Key Points
Alterations in RBC membranes contribute to dysregulated sphingolipid metabolism in sickle cell disease (SCD).
Increased RBC-derived MP production enhances monocyte adhesion and activation in SCD.
Abstract
Sphingolipids are a class of lipids containing a backbone of sphingoid bases that can be produced de novo through the reaction of palmitate and serine and further metabolized through the activity of various enzymes to produce intermediates with diverse roles in cellular processes and signal transduction. One of these intermediates, sphingosine 1-phosphate (S1P), is stored at high concentrations (1 μM) in red blood cells (RBCs) and directs a wide array of cellular processes mediated by 5 known G-protein coupled receptors (S1P1-S1P5). In this study, we show that RBC membrane alterations in sickle cell disease enhance the activation acid sphingomyelinase by 13%, resulting in increased production and storage of sphingosine (2.6-fold) and S1P (3.5-fold). We also show that acid sphingomyelinase enhances RBC-derived microparticle (MP) generation. These MPs are internalized by myeloid cells and promote proinflammatory cytokine secretion and endothelial cell adhesion, suggesting that potential crosstalk between circulating inflammatory cells and MPs may contribute to the inflammation-rooted pathogenesis of the disease. Treatment with amitriptyline reduces MP generation in vitro and in vivo and might be used to mitigate inflammatory processes in sickle cell disease.
Introduction
Sickle cell disease (SCD) is a hereditary blood disorder characterized by mutated hemoglobin molecules, which polymerize during deoxygenation to form fibers that deform the erythrocyte membrane. These red blood cells (RBCs) contribute to significant pathology including stroke, avascular necrosis, and vasculopathy.1 Because the sickling of the RBC membrane is pathognomonic in SCD, it is important to understand how modifications in membrane composition play a role in the disease.
Sphingolipids are a class of lipids containing a backbone of sphingoid bases. They play diverse roles in cellular processes and are significant components of cell membranes.2 Sphingomyelin (SM), which comprises 10% of the mammalian plasma membrane, is degraded by the hydrolyzing enzyme sphingomyelinase (SMase). In recent studies, membrane curvature and associated increases in mechanical bending stresses in RBCs, activated SMase, thereby reducing SM and increasing ceramide.3 Production of ceramide has a direct effect on a wide range of cellular processes and alters production of other immediately descendent metabolites such as sphingosine and sphingosine 1-phosphate (S1P), which are key regulators of inflammation.4-8 Furthermore, SMase has been implicated in lipid microdomain formation, membrane fragility, vesiculation, and MP formation.9,10 Although the orientation of membrane lipids in SCD and other hemolytic anemias has been studied, the consequences of altered distribution and metabolism of sphingolipids have been largely unexplored.
Sickle RBCs are a particularly interesting model system for the relationship between membrane stresses, inflammation, and sphingolipid metabolism. Acid SMase is secreted from endothelial and myeloid cells,11 and erythrocyte clearance and chronic inflammation, both of which occur in SCD and enhance the secretion of acid SMase.9,12-14 The role of acid SMase, and sphingolipid dysregulation more generally, has not been studied in the context of SCD.
In this study, we hypothesized that dynamic shape distortion of RBCs and the accompanying increases in RBC membrane stresses enhance the activity of acid SMase in the blood, plasma, and RBCs of people living with SCD. This results in a significant increase in sphingosine and S1P in RBCs, as well as the generation of RBC-derived MPs (Figure 1 and see supplemental Figure 1 on the Blood Web site). These factors enhance monocyte adhesion to endothelial cells and inflammatory cytokine production. Pharmacologic inhibition of acid SMase with a Food and Drug Administration-approved drug, amitriptyline, reduces MP generation and cytokine production. These results elucidate a novel mechanism for MP-mediated inflammatory cell activation in SCD.15 Additionally, these findings may be relevant to other hemolytic anemias and disorders rooted in aberrant RBC morphology.
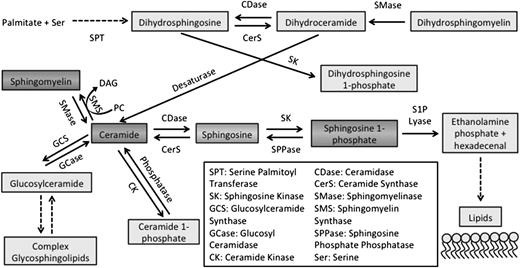
Sphingolipid metabolism. De novo sphingolipid production starts with the reaction of serine and palmitoyl CoA, mediated by serine palmitoyl transferase. Sphingolipids are metabolized in many reversible and irreversible reactions. Ceramide is at the center of the sphingolipid metabolism network and can result from the hydrolysis of SM, which in turn results in the production of sphingosine and S1P downstream.
Sphingolipid metabolism. De novo sphingolipid production starts with the reaction of serine and palmitoyl CoA, mediated by serine palmitoyl transferase. Sphingolipids are metabolized in many reversible and irreversible reactions. Ceramide is at the center of the sphingolipid metabolism network and can result from the hydrolysis of SM, which in turn results in the production of sphingosine and S1P downstream.
Methods
RBC and MP isolation and fractionation
Whole blood samples from donors homozygous for sickle (SS) or normal (AA) hemoglobin were centrifuged against a Ficoll-Paque density gradient (density: 1.077 g/mL; GE Healthcare) for 30 minutes at 400 relative centrifugal force (RCF) at 4°C to separate plasma, peripheral blood mononuclear cells (PBMCs), and packed RBCs (supplemental Figure 2). RBCs were separated into high- and low-density fractions using stacked Percoll solutions centrifuged at 41 000 rpm for 30 minutes using a Beckman SW 41 Ti Rotor.16 RBC-derived MPs were obtained by ultracentrifugation of packed RBCs at 10 000 (P2), 37 000 (P3), or 200 000 (P4) RCF for 1 hour at 4°C.
Flow cytometry for MP quantification
Whole blood, cultured RBCs, or isolated MPs were incubated with antibodies against glycophorin A (catalog #Ab91163, Abcam) and Annexin V (catalog #640906, BioLegend). Events were counted with AccuCheck counting beads (catalog #PCB100, Life Technologies) and analyzed on a BD FACSAria flow cytometer.
Protein and enzyme quantification
Enzyme expression and activation were quantified using the following kits: SMase activity (catalog #10006964, Cayman Chemical Company); Acid SMase expression (catalog #SEB360Hu, USCN Life Science); alkaline ceramidase (ACER1) expression (catalog #CSB-EL001151HU and CUSABIO); sphingosine kinase 1 and 2 (SK1/2) western blot (catalog #1000-6822, Cayman Chemical Company and catalog #AB37977, Abcam); intercellular adhesion molecule (ICAM)-4 antibody (catalog #ABIN901654, Antibodies Online); and total and phospho-extracellular signal-regulated kinase (p-ERK1/2) western blot (catalog #9102 and #9101, Cell Signaling). Western blot measurements were standardized by loading an equal volume of protein. An equal volume of blood was used for activity assays.
Lipid extraction and quantification
Lipids were extracted following a protocol from Shaner et al,17 and analyzed using a Shimadzu LC-10 AD VP binary pump system coupled to a PerkinElmer Series 200 autoinjector coupled to a 4000 quadrupole linear-ion trap (QTRAP) LC-MS/MS system.
Monocyte differentiation and MP internalization
THP-1 monocytes were seeded at 2 × 105 cells/well on poly-d-lysine–coated coverslips and treated with 100 nM phorbol myristate acetate (PMA) for 72 hours. Primary AA PBMCs were seeded at 1 × 106 cells/mL in 12-well plates in media supplemented with 20 ng/mL macrophage colony stimulating factor (Peprotech) for 24 hours. MPs were isolated from packed RBCs and incubated 1:1 with 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Abcam) for 20 minutes at room temperature, followed by ultracentrifugation at 37 000 RCF for 20 minutes at 4°C. The MP pellet was resuspended in phosphate-buffered saline (PBS) and seeded at 1 × 106 particles/well with THP-1 or primary isolated monocytes. After 30 minutes, 2 hours, or 24 hours, conditioned media was collected and the cells were washed three times with PBS before imaging or cell lysis with radioimmunoprecipitation assay buffer. Proteins were assessed for cytokines using a Milliplex MAP Human Cytokine/Chemokine Premixed 42 Plex Assay (catalog #MPXHCYTO60KPMX42, Millipore) on the Luminex platform.
Monocyte adhesion studies
GFP-HUVECs (angioproteomie) were grown to confluence in an 8-chamber slide or a 96-well plate. DRAQ5 (eBioscience) or DiI (Life Technologies) labeled THP-1 monocytes or primary isolated PBMCs were pretreated with either vehicle basal RPMI media, 1 μM S1P, or 10% AA/SS plasma for 1 hour, co-incubated with AA/SS RBCs (1:10 Monocyte:RBC) for 18 hours, or co-incubated with AA/SS MPs at high (50%), medium (20%), or low (10%) concentrations for 18 hours. Cells were washed and allowed to adhere to confluent HUVECs for 4 hours. Nonadherent cells were washed away with PBS three times and cells were stained with DAPI (4,6-diamindino-2-phenylindole), and imaged with fluorescence or near infrared microscopy.
Ex vivo RBC studies
RBCs were isolated and resuspended 1:100 in PBS + 20 mmol/L glucose with 0, 1, 10, or 100 μM amitriptyline and incubated at 37°C for 1, 2, or 24 hours. Cells were resuspended in diH20 for acid SMase activity measurement or PBS for flow cytometric measurement of MPs.
Animals
Wild-type (WT) C57BL/6 mice were obtained from The Jackson Laboratories; heterozygous (AS) and homozygous (SS) mice were initially obtained from the sickle transgenic breeding colony at Georgia Institute of Technology. Townes’ model sickle transgenic mice were AS or SS for the sickle mutation. Saline and 10 mpk amitriptyline (dissolved in sterile saline) injections were performed via intraperitoneal injection and peripheral blood was drawn via retro-orbital bleed. For hypoxia studies, mice were housed in polypropylene hypoxia chamber (Coy Laboratory Products) and kept at 8% oxygen (using pure O2 and N2 as background gas) for 24 hours. All surgical procedures and animal care protocols were approved by the Georgia Institute of Technology’s Animal Care and Use Committee.
Results
Sphingolipid metabolism is dysregulated in SCD
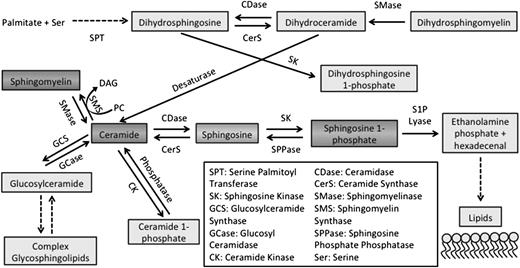
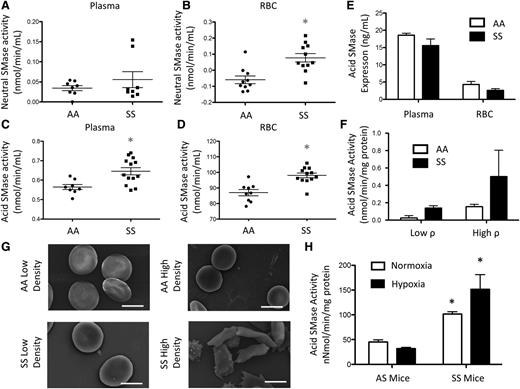
Neutral SMase is known to be activated upon membrane bending.3 Although neutral SMase activity was largely undetectable in plasma and AA RBCs, there was a small but significant (10 pmol/min per mL) increase in neutral SMase activity in SS RBCs but not in plasma (Figure 2A-B). Acid SMase activity in AA plasma was 0.56 nmol/min per mL but was significantly elevated (0.64 nmol/min per mL) in SS plasma (Figure 2C). Acid SMase activity in SS RBCs (98.06 nmol/min per mL) was also significantly elevated in comparison with AA RBCs (87 nmol/min per mL) (Figure 2D). There was a positive correlation (r2 = 0.84) between acid SMase activity in RBCs and plasma suggesting that RBCs may adopt secreted acid SMase from plasma (data not shown). Because both enzyme concentration and activation state can affect overall activity, we quantified acid SMase expression. It was not altered in plasma and RBCs, which suggests that its increased activity in SCD was due to activation (Figure 2E). We sought to determine if sickle RBCs had higher levels of acid SMase activity, so we used Percoll density centrifugation to separate high- (irreversible sickled) and low-density fractions of RBCs.18 Low-density SS RBCs displayed increased acid SMase activity relative to AA RBCs. Irreversible sickled SS RBCs, however, had the highest acid SMase activity (Figure 2F-G). To confirm the role of RBC sickling in acid SMase activation, we induced sickling in SS mice with hypoxic conditioning for 24 hours. Consistent with humans, SS mice displayed a significant increase in acid SMase activity in whole blood at normoxic baseline. Deoxygenation by hypoxic conditioning further enhanced acid SMase activity in SS mice (Figure 2H).
SMase activity in SCD. (A) Neutral SMase activity in plasma and (B) RBCs. (C) Acid SMase activity in plasma and (D) RBCs. (E) Acid SMase expression in plasma and RBCs. (F) Acid SMase activity in high- and low-density RBCs (labeled “high ρ” and “low ρ, ” respectively), and (G) representative SEM images of RBCs. (H) Acid SMase activity in whole blood of AS/SS mice immediately after 8 hours normoxic/hypoxic conditioning. *P < .05 measured with an analysis of variance (ANOVA) relative to AA or AS normoxia.
SMase activity in SCD. (A) Neutral SMase activity in plasma and (B) RBCs. (C) Acid SMase activity in plasma and (D) RBCs. (E) Acid SMase expression in plasma and RBCs. (F) Acid SMase activity in high- and low-density RBCs (labeled “high ρ” and “low ρ, ” respectively), and (G) representative SEM images of RBCs. (H) Acid SMase activity in whole blood of AS/SS mice immediately after 8 hours normoxic/hypoxic conditioning. *P < .05 measured with an analysis of variance (ANOVA) relative to AA or AS normoxia.
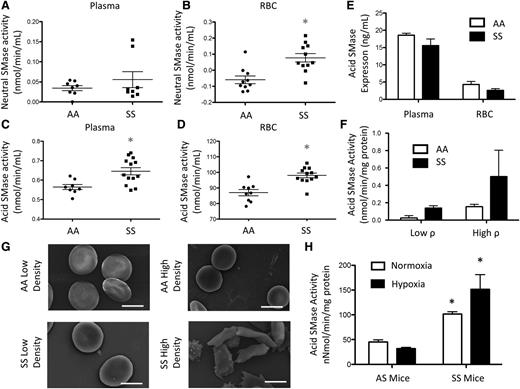
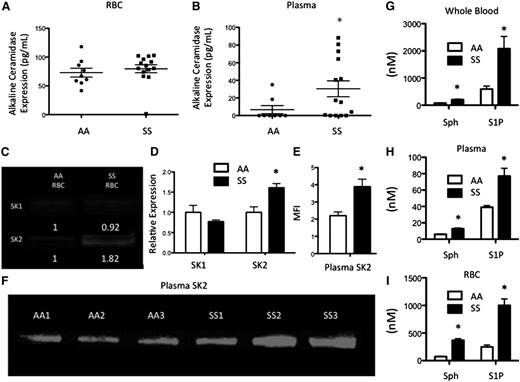
Multiple lipids can be metabolized downstream of SM (Figure 1). ACER1, which converts ceramide to sphingosine19 was measured in RBCs and plasma. ACER1 levels were not altered between AA (73.02 pg/mL) and SS (79.68 pg/mL) RBCs (Figure 3A). ACER1 was present at a concentration of 30.39 pg/mL in SS plasma, but was not detected in AA plasma (Figure 3B). Sphingosine is phosphorylated to form the bioactive signaling lipid, S1P, by SK1/2. Because S1P is stored at high concentrations in RBCs,20 we hypothesized that they would express SK1 and SK2. SK1 was expressed at low levels in both AA and SS RBCs. SK2 was also expressed by both cell types but was significantly elevated in SS RBCs (Figure 3C-D). SK2 levels in plasma were assessed via western blot and were significantly increased in SS relative to AA plasma (Figure 3E-F), suggesting that SK2 may be secreted into circulation in SCD. As these enzymes are upstream of sphingosine and S1P, we hypothesized that these sphingolipids would be elevated in SCD. High performance liquid chromatography mass spectrometry (HPLC-MS) was used to quantify lipids that were extracted from whole blood, plasma, or RBCs. Both sphingosine (202.84 nM vs 77.42 nM) and S1P (2080 nM vs 593.5 nM) were significantly elevated in SS whole blood relative to AA blood (Figure 3G). Sphingosine and S1P levels were much lower in the plasma, but there were still significant increases in sphingosine (12.82 nM vs 5.97 nM) and S1P (77.03 nM vs 35.01 nM) levels in SS plasma relative to AA plasma (Figure 3H). SS RBCs also had significantly more sphingosine (369.71 nM vs 73.41 nM) and S1P (1000.22 nM vs 249.3 nM) compared with AA RBCs (Figure 3I).
Alkaline CDase, SK, Sph, and S1P in SCD. (A) ACER1 expression in RBCs and (B) plasma. (C-D) SK1 and SK2 expression from equal amounts of RBC protein. (E-F) SK2 levels from equal amounts of plasma protein. (G-I) Sphingosine and S1P levels in whole blood (G), plasma (H), and RBCs (I). *P < .05 measured with a Student t test relative to AA.
Alkaline CDase, SK, Sph, and S1P in SCD. (A) ACER1 expression in RBCs and (B) plasma. (C-D) SK1 and SK2 expression from equal amounts of RBC protein. (E-F) SK2 levels from equal amounts of plasma protein. (G-I) Sphingosine and S1P levels in whole blood (G), plasma (H), and RBCs (I). *P < .05 measured with a Student t test relative to AA.
RBC-derived MPs express sphingolipids
We used the Townes’ mouse model of SCD to determine whether SS RBCs produced more MPs. SS mice have both mouse α and β hemoglobin knocked out and human α sickle β globin knocked in. Blood was collected from these mice and stained for CD41+ platelet MPs and glycophorin A+ RBC MPs.21 Although platelet MPs comprised the vast majority of blood-borne MPs, there was a significant increase in RBC-derived MPs in SS mice relative to AS mice (supplemental Figure 3), corroborating the finding that RBC-derived MPs are significantly elevated in SCD.22
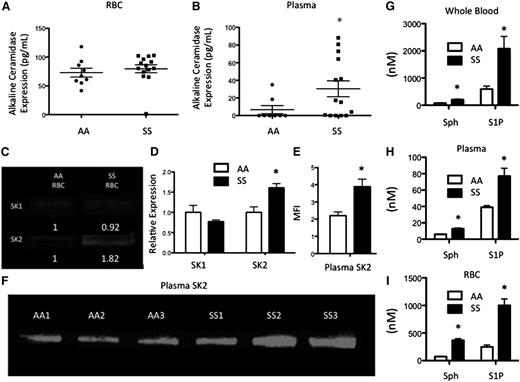
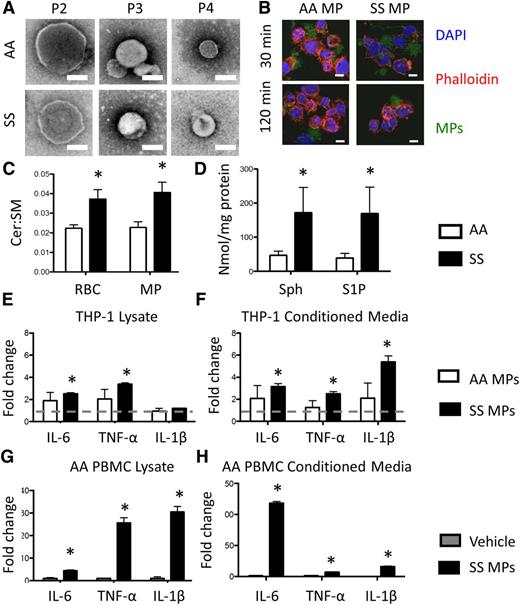
Preliminary image analysis of human RBC-derived MPs revealed a heterogeneous size distribution, so we employed ultracentrifugation at 10 000, 37 000, and 200 000 RCF to isolate P2, P3, and P4 MPs, respectively (Figure 4A). Their size distribution was between 150 and 400 nm (supplemental Figure 4A). Acid SMase activity was largely undetectable in MPs (supplemental Figure 4B), suggesting that the enzyme is not internalized within particles upon generation. The particles themselves, however, are internalized by macrophages (Figure 4B). SM and ceramide were measured in RBCs and MPs. As expected, both RBCs and MPs had an increase in the ceramide:SM ratio, indicating a shift toward ceramide production consistent with SMase activity (Figure 4C). Both AA- and SS-derived MPs expressed sphingosine and S1P, and similar to SS RBCs, SS RBC-derived MPs contained significantly more sphingosine and S1P (Figure 4D).
RBC-derived MPs and monocyte crosstalk. (A) Transmission electron microscopy images of P2 (10 000g), P3 (37 000g), and P4 (200 000g) MPs. Scale bar = 200 nm. (B) Internalization or CFSE-labeled P3 MPs by PMA-treated THP-1s. Scale bar = 10 μm. (C) Ceramide (Cer) and SM quantification with HPLC-MS. (D) Sphingosine and S1P quantification in RBCs and MPs. (E) Fold change over vehicle in cytokine production, and (F) secretion of PMA-treated THP-1s after non-SCD microparticle (AAMP) and SCD microparticle (SSMP) incubation relative to untreated cells (dotted line). (G) Fold change over vehicle in cytokine production, and (H) secretion of primary AA monocytes after SSMP incubation relative to untreated cells. *P < .05 measured in ANOVA relative to vehicle or AA.
RBC-derived MPs and monocyte crosstalk. (A) Transmission electron microscopy images of P2 (10 000g), P3 (37 000g), and P4 (200 000g) MPs. Scale bar = 200 nm. (B) Internalization or CFSE-labeled P3 MPs by PMA-treated THP-1s. Scale bar = 10 μm. (C) Ceramide (Cer) and SM quantification with HPLC-MS. (D) Sphingosine and S1P quantification in RBCs and MPs. (E) Fold change over vehicle in cytokine production, and (F) secretion of PMA-treated THP-1s after non-SCD microparticle (AAMP) and SCD microparticle (SSMP) incubation relative to untreated cells (dotted line). (G) Fold change over vehicle in cytokine production, and (H) secretion of primary AA monocytes after SSMP incubation relative to untreated cells. *P < .05 measured in ANOVA relative to vehicle or AA.
MPs are internalized and modulate cytokine production by monocytes
To determine whether monocytes internalized RBC-derived MPs, PMA-treated THP-1s were incubated with 1 × 106 CFSE-stained MPs for 30 or 120 minutes. Confocal microscopy revealed that MPs were internalized as early as 30 minutes (Figure 4B). Within 30 minutes, 75% of AA MPs and 84% of SS MPs were internalized (supplemental Figure 4C). Surprisingly, after 120 minutes, a smaller proportion of MPs (37% for AA and 40% for SS) was internalized by PMA-treated THP-1s, suggesting that MPs can be internalized and secreted as intact MPs (supplemental Figure 4C). MP internalization increased the production and secretion of many inflammatory cytokines after 24 hours. Interestingly, SS MPs, and not AA MPs, significantly enhanced the production and secretion of IL-6, TNF-α, and IL-1β from macrophages, markers of systemic inflammation during SCD,23 relative to vehicle-treated cells (Figure 4E-F). To interrogate the effects of SS MPs on primary AA PBMCs, we incubated PBMCs with SS MPs. SS MPs significantly enhanced the production and secretion of these cytokines in primary AA monocytes by as much as 120-fold (Figure 4G-H).
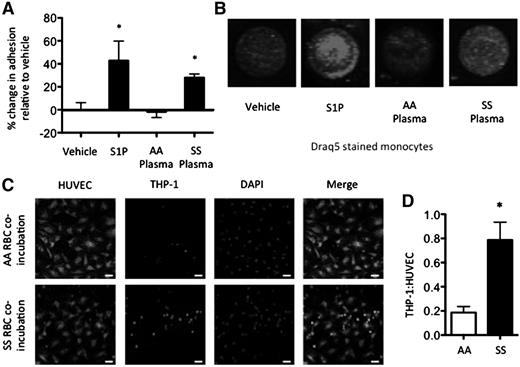
S1P, SS plasma, and SS RBC co-incubation enhances monocyte adhesion to endothelial cells
S1P, an immunomodulatory lipid, signals through the activation of 5 known G-protein coupled receptors (S1P1-5) with diverse biological functions.24 Because SS plasma and MPs express significantly more S1P than AA plasma and MPs, we wished to explore their effects on PBMC adhesion; 1 μM S1P or AA/SS plasma was used to pre-treat primary AA PBMCs for 1 hour before co-incubation with confluent HUVECs for 4 hours. S1P (43%) and SS plasma (28%) but not AA plasma (−1.7%) resulted in an elevation in endothelial adhesion relative to vehicle (Figure 5A-B and supplemental Figure 5).
Elevated monocyte adhesion in SCD. (A-B) Monocyte-adhesion after 1 hour with 1 μM S1P, AA plasma, or SS plasma treatment of AA PBMCs. Representative fluorescent images showing adherent DRAQ5 labeled THP-1 cells after 4 hours of adhesion to HUVECs (B). (C-D) RBCs co-incubated with THP-1 monocytes (10:1) for 18 hours. Representative confocal images (C) and monocyte:HUVEC ratio after 4-hour adhesion (D). *P < .05 measured with 1-way ANOVA or Student t test relative to vehicle or AA.
Elevated monocyte adhesion in SCD. (A-B) Monocyte-adhesion after 1 hour with 1 μM S1P, AA plasma, or SS plasma treatment of AA PBMCs. Representative fluorescent images showing adherent DRAQ5 labeled THP-1 cells after 4 hours of adhesion to HUVECs (B). (C-D) RBCs co-incubated with THP-1 monocytes (10:1) for 18 hours. Representative confocal images (C) and monocyte:HUVEC ratio after 4-hour adhesion (D). *P < .05 measured with 1-way ANOVA or Student t test relative to vehicle or AA.
Because RBCs are known to interact with monocytes in circulation,25,26 we sought to interrogate the potential of SS RBCs to directly alter monocyte-endothelial adhesion. AA or SS RBCs were incubated with THP-1s at a 10:1 ratio for 18 hours. Thereafter, THP-1s were co-incubated with HUVECs and allowed to adhere for 4 hours (Figure 5C). Coculture with SS RBCs significantly enhanced THP-1 adhesion to endothelial cells, as measured by the THP-1:HUVEC ratio (0.78) relative to coculture with AA RBCs (0.19) (Figure 5C-D).
SS RBC-derived MPs enhance PBMC adhesion and express ICAM-4 and elevated p-ERK1/2
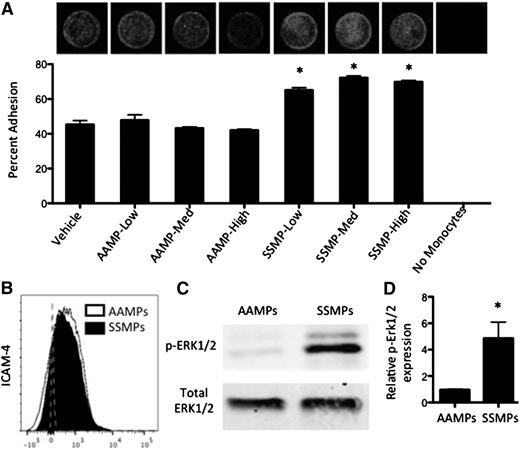
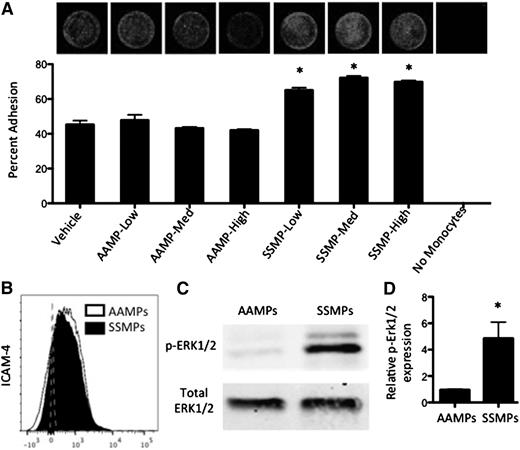
To confirm whether MPs alone could enhance monocyte-endothelial cell adhesion, we incubated primary AA PBMCs with AA- or SS RBC-derived MPs for 24 hours and allowed them to adhere to endothelial cells for 4 hours. Interestingly, AA RBC-derived MP incubation did not enhance adhesion, whereas SS RBC-derived MP incubation resulted in a significant increase in PBMC adhesion at all concentrations (Figure 6A). Zennadi et al established an ERK1/2-mediated mechanism for ICAM-4 activation on SS RBCs, which mediates RBC adhesion to both monocytes and endothelial cells.26,27 Both AA and SS RBC-derived MPs expressed ICAM-4 (Figure 6B). SS RBC-derived MPs, however, expressed significantly activated ERK1/2 as indicated by significantly higher p-ERK1/2 to total ERK1/2 than AA RBC-derived MPs (Figure 6C-D). These results suggest that, similar to SS RBCs, SS RBC-derived MPs may enhance monocyte adhesion in an ERK1/2–ICAM-4 mediated fashion.
SS RBC-derived MPs enhance endothelial adhesion, express LW, and express more p-ERK1/2 than AA RBC-derived MPs. (A) RBC-derived MPs were incubated with primary AA PBMCs at 3 different concentrations (low, medium, and high) for 18 hours before HUVEC adhesion. Representative fluorescent images show adherent DRAQ5-labeled THP-1 cells after 4 hours of adhesion to HUVECs. (B) AAMPs and SSMPs express surface ICAM-4 (LW). (C-D) p-ERK1/2 is significantly elevated in SSMPs relative to AAMPs (n = 6). P < .05 measured in ANOVA relative to vehicle or AAMPs.
SS RBC-derived MPs enhance endothelial adhesion, express LW, and express more p-ERK1/2 than AA RBC-derived MPs. (A) RBC-derived MPs were incubated with primary AA PBMCs at 3 different concentrations (low, medium, and high) for 18 hours before HUVEC adhesion. Representative fluorescent images show adherent DRAQ5-labeled THP-1 cells after 4 hours of adhesion to HUVECs. (B) AAMPs and SSMPs express surface ICAM-4 (LW). (C-D) p-ERK1/2 is significantly elevated in SSMPs relative to AAMPs (n = 6). P < .05 measured in ANOVA relative to vehicle or AAMPs.
Acid SMase inhibition reduces MP generation and proinflammatory cytokine production
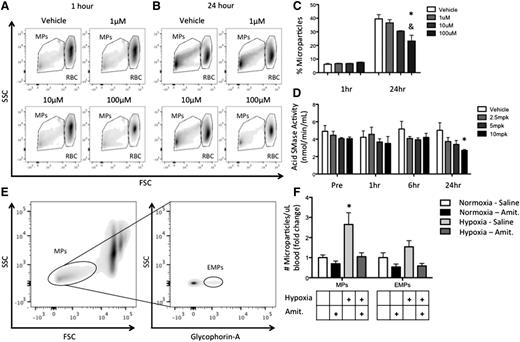
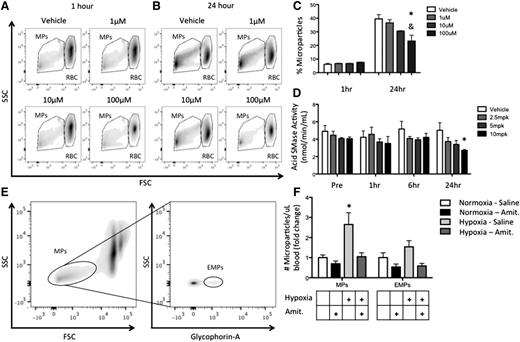
As spleen dysfunction and reduced MP clearance are characteristic of SCD, we sought to establish a unique role for SMase in MP generation. SS RBCs were cultured in media with amitriptyline, a tricyclic antidepressant and acid SMase inhibitor for 1 or 24 hours, and MPs were quantified by flow cytometry. Although 1-hour amitriptyline treatment did not alter the production of MPs at any dose, by 24 hours, amitriptyline had significantly reduced the generation of RBC-derived MPs in vitro in a dose-dependent fashion (Figure 7A-C).
Amitriptyline reduces MP generation. (A-B) Human SS RBCs were incubated in PBS with or without amitriptyline for 1 or 24 hours. Flow cytometry plots of MPs and RBCs 1 hour (A) and 24 hours (B) after amitriptyline treatment. (C) Quantification of MP percentage after treatment. (D) Acid SMase activity in mice after amitriptyline injection. (E) Gating strategy for identifying MPs and EMPs in vivo in WT mice. (F) Quantification of MP production in WT mice after normoxic/hypoxic conditioning with or without amitriptyline. *P < .05 compared with vehicle, and P < .05 compared with 1 μM.
Amitriptyline reduces MP generation. (A-B) Human SS RBCs were incubated in PBS with or without amitriptyline for 1 or 24 hours. Flow cytometry plots of MPs and RBCs 1 hour (A) and 24 hours (B) after amitriptyline treatment. (C) Quantification of MP percentage after treatment. (D) Acid SMase activity in mice after amitriptyline injection. (E) Gating strategy for identifying MPs and EMPs in vivo in WT mice. (F) Quantification of MP production in WT mice after normoxic/hypoxic conditioning with or without amitriptyline. *P < .05 compared with vehicle, and P < .05 compared with 1 μM.
We next injected amitriptyline at 4 concentrations into WT C57Bl/6 mice and found that acid SMase activity was significantly reduced after 24 hours of the highest dose (Figure 7D). Hypoxia has been shown to enhance MP generation in vivo.28 After 24 hours of hypoxic conditioning, MPs in blood and erythrocyte-derived MPs (EMPs) in blood were significantly elevated relative to prehypoxic conditioning. Amitriptyline reduced hypoxia-induced MP and EMP generation is shown in Figure 7E-F. Additionally, 2 of the 3 cytokines we showed to be modulated by MPs in monocytes in vitro (IL-6 and IL-1β) were reduced in amitriptyline-treated mice relative to saline-treated mice during normoxia (supplemental Figure 6). These results elucidate a role for acid SMase in RBC-derived MP generation and systemic inflammation in vivo, and establishes pharmacologic modulation of acid SMase as a strategy to modulate these phenomena in SCD.
Discussion
SCD is a complex disease with severe pathophysiological effects. In recent years, it has been discovered that MPs, which are elevated in SCD, contribute to SCD pathogenesis.22,29-31 In this study, we examined the dysregulation of sphingolipid metabolism, MP production, and inflammatory cell activation in SCD. López et al found that stresses in RBC membranes transiently enhanced the activation of neutral SMase.3 Similarly, Urbina et al found that SMase activity in unilamellar vesicles in Clostridium perfringens α-toxin increased with membrane curvature.32 Our study shows that SS RBCs, which may undergo membrane stresses due to sickling events, oxidative damage, ion fluxes, and enhanced adhesion, have a significant increase in acid SMase activity relative to AA RBCs (Figure 2D). Irreversibly sickled RBCs had the highest acid SMase activity, corroborating these findings (Figure 2F-G). Furthermore, when RBC sickling was induced in SS mice by hypoxic deoxygenation, there was an increase in acid SMase activity confirming that sickling is upstream of acid SMase (Figure 2H). More studies need to be performed to elucidate the mechanism underlying the sensing of local forces and the subsequent activation of SMase in cell membranes.
SMase activity has been implicated in vascular inflammation.33,34 Becker et al discovered that ceramide production in the lungs contributed to inflammatory cytokine production during cystic fibrosis,35 and amitriptyline reduced ceramide production, inflammatory cytokine production, and inflammatory cell recruitment in a mouse model of cystic fibrosis.35,36 Other studies have shown that SMase-mediated ceramide synthesis in plasma membranes promotes microvessel formation.9,37 We found that the ceramide:SM ratio in SS RBCs and MPs was increased, which corroborates the finding that acid SMase is activated in SS RBCs (Figure 4C).
MPs have been shown to enhance atherosclerosis,31 coagulation,30 vaso-occlusion in the kidneys,38 inflammation,39,40 and monocyte adhesion.41 RBC-derived MPs from AA and SS donors were both internalized by THP-1s (Figure 4B). The internalization, processing, and secretion of RBC-derived particles deserve significant attention, and future studies are needed to completely understand these mechanisms. Interestingly, SS MPs enhanced the production and secretion of 3 cytokines consistently associated with inflammation in SCD (ie, TNF-α, IL-1β, and IL-623 ) to a much larger extent than AA MPs (Figure 4E-H). Additionally, we show that SS MPs, and not AA MPs, enhance monocyte adhesion to endothelial cells (Figure 6A), and similarly, co-incubation of monocytes with SS RBCs enhances monocyte-endothelial adhesion relative to AA RBCs (Figure 5C). Zennadi et al established an ERK1/2-mediated mechanism for ICAM-4 activation on SS RBCs, which mediates monocyte adhesion and activation26,27 ; therefore, we wished to determine whether RBC-derived MPs expressed both ICAM-4 and p-ERK1/2. Interestingly, while both AA and SS RBC-derived MPs expressed roughly the same level of ICAM-4, SS RBC-derived MPs expressed significantly more activated ERK1/2. Taken together, these results suggest that, similar to SS RBCs, SS MPs may enhance monocyte adhesion in an ERK1/2–ICAM-4 mediated fashion. Additional studies are needed to elucidate this mechanism, and lipidomic and proteomic profiling of RBC-derived MPs might provide critical insights into the molecules contributing to these phenotype-specific alterations in inflammatory cell adhesion and cytokine secretion.
Other studies have described inflammatory cell activation in SCD,15 and recently, a group discovered that S1P was elevated in SCD and promoted sickling.42 Because both SS plasma, RBCs, and MPs contain significantly more S1P than AA plasma, RBCs, and MPs (Figure 3H-I and Figure 4D), we wished to explore the direct effects of S1P on monocyte adhesion. S1P can be proinflammatory by activating S1P receptors on monocytes,43 inducing E-selectin expression and the adhesion of monocytes to endothelial cells,44 and cathepsins-mediated endothelial cell activation.45 We recently showed that S1P receptor 3 activation enhances the recruitment of anti-inflammatory monocytes to inflamed tissues.7 In this study, we found that both SS plasma and S1P directly enhanced monocyte-endothelial cell adhesion (Figure 5A-B). Although S1P has been shown to alter monocyte morphology and motility through the activation of ρ-kinase46 and receptor tyrosine kinases,47 more comprehensive studies need to be performed to elucidate the receptor-specific signaling mechanism behind S1P-mediated monocyte adhesion.
It is important to note that SCD is characterized by significant hyposplenism. As the spleen is responsible for the removal of cellular debris, the increase in blood MPs observed in SCD is also a result of impaired spleen function.48 We wished to establish a nonredundant role for acid SMase in RBC-derived MP generation, therefore, we interrogated MP generation from SS RBCs in vitro, in the absence of spleen clearance. Amitriptyline and other tricyclic antidepressants have been shown to reduce acid SMase activity and exosome formation.35,36,49 Amitriptyline significantly reduced MP generation from SS RBCs in vitro (Figure 7A-C). Hypoxia has been shown to enhance MP generation28 and we confirmed that in nonsickle mice with normal functioning spleens, MP production was significantly elevated after 24 hours hypoxia. Amitriptyline significantly reduced MP generation in normoxic and hypoxic conditioned WT mice, suggesting a role for acid SMase in MP generation independent of dysfunctional spleen clearance (Figure 7F). Because amitriptyline is not specific to acid SMase and has other targets (ie, NO and PGE2), these studies should be repeated with a putative acid SMase inhibitor or with genetic knockdown. Additionally, these changes occurred in concert with proinflammatory cytokine reduction in vivo (supplemental Figure 6). However, additional studies will be required to demonstrate whether more selective pharmacologic inhibition of SMase is effective in reducing the generation of RBC MPs in SCD. Since amitriptyline acts indirectly on acid SMase by disrupting the electrostatic attraction that attaches SMase to the inner lysosomal membrane, the inhibitory effects of the drug are not specific to acid SMase. Indeed, the drug may act on acid lipase, phospholipase A and C, and other sphingolipid hydrolases by a similar mechanism. Furthermore, amitriptyline, as a tricyclic antidepressant, inhibits the uptake of adrenaline, dopamine, and serotonin, and affects the cholinergic and histaminergic systems.36 The role of systemic hypoxia and local hypoxia in sickle cells due to transient vaso-occlusions in acid SMase activation, cannot be ruled out by these studies and needs to be explored further.
There are multiple lipids metabolically downstream of SM that have diverse biological effects. Erythrocytes convert ceramide into sphingosine with ACER1.19 To date, this enzyme has not been detected in plasma; however, we detected ACER1 in SS plasma (Figure 3B), suggesting that it can be released into circulation through secretion or hemolysis. Ceramide synthesis in erythrocyte membranes is associated with phosphatidylserine exposure and macrophage clearance.50 Additionally, the chronic stress induced by sickled RBCs in circulation during SCD results in an increase in the apoptosis of white blood cells.51 Weigert et al found that cellular apoptosis leads to the activation and secretion of SK2.52 Consistent with these findings, we found that SK2 is increased in both the plasma and RBCs of SCD donors (Figure 3C-F). Although more studies are needed to fully understand the source and mechanism of ACER1 and SK2 secretion into plasma, it is apparent that complex mechanical and biological cues in SCD result in dysregulated sphingolipid enzyme expression and activation, causing a significant increase in sphingosine and S1P (Figure 3G-I).
Our findings reveal, for the first time, that sphingolipid metabolism is dysregulated in SCD. Because this dysregulation is a result of altered enzyme activation and expression in damaged cells, these results may not be specific only to SCD, where the damage is due to membrane deformation, but may also be applicable to other RBC disorders. Additionally, they elucidate a novel mechanism for MP generation in SS RBCs. Membrane stresses imposed on RBCs in SCD activate acid SMase, which, in concert with other sphingolipid enzymes, results in elevated S1P and MP production (supplemental Figure 1). SS RBC-derived MPs, containing S1P, activate monocytes by enhancing endothelial cell adhesion and proinflammatory cytokine production. These findings elucidate potential new strategies to regulate inflammatory processes in SCD through modulating sphingolipid metabolism. Modulating sphingolipid metabolism may be a novel way to pharmacologically control systemic inflammation present in many human diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jingjing Duan, Dr Al Merrill, and Yugesh Kharel for assistance with lipid extraction and HPLC-MS. The authors also thank the Sickle Cell Foundation of Georgia for collecting and recruiting blood donors for these studies.
This study was supported in part by grants from the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R01DE019935-01), National Institute of Dental and Craniofacial Research (1R01AR056445-01A2), National Institute of General Medical Sciences (R01GM067958), and Office of the Director (1DP2OD007433-01), and the National Science Foundation (0933643).
Authorship
Contribution: A.O.A. and E.A.B. conceived of and designed the study, and wrote the manuscript; P.M.K. and M.O.P. helped with experimental design and data interpretation; A.O.A. and P.M.K. isolated RBCs and plasma from donors; A.O.A., A.R.L., and Y.Z. performed in vitro adhesion and enzyme assays; and A.O.A. and K.R.L. performed lipidomic analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward A. Botchwey, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, 315 Ferst Dr. NW, Atlanta, GA 30332; e-mail: edward.botchwey@bme.gatech.edu.