Key Points
Optimization of IUHCT in a preclinical canine model yields stable long-term donor engraftment.
Clinically significant levels of chimerism can be achieved without conditioning, immunosuppression, or graft-versus-host disease.
Abstract
Evidence supporting the efficacy of in utero hematopoietic cell transplantation (IUHCT) in a valid large animal model is needed prior to clinical application. The objective of this study was to establish clinically relevant levels of hematopoietic chimerism in a canine model of maternal-to-fetal IUHCT. We first assessed immune and hematopoietic ontogeny relevant to IUHCT in the canine model and identified 40 days’ gestation (term 63 days) as a time point at the initiation of thymic selection, and prior to bone marrow hematopoiesis, that might be optimal for IUHCT. We next determined that intravascular administration of donor cells via intracardiac injection was far more efficient and resulted in much higher levels of donor cell engraftment than intraperitoneal injection. By applying these findings, we achieved stable long-term multilineage engraftment in 21 of 24 surviving recipients with an average level of initial chimerism of 11.7% (range 3% to 39%) without conditioning or evidence of graft-versus-host disease. Donor cell chimerism remained stable for up to 2 years and was associated with donor-specific tolerance for renal transplantation. The levels of donor cell chimerism achieved in this study would be therapeutic for many hematopoietic disorders and are supportive of a clinical trial of IUHCT.
Introduction
In utero hematopoietic cell transplantation (IUHCT) is a nonmyeloablative approach capable of achieving allogeneic mixed hematopoietic chimerism and associated donor-specific tolerance (DST) without conditioning under specific experimental circumstances.1 The clinical promise of IUHCT has not yet been realized because of significant barriers to donor cell engraftment within the fetal environment.2 In the murine model, the primary barriers include a formidable competitive barrier from the healthy, nonmyeloablated host hematopoietic compartment and the maternal immune response.3,4 Using an intravascular injection technique and maternal fostering, we can now routinely achieve allogeneic mixed hematopoietic chimerism in the mouse at levels of engraftment uniformly associated with DST.5-7 However, prior to clinical application, this success must be replicated in a valid, preclinical large animal model.
Our initial experience with IUHCT in the canine used the canine leukocyte adhesion deficiency model,8 analogous to human leukocyte adhesion deficiency-1.9 In that study, we demonstrated that intraperitoneal (IP) paternal or maternal-to-fetal IUHCT resulted in stable, low-level (0.5% to 1.7%) donor chimerism without evidence of graft-versus-host disease (GVHD). Furthermore, postnatal enhancement of chimerism in 2 of 5 carriers after same-donor hematopoietic stem cell (HSC) transplantation (HSCT) with minimal conditioning provided evidence of DST. Although promising, higher initial chimerism levels are necessary to ensure uniform DST and justify a clinical trial of IUHCT. In this study, we optimized the (1) timing of IUHCT by assessment of the immune and hematopoietic ontogeny of the canine fetus in the context of IUHCT and (2) the mode of injection by tracking labeled donor cells at short time intervals after either IP or intracardiac (IC) administration. We now report the consistent achievement of stable engraftment in the canine model with levels of hematopoietic chimerism that are associated with DST, and potentially therapeutic for many hematopoietic disorders.
Materials and methods
Animals
Time-dated pregnant female beagles were purchased from Covance Research Products Inc. (Cumberland, VA), which maintains a closed colony with an inbreeding coefficient of <0.3%. Pregnancy was confirmed by transabdominal ultrasound (Acuson Sequoia; Siemens, Malvern, PA) upon arrival. Animals were housed in the Laboratory Animal Facility of the Abramson Research Center at the Children’s Hospital of Philadelphia, which is approved by the Association for Assessment and Accreditation of Laboratory Animal Care. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Children’s Hospital of Philadelphia and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Ontogeny analysis
Fetal tissues were harvested at gestational age (GA) 33-46 days, and analyzed for CD45 expression (liver, bone marrow [BM]) or CD4/CD8 double positivity (thymus). In separate incubations, 1 × 106 BM mononuclear cells (MNCs) were stained with phycoerythrin (PE)–conjugated anti-canine CD45 (YKIX16.13) (AbD Serotec, Raleigh, NC) or fluorescein isothiocyanate (FITC)–conjugated anti-canine CD4 (YKIX302.9)/PE-conjugated anti-canine CD8 (YCATE55.9) (AbD Serotec). Following a 30-minute incubation, cells were stained with propidium iodide to confirm viability and analyzed by flow cytometry on a FACSCalibur (BD Pharmingen, Franklin Lakes, NJ).
BM harvest and HSC enrichment
BM was harvested under sterile conditions from the pregnant mother under general anesthesia (supplemental Methods; see the Blood Web site). MNCs were isolated using a Nycoprep 1.077A (Accurate Chemical, Westbury, NY) density gradient. CD34 selection was carried out using monoclonal anti-canine CD34 1H6 (AbD Serotec), and CD3 depletion used monoclonal anti-canine CD3 CA17.2A12 (AbD Serotec). Cells were then incubated with rat anti-mouse IgG1 microbeads and sorted via AutoMACS (Miltenyi, Auburn, CA). CD26 inhibition was performed as previously described8 (supplemental Methods).
IUHCT
Enriched and depleted fractions were each analyzed for CD3 and CD34 content by flow cytometry, using anti-canine CD34PE (1H6) (BD Pharmingen, San Diego, CA) or anti-canine CD34FITC (AbD Serotec), and anti-canine CD3FITC (AbD Serotec). For CD34 enrichment, a portion of the CD34− population was added back to achieve a final CD3+ concentration of ∼1%. For CD3-depleted cells, CD3 content was confirmed to be ≤1% and injected without addback. For injection, cells were resuspended in normal saline with 1% heat-inactivated donor serum, 0.03% DNase, and 0.1% heparin. Injections were performed via laparotomy under ultrasound guidance (see supplemental Video), and fetal viability was confirmed.
Tracking studies
Maternal BM-MNCs were stained with PKH-67 (Sigma-Aldrich, St. Louis, MO). Following injection, litters were analyzed at 1, 24, and 48 hours postinjection. Tissues were examined grossly and under fluorescence stereomicroscopy (Leica Microsystems MZ16FA; Leica Microsystems, Buffalo Grove, IL). Liver and thymus were isolated; BM was collected by crushing the entire skeleton and passing the digest through a 70-µM filter. Liver and BM cells were stained with anti-canine CD45PE (AbD Serotec), whereas thymus was stained with anti-canine CD4FITC/CD8PE (AbD Serotec).
Chimerism analysis
Chimerism was assessed using the variable number of tandem repeats (VNTR) assay, which identifies microsatellite repeats with a sensitivity of ∼2%, as previously described8 (supplemental Methods). Because of overlap between donor peak and stutter patterns, no more than 2 primers were informative for any pairing; in most cases, only 1 could be used. This assay was validated against both quantitative Taqman polymerase chain reaction for the SRY gene and CD18 fluorescence-activated cell sorter (FACS), using long-term chimeras from our previous study.8 VNTR results correlated well with both techniques (supplemental Figure 1).
For multilineage chimerism analysis, after CD45 gating (FITC; BD Pharmingen; PE, AbD Serotec), peripheral blood (PB) MNCs were sorted using a FACSAria (BD Pharmingen) to isolate CD3FITC (AbD Serotec), CD21PE (AbD Serotec), CD11bPE-Cy5 (Abcam, Cambridge, MA), and CD11cFITC (Abcam) positive cells. DNA isolation and VNTR were performed on each sorted population (supplemental Figure 2).
Immunohistochemistry
Tissue specimens were fixed in 10% buffered formalin and embedded in paraffin using a Sakura Tissue-Tek embedder (Sakura Finetek USA, Torrance, CA). Using a paraffin microtome (Leica RM2035; Instrument GmbH, Germany), 4-µm sections were obtained. Paraffin sections were incubated overnight at 55°C and deparaffinized in serial xylene washes, followed by rehydration through a graded alcohol series to deionized water. Slides were blocked for specific protein for 30 minutes at room temperature (RT). Primary antibody was applied (mouse anti-canine CD45 [AbD Serotec], goat anti-canine CD4 [R&D Systems, Minneapolis, MN], or mouse anti-canine CD8 [Lifespan, Seattle, WA]) and incubated overnight at 4°C. The slides were washed with phosphate-buffered saline (PBS) at RT for 10 minutes, and species-specific secondary antibodies were applied (anti-mouse IgG, anti-goat IgG; Vector Laboratory, Burlingame, CA). Following RT incubation for 30 minutes, slides were rinsed in PBS for 10 minutes, and avidin-biotin complex (1:200 dilution; Vector Laboratory) was added for 30 minutes. Following a PBS rinse, the slides were developed with peroxidase substrate kit SK-4100 (Vector Laboratory), lightly stained with Harris hematoxylin, dehydrated in alcohol, cleared in xylene, and mounted using Acrymount (Statlab Medical Products, Lewisville, TX). Slides were imaged using a Leica DMR Microscope (Leica Microsystems, Wentzler, Germany).
Renal transplantation
Following left nephrectomy, the maternal donor organ was immediately flushed with ice-cold University of Wisconsin solution. Renal arterial anastomosis was performed to recipient aorta, while the renal vein was joined to recipient inferior vena cava in end-to-side fashion. The donor ureter was anastomosed to recipient bladder by creating a tunneled neoureterovesicostomy. Postoperatively, transabdominal Doppler ultrasound confirmed perfusion to the kidney. Donor allografts were biopsied 60 days after transplantation, and transplant nephrectomy was performed at 6 months for immunohistochemical analysis. Tissues were stained with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), trichrome, anti-canine CD3 (CA17.2A12; AbD Serotec), and anti-human c4d (BI-RC4D; Biomedica). Coded samples were examined by a blinded pathologist and graded according to a standardized (Banff) grading system.
Statistical analysis
Statistical analysis was performed via logistic regression using exponential curve fit. Statistical significance was accepted at P < .05, and data were analyzed using SPSS v.19 (IBM, Armonk, NY).
Results
Canine ontogeny
We undertook a focused analysis of immunologic and hematopoietic events relevant to engraftment and tolerance after IUHCT. From an immunologic perspective, initiation of thymic processing of self-antigen in the form of positive and negative selection coincides with the appearance and expansion of double-positive (DP; CD4+/CD8+) lymphocytes in the fetal thymus. Previous work suggests that the dog thymic anlage appears during days 27-28 of the 63-day gestation, lymphocytes occupy the thymus between GA35 and GA40, and a postnatal histologic appearance develops by GA45.10 Results in our model confirm these findings, with the thymus appearing relatively devoid of lymphocytes until around GA37. Significant lymphocytic expansion was observed after GA39, consisting primarily of DP lymphocytes. Subsequently, the thymus rapidly developed a normal degree of cellularity taking on the histologic appearance of normal postnatal thymus by GA46 (Figure 1A-F). Logistic regression confirmed a highly significant exponential curve fit (R2 = 0.928, P = .008), with profound expansion of DP lymphocytes beginning between GA39 and GA42 (Figure 1G).
Development of immune profile in fetal thymus by immunohistochemistry. (A,C,E; ×20, H&E) Shows the evolution in tissue architecture, as well as the appearance and expansion of CD4/8 DP cells (B,D,F; ×20, CD4+ [blue], CD8+ [red], CD4/8 DP [purple]). At 33 days (A-B), the thymus is relatively devoid of T cells or their precursors. By 39 days (C-D), DP cells begin to appear and undergo significant expansion by 46 days (E-F), when the tissue architecture takes on a relatively normal postnatal appearance. Scale bars represent 50 μm. Flow cytometry (G) confirms rapid proliferation of DP thymocytes between 39 and 42 days, with even more significant increase by 46 days.
Development of immune profile in fetal thymus by immunohistochemistry. (A,C,E; ×20, H&E) Shows the evolution in tissue architecture, as well as the appearance and expansion of CD4/8 DP cells (B,D,F; ×20, CD4+ [blue], CD8+ [red], CD4/8 DP [purple]). At 33 days (A-B), the thymus is relatively devoid of T cells or their precursors. By 39 days (C-D), DP cells begin to appear and undergo significant expansion by 46 days (E-F), when the tissue architecture takes on a relatively normal postnatal appearance. Scale bars represent 50 μm. Flow cytometry (G) confirms rapid proliferation of DP thymocytes between 39 and 42 days, with even more significant increase by 46 days.
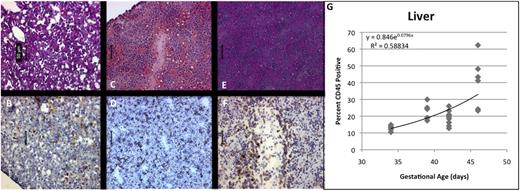
From the hematopoietic perspective, we reasoned that an ideal point for IUHCT would be during the period of fetal liver (FL) hematopoiesis when the BM niche was beginning to form and would be potentially receptive to circulating HSCs. Histologically, we observed CD45+ cells in FL throughout the period of analysis. The immature appearance of the tissue at GA33 was associated with a relatively low frequency of CD45+ cells (Figure 2A-B). We observed a progressive increase in CD45+ cells as a percentage of total FL-MNCs between GA39 and GA46 (Figure 2C-F), shown on logistic regression to be a significant exponential expansion (R2 = 0.741, P < .001) (Figure 2G). By GA46, FL had a more mature appearance, with increased cellularity, obvious vascular architecture (Figure 2E-F), and an overall increase in CD45+ hematopoietic cells.
Development of hematopoietic ontogeny in FL by immunohistochemistry. (A,C,E; ×20, H&E) Shows the structural maturation and expansion of CD45+ cells (B,D,F; ×20, anti-CD45 immunoperoxidase). At 33 days (A-B), the tissue appears immature, and relatively few CD45+ cells are apparent. By 39 days (C-D), CD45+ cells begin to appear, and by 46 days (E-F), obvious vascular architecture and a more mature histologic appearance correspond with a significantly increased concentration of CD45 hematopoietic cells and CD34+ progenitors. Scale bars represent 50 μm. Again, flow cytometry confirms the presence of CD45+ hematopoietic progenitors throughout the period of analysis, increasing exponentially with cellular division (G).
Development of hematopoietic ontogeny in FL by immunohistochemistry. (A,C,E; ×20, H&E) Shows the structural maturation and expansion of CD45+ cells (B,D,F; ×20, anti-CD45 immunoperoxidase). At 33 days (A-B), the tissue appears immature, and relatively few CD45+ cells are apparent. By 39 days (C-D), CD45+ cells begin to appear, and by 46 days (E-F), obvious vascular architecture and a more mature histologic appearance correspond with a significantly increased concentration of CD45 hematopoietic cells and CD34+ progenitors. Scale bars represent 50 μm. Again, flow cytometry confirms the presence of CD45+ hematopoietic progenitors throughout the period of analysis, increasing exponentially with cellular division (G).
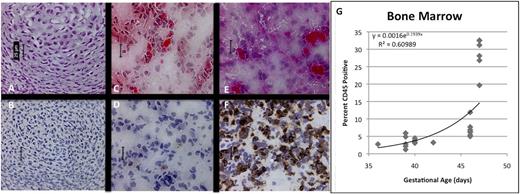
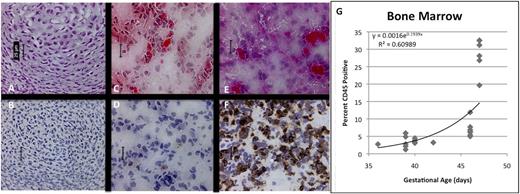
Of greater interest were changes observed in the BM, where very few CD45+ cells were noted histologically at GA33 (Figure 3A-B). A steady increase in cellularity and CD45 content was observed until GA46 (Figure 3C-F), when BM demonstrates lacunar architecture and is filled with hematopoietic cells. Although clearly absent at GA39, osteogenesis is well underway at GA46, suggesting development of the osteogenic niche during that interval. By flow cytometry, we confirmed a significant increase in the number of CD45+ hematopoietic cells around GA46-47, consistent with onset of HSC/progenitor migration from the FL (R2 = 0.610, P < .001) (Figure 3G).
Development of hematopoietic ontogeny in fetal BM by immunohistochemistry. (A,C,E; ×40, H&E) Shows increasing cellularity, the beginning of hematopoiesis and the appearance of CD45+ cells (B,D,F; ×40, anti-CD45 immunoperoxidase). At 33 (A-B) and 39 (C-D) days, very few CD45+ cells are apparent. FACS shows evidence of hematopoietic migration by GA37 days, but cell content within the BM does not significantly change until 46 days (E-F), when tissue architecture begins to evolve, demonstrating a slight increase in content of CD45+ hematopoietic cells. Scale bars represent 25 μm. Flow cytometry (G) confirms significant expansion of CD45+ cells only at the latest time point, with a relatively flat curve throughout the early portion of the analysis.
Development of hematopoietic ontogeny in fetal BM by immunohistochemistry. (A,C,E; ×40, H&E) Shows increasing cellularity, the beginning of hematopoiesis and the appearance of CD45+ cells (B,D,F; ×40, anti-CD45 immunoperoxidase). At 33 (A-B) and 39 (C-D) days, very few CD45+ cells are apparent. FACS shows evidence of hematopoietic migration by GA37 days, but cell content within the BM does not significantly change until 46 days (E-F), when tissue architecture begins to evolve, demonstrating a slight increase in content of CD45+ hematopoietic cells. Scale bars represent 25 μm. Flow cytometry (G) confirms significant expansion of CD45+ cells only at the latest time point, with a relatively flat curve throughout the early portion of the analysis.
Tracking studies
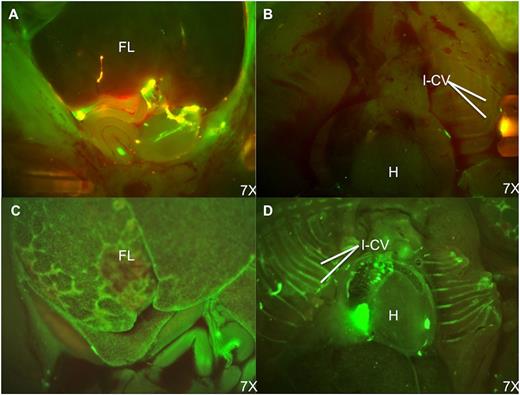
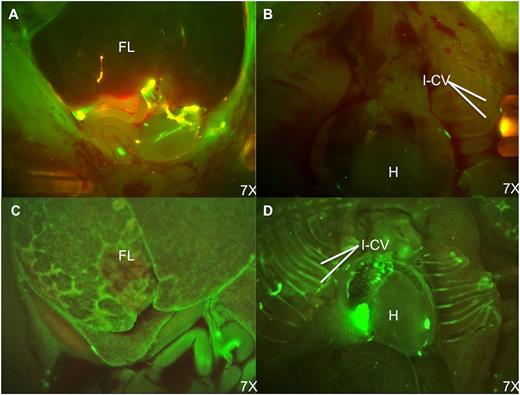
In our tracking studies, we compared 4-hour homing and 48-hour engraftment after IP or IC injection. Although direct quantitative comparison is limited by differences in cell dose, the lower IC dose yielded a much higher percentage of PKH+ cells within FL by stereoscopic fluorescent microscopy and flow cytometry (GA42; average 1.22% [IP] vs 21.89% [IC] at 48 hours). Fluorescent microscopy performed 4 and 48 hours after IP injection showed donor cells pooled in the peritoneal cavity with minimal FL engraftment. In contrast, IC injections resulted in obvious circulating donor cells with far higher levels of FL engraftment (Figure 4A-D).
Fluorescence stereomicroscopic comparison of donor cell distribution at 48 hours postinjection of PKH-67–labeled BM cells. (A) Fetal abdomen after IP injection. (B) Fetal thorax after IP injection. (C) Fetal abdomen after IC injection. (D) Fetal thorax after IC injection. Note the persistence of donor cells in the peritoneal cavity and paucity of engrafted donor cells in the FL after IP injection. In contrast, the FL and intercostal vascular bundles have large numbers of engrafted and circulating PKH+ cells following IC injection. H, heart; I-CV, intercostal vessels.
Fluorescence stereomicroscopic comparison of donor cell distribution at 48 hours postinjection of PKH-67–labeled BM cells. (A) Fetal abdomen after IP injection. (B) Fetal thorax after IP injection. (C) Fetal abdomen after IC injection. (D) Fetal thorax after IC injection. Note the persistence of donor cells in the peritoneal cavity and paucity of engrafted donor cells in the FL after IP injection. In contrast, the FL and intercostal vascular bundles have large numbers of engrafted and circulating PKH+ cells following IC injection. H, heart; I-CV, intercostal vessels.
Results in the optimized IUHCT canine model
Given the results of the ontogeny and tracking studies, we elected to target GA40 using IC administration to optimize engraftment. To avoid the confounding effects of maternal immunization, donor cells were isolated exclusively from maternal BM, preventing any limitation of chimerism through transplacental or breast milk–mediated transfer of maternal antibodies.4 We did not perform preemptive DLA testing on breeding pairs.11 However, based on review of 5-generation pedigrees, pups have no shared ancestors at 4 generations removed, with 2 sharing 1 at the fifth generation. Twenty-nine fetuses of 6 pregnant dogs underwent IC injection at GA39-42.5 (Table 1).12 In our first pregnant dog, HSC enrichment was accomplished via CD34 selection as we and others have previously reported,8 but we found that this approach was associated with insufficient cell yield to inject the entire litter. Subsequent injections were performed using CD3 depletion to achieve a T-cell dose of 1%. This method was associated with a modest enrichment of CD34+ cells to between 2% and 4% and a vastly improved cell yield. Flow cytometric analysis of the donor inoculum revealed ∼1% (0.2% to 2%) CD21, ∼45% (25% to 70%) CD11b, and ∼1% (0.2% to 1.5%) CD11c. Our strategy maximized yield to allow injection of the entire litter, resulting in some variation in absolute cell dose delivered (Table 1).
Survival to birth among all fetuses in whom injection was attempted was 86% (25/29), and postinjection viability per litter as determined by ultrasound confirmation of heartbeat averaged 90%. Overall survival of injected pups to weaning was 83% (24/29); 1 pup that died on day of life 13 was found to have a small bowel volvulus with necrosis. These data compare favorably to our historical IP-injected animals, in whom survival to weaning was 67%,8 and with survival rates reported previously by Blakemore et al.13
Chimerism following IUHCT
Of 24 long-term survivors, 21 were chimeric (>2% PB donor cells) at 2 months of age. Additionally, analysis of the pup that succumbed to volvulus raised the overall rate of detectable chimerism in injected pups of 22/25 (88%). Because maternal donors were used, no more sensitive methodology was applicable for identification of chimerism levels <2%. We did not find any dose effect related to either CD3 or CD34 content, although both remained within a relatively narrow range.
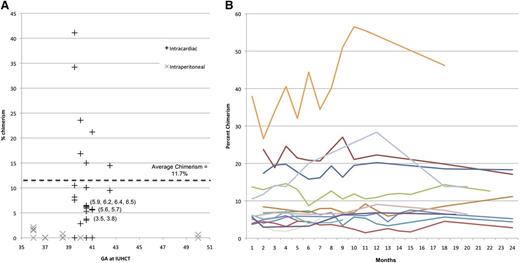
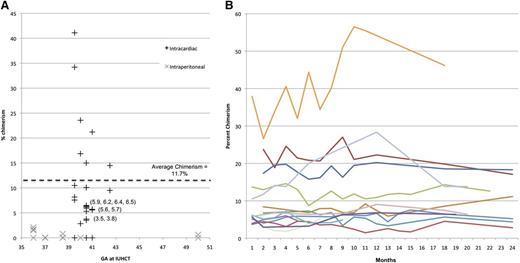
Levels of PB chimerism among the 22 chimeras ranged from 3% to 39% at initial measurement (1-2 months; Figure 5A). All animals followed for long-term chimerism (14 total for >6 months) demonstrated stable engraftment through 6-24 months with an average length of follow-up of 18 months (Figure 5B). PB from selected animals underwent flow cytometric sorting for CD3 (T cells), CD21 (B cells), CD11b (monocytes/macrophages), and CD11c (dendritic cells), and VNTR performed on each fraction confirmed balanced multilineage engraftment (supplemental Figure 2).
Levels of PB chimeriam. Initial chimerism following IUHCT alone as compared with historical IP-injected controls8 (A), and long-term stable engraftment (B) as measured by VNTR. All animals were followed until 2 years or earlier end point dictated by entry into a related protocol, including renal transplant.
Levels of PB chimeriam. Initial chimerism following IUHCT alone as compared with historical IP-injected controls8 (A), and long-term stable engraftment (B) as measured by VNTR. All animals were followed until 2 years or earlier end point dictated by entry into a related protocol, including renal transplant.
Renal transplantation following IUHCT
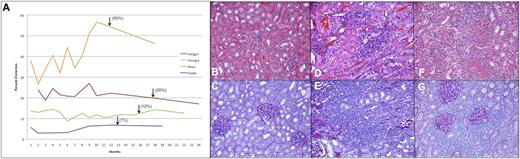
Four recipients with chimerism levels ranging from ∼7% to 55% were selected to undergo renal transplant from their maternal BM donor, as was a nonchimeric IUHCT recipient control (Figure 6A). Postprocedural ultrasound demonstrated excellent flow to the kidney in all cases. No immunosuppression or conditioning was used in any recipient. All 4 chimeric animals remained clinically well throughout the experimental period without clinical evidence of rejection, whereas the control animal showed signs of acute rejection by day 5, including fever, lethargy, and anorexia. Following removal of an inflamed, hemorrhagic donor kidney without evidence of vascular compromise, these symptoms entirely resolved.
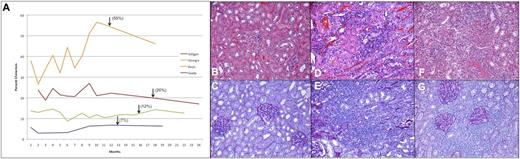
Renal transplantation following IUHCT. Chimerism profile of renal allograft recipients, with arrows marking the time of transplantation (A). Evaluation of the kidney allograft in tolerant animals at 6 months reveals no evidence of rejection on H&E (B) or PAS (C). In the nonchimeric control dog, there is evidence of acute rejection with significant and severe lymphocytic infiltrate, inflammation, and tubulitis on H&E (D) and PAS (E) upon transplant nephrectomy 5 days following transplantation. This animal manifested an early and acute Systemic Inflammatory Response Syndrome reaction characterized by fevers, anorexia, and weight loss, consistent with acute rejection observed histologically and requiring urgent explant, upon which all symptoms resolved. In this animal, gross examination of the kidney revealed a markedly shrunken and discolored appearance. In the animal with the lowest chimerism levels (3% to 7%), there was persistent lymphocytic infiltrate on both H&E (F) and PAS (G) at 6 months, consistent with Banff class 3 subclinical chronic rejection; no clinical sequelae were noted, and the animal remained healthy throughout the planned 6-month observation. Upon gross examination, this allograft had a normal appearance. All images ×20.
Renal transplantation following IUHCT. Chimerism profile of renal allograft recipients, with arrows marking the time of transplantation (A). Evaluation of the kidney allograft in tolerant animals at 6 months reveals no evidence of rejection on H&E (B) or PAS (C). In the nonchimeric control dog, there is evidence of acute rejection with significant and severe lymphocytic infiltrate, inflammation, and tubulitis on H&E (D) and PAS (E) upon transplant nephrectomy 5 days following transplantation. This animal manifested an early and acute Systemic Inflammatory Response Syndrome reaction characterized by fevers, anorexia, and weight loss, consistent with acute rejection observed histologically and requiring urgent explant, upon which all symptoms resolved. In this animal, gross examination of the kidney revealed a markedly shrunken and discolored appearance. In the animal with the lowest chimerism levels (3% to 7%), there was persistent lymphocytic infiltrate on both H&E (F) and PAS (G) at 6 months, consistent with Banff class 3 subclinical chronic rejection; no clinical sequelae were noted, and the animal remained healthy throughout the planned 6-month observation. Upon gross examination, this allograft had a normal appearance. All images ×20.
Immunohistochemistry revealed no evidence of cellular or humoral rejection (Banff 0) at either biopsy or transplant nephrectomy in the 3 higher-level chimeras (>10%) (Figure 6B-C). The low-level chimeric recipient (3% to 7%) had evidence of mild chronic rejection upon 60-day biopsy (Banff 1), which progressed to Banff 3 by 6-month nephrectomy (Figure 6D-E). Histology confirmed acute cellular rejection in the control animal by nephrectomy at 5 days posttransplant (Figure 6F-G).
GVHD following IUHCT
No dogs showed clinical evidence of GVHD following IUHCT; all dogs gained and maintained weight appropriately throughout the study.
Discussion
Although the murine model has allowed systematic investigation of the requirements for successful IUHCT, translation of the success in this model to a relevant large animal model is required prior to clinical application. Previous large animal studies in sheep,14 pigs,15 and nonhuman primates16-18 have investigated the potential for engraftment following IUHCT, yielding inconsistent results. With the exception of the sheep, levels of chimerism have been quite low, and multilineage, long-term engraftment has not been demonstrated. Promising early results in the allogeneic and xenogeneic ovine models did not translate to clinical success despite many attempts,19 raising questions regarding the clinical relevance of this model.
Canines have historically proved difficult to engraft by IUHCT, suggesting the presence of a robust and competitive fetal hematopoietic compartment.13,20,21 Stage for stage, the dog mimics human hematopoietic and immune ontogeny with similar genetic diversity.10,22 Most importantly, the canine model has been used extensively for preclinical testing of HSCT regimens and has proved a reliable predictor of clinical results,23,24 particularly with respect to the risk of GVHD.23-31 Finally, a number of valuable canine models of human disease have been described.32-39 Historically, the canine model of allogeneic IUHCT was associated with very low levels of chimerism,13 as was our initial work in canine leukocyte adhesion deficiency dogs.8 However, despite the low levels of chimerism, our previous study confirmed the feasibility of postnatal minimally conditioned same-donor HSCT in animals with IUHCT-induced DST.40-42
Studies in the murine model support competition from the nonmyeloablated host hematopoietic compartment as the primary barrier to engraftment after IUHCT.4 We have overcome that barrier primarily by consistent delivery of much higher donor cell doses by an intravascular (vitelline vein) injection technique.43 In our assessment of the early homing and engraftment events after IUHCT, the most striking finding was the inefficiency of cell uptake from the peritoneum after IP injection. In contrast, IC injection resulted in a dramatic increase in engrafted cells with far higher donor cell frequencies in the FL and other hematopoietic organs. Our results in this model support our hypothesis that intravascular delivery of a very large dose of maternal HSCs can overcome the competitive barrier of fetal hematopoiesis and achieve significant levels of donor chimerism. Although the size of the canine fetus requires that IC injection be used as a surrogate, clinical IUHCT will likely be performed using ultrasound-guided, fetoscopic intravascular injection, a modification of the well-established technique for treatment of Rh disease and fetal blood sampling.44-46
The other primary impediment to successful allogeneic IUHCT is the immune barrier. The rationale for IUHCT is based on exploitation of normal immune ontogeny, with delivery of donor cells prior to the development of self-tolerance, a series of positive and negative selection events in the fetal thymus that result in a repertoire of lymphocytes reactive to foreign antigen but not to “self.”47-50 In addition, T-regulatory cells (Treg) are generated in the thymus and peripheral tissues to suppress autoimmune activity from self-reactive cells that escape thymic deletion.51-53 In the murine system, we have demonstrated that, in the absence of maternal immunization, the mechanisms of clonal deletion and Treg suppression apply to tolerance induction for donor cells after IUHCT.4 As thymic selection occurs predominantly at the stage of the DP thymocyte,54 we hypothesized that the appearance and early expansion of DP thymocytes would represent optimal timing of IUHCT from the perspective of immune ontogeny, and that use of maternal donor cells would avoid confounding effects of maternal immunization.4 Transplantation around GA40 stably engrafted nearly all animals, validating that hypothesis. The existence and stability of the chimerism observed in this study is a powerful argument against the presence of any nonregulated mechanism of allogeneic response and would be expected to be associated with DST for either same-donor HSCT or organ transplantation. We assessed DST using a renal transplant model both to demonstrate the potential for solid organ transplantation without immunosuppression and to perform a direct assessment of immunologic response. Renal transplant has been used previously to demonstrate tolerance in mixed chimeras after BMT,55,56 and rejection of haploidentical and DLA-matched organs is well-established in the absence of mixed chimerism or immunosuppression.57,58 Indeed, our control animal became clinically unwell within 5 days of transplantation and required urgent explant for acute cellular rejection associated with a Systemic Inflammatory Response Syndrome response. Although the presence of low-grade chronic rejection in a low-level chimera suggests that the threshold for solid organ tolerance in our system may be higher than that required for hematopoietic tolerance, all 3 recipients with sustained chimerism levels >10% showed no evidence of acute or chronic rejection at any point. These results confirm that IUHCT induces tolerance to same-donor antigens with adequate donor chimerism levels. Importantly, we have not defined the late limit for the immunologic window of opportunity. Blakemore et al performed IUHCT by IP injection in the canine model between GA31 and GA50.13,59 Interestingly, the best GA in their study was GA42, similar to our results.
From the perspective of hematopoietic ontogeny, less is known regarding the requirements for receptivity to donor cells. It is well documented that donor cells engraft in the FL after IUHCT, and earlier attempts have generally resulted in poorer engraftment.59,60 Our analysis in the canine model confirms predominantly FL hematopoiesis until after GA40 with population of the BM by hematopoietic cells and maturation of BM architecture between GA39 and GA46. Although we have not attempted to define the optimal time from a hematopoietic perspective, our results demonstrate that delivery of a large dose of donor cells during the period of predominantly FL hematopoiesis results in FL engraftment with subsequent long-term BM engraftment.
These results represent a dramatic improvement over previous results in the canine and other large animal models with respect to both the frequency and level of donor cell chimerism. Using our optimized protocol, we achieved an 88% frequency of engraftment in the absence of any form of conditioning with levels of chimerism in all animals above the expected threshold for DST and, often, high enough to treat many target disorders, including immunodeficiencies61-63 and hemoglobinopathies.64-66 Most importantly, levels of mixed hematopoietic chimerism have remained stable on long-term analysis with no instance of lost chimerism in our cohort. The 3/25 animals that had no VNTR-detectable chimerism likely represent technical misses rather than failed biology, based on our observations at the time of injection.
Once engraftment has been achieved, the greatest anticipated risk of clinical IUHCT is GVHD. Billingham’s tenets would predict a high risk of GVHD in fetal recipients: (1) IUHCT grafts contain immunologically competent cells; (2) recipients express tissue antigens not present in the donor; and (3) recipients are incapable of mounting an effective response to eliminate the transplanted cells.67 However, in primates, GVHD was not observed until doses of 108 CD2+ cells per kg fetal weight were administered16 ; an early study in sheep using adult BM without T-cell depletion resulted in engraftment without GVHD68 ; and a subsequent study assessing the effect of T-cells on engraftment observed no GVHD until >3% of donor cells were T cells.69 In the pig model, multilineage chimerism with DST resulted after BM was T-cell depleted to 1.5% with no evidence of GVHD.15 Our study evaluated GVHD primarily as a clinical diagnosis, although every stillborn fetus underwent full necropsy and a few clinical situations warranted skin biopsy; no GVHD was seen on pathological evaluation, despite the use of CD3+ doses from 2.5 × 108 to 4.1 × 108 cells per kg estimated fetal weight. Our results in this preclinical large animal model support previous studies and our own experience with many years of experimental work in IUHCT, which suggests that the fetus is somewhat resistant to GVHD relative to recipients of postnatal myeloablative HSCT. Although speculative, this is likely because of a vigorous Treg response from a healthy fetal thymus.4,70,71 We are currently performing studies to define the threshold for GVHD in this model and establish a safety margin prior to clinical application.
Clinical experience with IUHCT, with the exception of success in treating severe combined immunodeficiency syndrome,72 has been disappointing.2,19,73-75 In view of our experience in both murine and canine models, we believe that clinical efforts have failed because of inadequate delivery of donor cells, a potential maternal immune barrier, and, in many cases, suboptimal timing of IUHCT, rather than for inherent biological reasons. Prior to clinical application, other methods of intravascular injection using ultrasound and/or fetoscopic guidance need to be evaluated in the equivalent-size nonhuman primate model. The risk of IUHCT-induced GVHD requires clear definition to identify the margin of safety for donor T-cell content. In addition, we have little insight into the importance of the maternal immune system or MHC matching of the graft in large animal systems. The development of a consistent canine model of allogeneic engraftment and long-term chimerism after IUHCT should facilitate future investigations of these questions. Finally, the generation of stable, long-term hematopoietic chimerism in the previously resistant canine model, at donor cell levels associated with DST and potentially therapeutic for many target disorders, represents a major step toward adequate experimental justification for clinical trials of IUHCT for inherited hematologic disorders.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Keith Alcorn, Aaron Weilerstein, and Laboratory Animal Services staff for their assistance in animal care, and Jane Wright for her sonographic expertise.
This work was supported by a grant from the Pennsylvania Department of Health (SAP#4100043360) (A.W.F.), charitable contributions from the Arthur M. Greenfield Foundation (A.W.F.), and the Ruth and Tristram C. Colket Jr Endowed Chair (A.W.F.).
Authorship
Contribution: J.D.V. participated in experimental design, performed experiments, analyzed data, and wrote the manuscript; E.G.P. participated in experimental design, performed experiments, and analyzed data; M.T.S. participated in experimental design and performed experiments; C.A.T., H.L., and A.R. performed experiments; T.B. analyzed data; W.H.P. and M.P.J. performed experiments; and A.W.F. participated in experimental design, performed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan W. Flake, Department of Surgery, Abramson Research Building, Room 1116B, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: flake@email.chop.edu.
References
Author notes
J.D.V. and E.G.P. contributed equally to this study.

![Figure 1. Development of immune profile in fetal thymus by immunohistochemistry. (A,C,E; ×20, H&E) Shows the evolution in tissue architecture, as well as the appearance and expansion of CD4/8 DP cells (B,D,F; ×20, CD4+ [blue], CD8+ [red], CD4/8 DP [purple]). At 33 days (A-B), the thymus is relatively devoid of T cells or their precursors. By 39 days (C-D), DP cells begin to appear and undergo significant expansion by 46 days (E-F), when the tissue architecture takes on a relatively normal postnatal appearance. Scale bars represent 50 μm. Flow cytometry (G) confirms rapid proliferation of DP thymocytes between 39 and 42 days, with even more significant increase by 46 days.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/12/10.1182_blood-2013-11-537571/4/m_1987f1.jpeg?Expires=1765966404&Signature=datVQU8OnIHHkOGkN1YHqXdW-jWLNX804LnhV4Dgy-Gm2sYiaciF4O2iPM1ZcvdJ1l7cs4FnFoq5ENjdVDRkjHwhXRR4WHcqHXsOYKqivkxRDd881ZafhldVupWZPQ~cxaylThKRplur5LUcAIZyGabtt0X1NH-00yjGkjjzxAhV2OWrei46Bm-gRlF6f4iQotAs-QV9j5iugbR4pSTQguwDHOLdgxzkU5xVmOeFqAcFYOQSei9rCET33TqmJARREH13jC4gHjVOJkraWmTwskNoaJ7lifxLwD4P0PJt9Kl7J1cQM2rVRnCj~eNs4BgR2nikvpYvjxyhwF7qjBv1ng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Development of immune profile in fetal thymus by immunohistochemistry. (A,C,E; ×20, H&E) Shows the evolution in tissue architecture, as well as the appearance and expansion of CD4/8 DP cells (B,D,F; ×20, CD4+ [blue], CD8+ [red], CD4/8 DP [purple]). At 33 days (A-B), the thymus is relatively devoid of T cells or their precursors. By 39 days (C-D), DP cells begin to appear and undergo significant expansion by 46 days (E-F), when the tissue architecture takes on a relatively normal postnatal appearance. Scale bars represent 50 μm. Flow cytometry (G) confirms rapid proliferation of DP thymocytes between 39 and 42 days, with even more significant increase by 46 days.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/12/10.1182_blood-2013-11-537571/4/m_1987f1.jpeg?Expires=1766167204&Signature=WxrngTsdGZ~SSbhw9l0JTmhBbL5yU4l5Sn2RWpwaKbNmI7Khboj234vjDv38pbujKEnn6pEAm9uRpg7USHkHD8h8oqEGmT8xG5DQE7XeN99Ydtufd8YM0jXPPp~Yxyv5VLspefUMusfxzcBWuUi7jmrQq6hFkGDEvY-VpLg-0UGnhQ-hixIFwIu7jw49vxQY5FDz~3oBhQYVVxllaR36ATdD8znQ9ckNz9zAWe-dQGlaO0omwZ7tsK~4znn~2X-iCgE0IOAcf9pJDeWTiCe~BFNaf8MmrOhw5Mda~GtNLd1tB~4N2ejffVNexoxJgm7szXrXokcd9AAjQq9LvlFyEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)