at study entry were similar irrespective of the bleed precipitant and whether women had ongoing bleeding at study entry (see supplemental Table 1, available at the Blood Web site).
All women received a prophylactic uterotonic, 238 women required a second, 130 a third, and 65 a fourth uterotonic. There were 19 invasive procedures in 17 women (4.6%) (3 uterine compression sutures and 16 intrauterine balloons). No woman had interventional radiology or hysterectomy. Tranexamic acid was administered in 61 out of 347 women (18%). No woman received cryoprecipitate or rFVIIa (Novonordisk, Denmark), 8 women received between 2 and 7 g fibrinogen concentrate (CSL Behring, Marburg, Germany), and 292 out of 347 women (84%) were admitted to the HDU (4 were also admitted to intensive care). Transfusions of RBCs and blood components are shown in Table 1.
Hemostatic test results at study entry and associated outcomes.
The lowest fibrinogen and Fibtem levels at study entry were associated with placental abruption, where the median (25th-75th centiles) fibrinogen was 2.2 (1.9-2.9) g/L and Fibtem A5 14 (10-17) mm. Uterine atony, adherent/retained placenta, and surgical/trauma-induced bleeds had higher levels (median 3.9 g/L and 19 mm for fibrinogen and Fibtem A5, respectively; supplemental Table 1). Crystalloid had been infused into 82% (n = 284) and colloid 39% (n = 135) of women before study blood samples were taken (no woman received hydroxyethyl starch). Three women had started the first unit of RBC before the study blood samples were taken.
Blood loss at study entry was not significantly different between women who subsequently required ≥4 U RBCs, FFP, or ≥8 U allogeneic blood products or those who progressed to an invasive procedure but was higher in women who progressed to >2500 mL total blood loss. At study entry, the Hb was lower in women who progressed to more severe outcomes (Table 2). There was no correlation between Hb and estimated blood loss at study entry (r = 0.2) or blood loss and fibrinogen at study entry (r = 0.1).
Fibrinogen was lower in women subsequently transfused with RBCs (median 3.2 g/L) than those who were not (3.9 g/L) (Table 2). The difference between fibrinogen levels was more marked for larger transfusions and larger bleeds. There was a small but statistically significant difference between the Fibtem A5 comparing women who were transfused (18 mm) with those who were not (19 mm) (P < .001). The differences in Fibtem A5 were more marked in women who received ≥4 U RBCs or ≥8 U allogeneic blood products (Table 2). The Fibtem A5 was significantly lower in women who required an invasive procedure or progressed to total blood loss >2500 mL. Small but statistically significant differences in the PT and aPTT were seen for progression to ≥4 U RBCs, any FFP, or ≥8 U allogeneic blood products and total bleed volume >2500 mL, but not for the need for an invasive procedure. Platelet number at study entry was lower in women who required an invasive procedure compared with those who did not but was not different for other outcomes (Table 2).
Lower fibrinogen and Fibtem levels were associated with larger bleeds and higher transfusion rates. Women with fibrinogen ≥4 and 3 to 3.9 g/L and Fibtem ≥23 and 16 to 22 mm had similar outcomes, with low rates of transfusion and few bleeds >2500 mL. Women with a fibrinogen 2 to 2.9 g/L or Fibtem 10 to 15 mm had a higher proportion of bleeds >2500 mL and requirement for transfusion, and this further increased for women with fibrinogen <2 g/L or Fibtem <10 mm (Table 3).
Utility of Clauss fibrinogen and Fibtem for predicting progression from moderate to severe PPH
There were 312 paired Clauss fibrinogen and Fibtem assays at study entry, and these were moderately correlated (r = 0.59). A5 and MCF correlated well (r = 0.94). The Fibtem A5 was available about 10 minutes after venepuncture and at a median (25th-75th centiles) of 19 (13-24) minutes earlier than MCF. Audit at the institution had shown that Clauss fibrinogen was available at a median of 65 minutes after venepuncture.
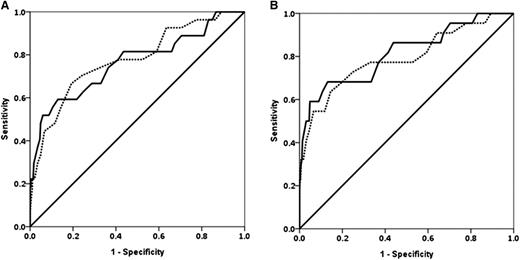
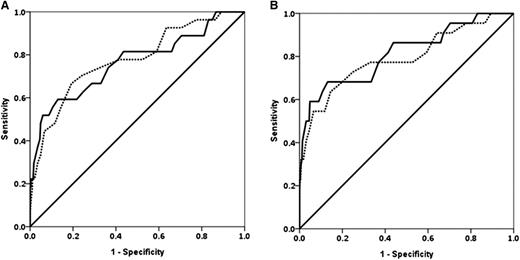
Fibrinogen and Fibtem A5 had similar ROC AUCs for progression to study end points except invasive procedure, where fibrinogen was higher (Table 4 and Figure 2). The prespecified primary outcome measures are shown in Table 4; the ROC (AUC) for progression to any RBC transfusion for fibrinogen was 0.67 (0.60-0.74) and for Fibtem A5 was 0.61 (0.54-0.68). The ROC AUCs for Fibtem A5 and Fibtem MCF were indistinguishable for all outcomes.
ROC curves for fibrinogen and Fibtem A5. (A) ROC curves for fibrinogen (solid line) and Fibtem A5 (dashed line) for progression to ≥4 U RBCs. (B) Progression to ≥8 U allogeneic blood products (RBCs + FFP + platelets).
ROC curves for fibrinogen and Fibtem A5. (A) ROC curves for fibrinogen (solid line) and Fibtem A5 (dashed line) for progression to ≥4 U RBCs. (B) Progression to ≥8 U allogeneic blood products (RBCs + FFP + platelets).
The PPV (95% CI) of fibrinogen <2 g/L or Fibtem A5 <10 mm for progression to any transfusion was 75 (43-94) and 71 (42-91), respectively. For women with ongoing bleeding at study entry, the PPV of a fibrinogen <2 g/L or Fibtem A5 <10 mm for any transfusion was 100 (63-100) and 100 (69-100), respectively. The NPV of fibrinogen >4 g/L or Fibtem A5 >22 mm was 80 (73-87) and 75 (66-82), respectively.
Investigation of predictors for progression of PPH
To investigate independent predictors for progression, a multivariate logistic regression analysis was performed. Age at delivery and BMI were not significantly associated with any outcome. For progression to PPH >2500 mL, several factors were predictive at the univariate level (Table 5); however, in the final multivariate model, only Fibtem A5 was an independent predictor of >2500 mL bleeds (0.85, 0.77-0.95).
Several factors were also predictive of progression to transfusion of any RBCs at the univariate level (supplemental Table 2). In the multivariate model, Hb at study entry, mode of delivery, and primary cause of PPH were predictive of RBC transfusion. Although significant in the univariate analyses, fibrinogen and Fibtem A5 were not independently predictive of progression to any transfusion of RBCs.
For progression to the need for ≥8 U allogeneic blood products, the independent predictors were Fibtem A5 (0.82, 0.70-0.96), Hb at study entry (0.51, 0.32-0.83), and uterine atony (5.93, 1.03-34.13). Fibrinogen was not an independent predictor in this model (1.02, 0.41-2.53); however, if Fibtem A5 was omitted from the model, fibrinogen was an independent predictor. Similar results were found for transfusion of ≥4 U RBC with Fibtem A5 (0.84, 0.74-0.96), Hb at study entry (0.66, 0.44-0.99), and uterine atony (3.70, 1.03-13.37) being independently associated.
Association of Clauss fibrinogen and Fibtem with time to study outcomes
The median (25th-75th centiles) time from recognition of abnormal bleeding to clinically accessed hemostasis was 68 (46-149) minutes. In women transfused, the time from onset of abnormal bleeding to the first unit of RBCs was 225 (80-1327) minutes. In women who received ≥4 U RBCs, transfusion started sooner at 80 (41-180) minutes and the time to the fourth unit was 213 (98-960) minutes. The time to the first unit of FFP was 132 (82-229) minutes.
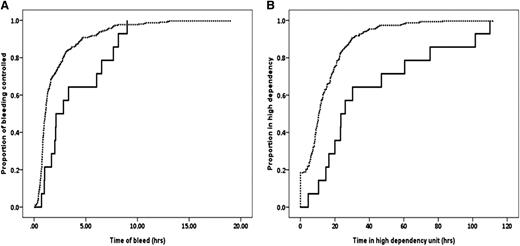
Lower fibrinogen and Fibtem A5 were associated with more prolonged bleeds and longer stays in the HDU. In women with a Fibtem <10 mm, the median (95% CI) time of abnormal bleeding was 127 (44-210) minutes compared with 65 (59-71) minutes in women with A5 ≥10 mm (P < .022) (Figure 3A). For women with a fibrinogen <2 g/L (equivalent to a Fibtem A5 of about 10 mm), the time of abnormal bleeding was 125 (91-304) compared with 66 (60-72) minutes for those with fibrinogen ≥2 g/L (P = .011). The median (95% CI) time in the HDU for women with a Fibtem A5 < or ≥10 mm was 23.5 (18.4-28.5) and 10.8 (9.7-11.8) hours, respectively (P = .001) (Figure 3B), and for women with fibrinogen < or ≥2g/L it was 22.0 (18.6-25.4) and 11.0 (10.0-12.0) hours, respectively (P = .019).
Time of bleeds and stay on HDU. (A) Time from the recognition of abnormal bleeding to hemostatic control. (B) Time spent in high-dependency care for Fibtem A5 <10 mm (solid line) and ≥10 mm (dashed line).
Time of bleeds and stay on HDU. (A) Time from the recognition of abnormal bleeding to hemostatic control. (B) Time spent in high-dependency care for Fibtem A5 <10 mm (solid line) and ≥10 mm (dashed line).
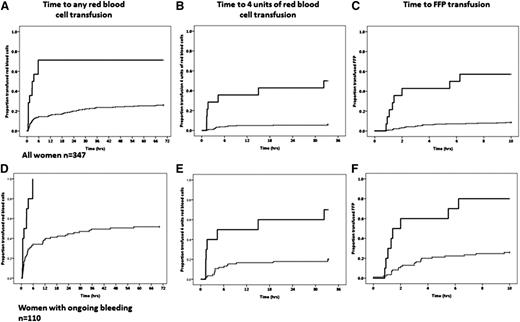
There was a shorter time from the onset of bleeding to first RBC transfusion, 4 U RBCs, and FFP transfusion for women with lower fibrinogen and Fibtem A5 levels (P < .001 for all) (Figure 4A-C). The proportion of women transfused was higher for women with ongoing bleeding, but the time to transfusion was similar (Figure 4D-F).
The time to first unit of RBC, fourth unit of RBC, and first unit of FFP. (A-C) All women in the study. (D-F) Women with ongoing bleeding at study entry. The solid line is women with a Fibtem A5 <10 mm, and the dashed line is Fibtem A5 ≥10 mm.
The time to first unit of RBC, fourth unit of RBC, and first unit of FFP. (A-C) All women in the study. (D-F) Women with ongoing bleeding at study entry. The solid line is women with a Fibtem A5 <10 mm, and the dashed line is Fibtem A5 ≥10 mm.
Discussion
A POCT of fibrin-based clot formation, taken early and at prespecified times during PPH, was a predictive biomarker for progression to severe bleeding and the need for transfusion. Fibtem and fibrinogen had similar ROC AUCs for predicting progression to transfusion of RBCs, at least 4 U RBCs, 8 U allogeneic products, and blood loss >2500 mL. On multivariate analysis, Fibtem, but not fibrinogen, was independently associated with progression to bleeds >2500 mL and transfusion of at least 4 U RBCs and 8 U allogeneic products. Lower fibrinogen and Fibtem levels were associated with more prolonged bleeds, the need for invasive procedures, a longer time in the HDU, and earlier transfusion, especially when Fibtem was <10 mm or fibrinogen <2 g/L.
This was a prospective study in a well-characterized consecutive cohort. All women with bleeds >2000 mL were included. Some smaller bleeds were not included, because women who stopped bleeding at the time of fulfilling the study entry criteria were not all approached. The incidence of bleeds >2500 mL (6/1000) was similar to a recent confidential audit (5.9/1000)4 and the proportion transfused comparable to national reports.6,8,23-26 Women were managed on routine protocols and had received variable amounts of crystalloid and colloid before inclusion; therefore, the results are applicable to many maternity units and different clinical situations. Limitations are that blood loss at study enrolment is difficult to quantify and that, although the Fibtem result was not known by the clinicians, they could react to the fibrinogen result when it became available. This would have affected FFP transfusion, because women with low levels would have been infused, and so it is inevitable that low fibrinogen was predictive of FFP infusion. Knowledge of fibrinogen, however, is very unlikely to have affected RBC transfusion (based on near-patient Hb), the need for invasive procedures, bleed size, time of bleed, or length of HDU stay, supporting the conclusion that low Fibtem was predictive of progression.
A previous study, including 128 women recruited at variable times after the bleed started, reported that fibrinogen predicted progression to severe PPH (defined in part as transfusion of 4 U RBCs)15 with an ROC AUC (0.75) similar to that reported here for 4 U RBCs (0.78). In a retrospective study, 249 women with PPH >1000 mL had fibrinogen taken at the time of clinical concern, and this was predictive of progression to 4 U RBCs (ROC AUC, 0.8 [0.73-0.86]).27 Post hoc analysis of 738 women reported an ROC AUC of 0.66 (0.64-0.68) for progression to RBC transfusion,16 similar to our result (AUC 0.67).
In our study, Fibtem A5, but not fibrinogen, was an independent predictor of progression to larger bleeds (>2500 mL) and ≥4 U RBCs and 8 U allogeneic products. Previous studies had reported fibrinogen as an independent predictor15,16 but had not measured Fibtem. If Fibtem was removed from our model, fibrinogen became an independent predictor, though the −2 log likelihood increased. Despite this, the similar ROC curves demonstrate that fibrinogen and Fibtem have similar utility for predicting progression. On multivariate analysis, fibrinogen and Fibtem were not independently associated with progression to transfusion of RBCs, whereas Hb at study entry, mode of delivery, and cause of bleed were, possibly suggesting that hemostatic impairment is more implicated in progression to larger bleeds. The clinical utility of fibrinogen as a biomarker for progression is limited because of the time taken for a result to become available. Fibtem A5, available within 10 minutes, can alert clinicians to the potential for progression. The time for laboratory results to become available has contributed to the use of empirical, formulaic replacement therapy.9,10,12 This may lead to under- and overtransfusion of FFP, because early PPHs of similar size are associated with widely different fibrinogen/Fibtem levels and transfusion requirements, depending on the precipitant. Avoiding blood products in women bleeding for reasons other than hemostatic impairment will reduce morbidity,28 but delaying blood products in women with hemostatic impairment may worsen bleeding.
In this study, the median fibrinogen of women who received ≥4 U RBCs was 2.6 g/L compared with 3.9 g/L for those who did not. Similar results were found in other studies in which women who progressed had average fibrinogen levels of 3.1, 3.3, and 3.4 g/L compared with 4.4, 4.4, and 4.2 g/L in women who did not progress.15,16,27 These studies of >1400 women have remarkably consistent results demonstrating that progression of PPH is associated with fibrinogen within the nonpregnant normal range29 and that fibrinogen >4 g/L was not associated with progression, irrespective of the cause of the bleed.
Although raised PT and aPTT were associated with progression of PPH, the actual differences were too small to translate into treatment algorithms. Furthermore, in multivariate analysis, they were not independent predictors, possibly because they increase as fibrinogen falls. Hb at study entry was an independent predictor for transfusion of RBCs, although this is almost inevitable because RBCs were transfused at a predetermined Hb. It is not possible to assess the impact of tranexamic within the study cohort. Women who received tranexamic tended to have more severe bleeds. How these bleeds were affected by an antifibrinolytic cannot be measured, although previous studies have reported reduced transfusion.30 The women who received fibrinogen concentrate were all treated as rescue therapy after FFP and obstetric measures had been unsuccessful in controlling bleeding. The effect of the fibrinogen concentrate on the bleeding is not known, although many study end points had occurred before infusion.
Current UK guidelines, based on data derived from trauma, recommend that fibrinogen should be maintained >1 g/L,10 whereas others recommend 1.5 g/L12 or 1.5 to 2 g/L.9 All studies in PPH report that progression is associated with fibrinogen <3 and especially <2 g/L, whereas lack of progression is associated with fibrinogen >4 g/L.15-17,27 The validity of the target fibrinogen in current PPH guidelines has been questioned.22 Whether increasing fibrinogen or correcting Fibtem into the normal range for term, early during PPH, would reduce bleeding is unknown, and studies are required to investigate this. Anecdotal, uncontrolled reports suggest improved hemostasis associated with increasing fibrinogen levels.31-35
It is not known whether a low fibrinogen or fibrin-based clot formation causes progression of PPH or reflects the severity of the bleed. There is biological plausibility to support causality, because these factors are critical for clot formation. Fibtem may also give an assessment of severity of the PPH at study entry. Estimated blood loss is notoriously inaccurate,36 and women recruited to this study may have wider variation in blood losses than recorded. Abnormal fibrin-based clot formation may be both a cause and effect of severe blood loss, and the predictive utility of Fibtem might have a role in directing the intensity of obstetric management.
Based on these results, we are enrolling into a double-blind, multicenter study that randomizes women with moderate PPH (1000-1500 mL) and a reduced Fibtem to fibrinogen concentrate vs placebo (ISRCTN46295339). In another study, women experiencing PPH of 500 to 1000 mL were randomized to 2 g fibrinogen concentrate or placebo (NCT01359878).37 These studies will help to establish whether early fibrinogen reduces bleeding and define an appropriate trigger and target fibrinogen/thromboelastometry levels during PPH.
Fibtem A5 and fibrinogen are useful early biomarkers for predicting progression of PPH. Fibtem, however, is available within 10 minutes and hence may have more clinical utility than fibrinogen. The fibrinogen level associated with progression of PPH is significantly higher than that recommended for intervention in current guidelines. Whether early correction of fibrinogen improves outcomes is being investigated in prospective trials.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the delivery suite at University Hospital of Wales for their many contributions to the study.
Authorship
Contribution: P.C. designed the study, interpreted and analyzed data, and wrote the first draft of the manuscript; G.L., D.B., D.B.-S.L., E.P., V.H., and A.K. collected and interpreted data and reviewed the manuscript; R.C.-J. analyzed and interpreted data and reviewed the manuscript; and J.S., R.A., R.R., S.P., A.W., J.H., and R.C. designed the study, interpreted data, and reviewed the manuscript; and A.R. interpreted data and reviewed the manuscript.
Conflict-of-interest disclosure: The Rotem machine and reagents used in the study were loaned without charge by TEM International. TEM International had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The study received no other source of funding. P.W.C. has received honoraria for talks at symposia funded by CSL Behring and acted as a paid consultant to CSL Behring on the subject of postpartum hemorrhage. R.C. has received honoraria for talks at symposia funded by CSL Behring. Based on the results of this study, a prospective randomized trial (ISRCTN46295339) has been initiated. This investigator-led study is funded by a grant from CSL Behring to Cardiff University with P.W.C. as principle investigator and involving all authors.
Correspondence: Peter Collins, Department of Haematology, University Hospital of Wales, Heath Park Cardiff, United Kingdom CF14 4XN; e-mail: peter.collins@wales.nhs.uk.