Key Points
STAT1−/− BM prevents GVHD induced by delayed donor lymphocyte infusion via the expansion of CD9−Siglec Hhi pDCs, which are low producers of IFNα and IL-12.
pDCs recovered from STAT1−/− BM chimeras show increased expression of S100A8, S100A9, and STAT3.
Abstract
Selective targeting of non-T cells, including antigen-presenting cells (APCs), is a potential strategy to prevent graft-versus-host-disease (GVHD) but to maintain graft-versus-tumor (GVT) effects. Because type I and II interferons signal through signal transducer and activator of transcription-1 (STAT1), and contribute to activation of APCs after allogeneic bone marrow transplant (alloBMT), we examined whether the absence of STAT1 in donor APCs could prevent GVHD while preserving immune competence. Transplantation of STAT1−/− bone marrow (BM) prevented GVHD induced by STAT1+/+ T cells, leading to expansion of B220+ cells and regulatory T cells. STAT1−/− BM also preserved GVT activity and enhanced overall survival of tumor-challenged mice in the setting of GVHD. Furthermore, recipients of allogeneic STAT1−/− BM demonstrated increased CD9−Siglec Hhi plasmacytoid dendritic cells (pDCs), and depletion of pDCs after STAT1−/− BM transplantation prevented GVHD resistance. STAT1−/− pDCs were found to produce decreased free radicals, IFNα, and interleukin (IL)-12, and increased IL-10. Additionally, STAT1−/− pDCs that were isolated after alloBMT showed increased gene expression of S100A8 and S100A9, and transplantation of S100A9−/− BM reduced GVHD-free survival. Finally, elevated STAT3 was found in STAT1−/− pDCs isolated after alloBMT. We conclude that interfering with interferon signaling in APCs such as pDCs provides a novel approach to regulate the GVHD/GVT axis.
Introduction
Allogeneic hematopoietic stem cell transplant (alloHSCT) is curative for multiple malignant and nonmalignant diseases. T cells in the donor graft play a critical role in engraftment, antiviral immunity, and graft-versus-tumor (GVT) effects, contributing to remissions and cures.1 However, the therapeutic benefit of increasing T-cell dose in the stem cell graft or as a donor lymphocyte infusion (DLI) is limited by graft-versus-host-disease (GVHD), a major life-threatening complication subsequent to alloHSCT. Despite advances in the matching of donors and recipients at major histocompatibility complex (MHC) alleles and improvements in immunoprophylaxis, GVHD from mismatched minor histocompatibility antigens (miHAs) occurs in up to 65% of recipients, leading to significant morbidity and mortality.2 Although there is a variety of medications available to prevent and treat GVHD, these drugs mainly target global T-cell function, impairing immunity to pathogens and to malignancy, resulting in increased incidence of infection and relapse.3 Thus novel strategies are needed that can prevent GVHD and can preserve immune competence.
After conditioning with cytotoxic chemotherapy and/or total body irradiation for alloHSCT, there is a “cytokine storm” reflected by an increase in inflammatory cytokines. These cytokines activate APCs in the recipient,2 which act to prime donor T cells against host antigens, inducing GVHD.4 One family of cytokines that has drawn considerable interest for its role in GVHD/GVT is the interferon family, which serves as a critical interface between innate and adaptive immunity and is a major component of the alloreactive environment.5 Interferon signaling can produce differential effects on GVHD depending on whether responding cells are of donor6-8 or host origin,7,9,10 and if they are interrupted in transplanted T cells6-8,11,12 or non-T cells.6,7
Because both type I and type II interferons signal through the transcription factor signal transducer and activator of transcription-1 (STAT1),13 there is interest in the role of this transcription factor in GVHD.2,5,14 Furthermore, developing therapies for GVHD that spare T cells are appealing, because they may preserve or even enhance GVT effects. STAT1 gene expression is elevated in GVHD,15 and data suggest that adenosine triphosphate stimulates APCs through STAT1 to exacerbate GVHD.16 In addition, correlative evidence in adults with chronic GVHD demonstrates elevated expression of STAT1.17 Finally, infusion of donor CD4+ T cells deficient in STAT1 were shown to abrogate GVHD through the expansion of regulatory T cells (Tregs).12 Although effective in preventing GVHD, targeting STAT1 solely in donor T cells may unintentionally impair overall immunity because deficiency of type I7 and type II6 interferon signaling in donor T cells attenuates their ability to cause GVT. The goal of this study was to assess the effects of STAT1 modulation in donor-derived APCs as an innovative means of preventing GVHD by leaving T-cell function intact and thus potentially preserving GVT.
Materials and methods
Mice
C3H.SW (H-2b), C57BL/6 (B6) × C3H.SW (H-2b), B6 IFNγR1−/− (H-2b), B6.CD11c-cre (H-2b), B6.129P2 CD19-cre (H-2b), and B6.129P2 Lysozyme (Lys)-cre (H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). 129S6 (H-2b) and 129S6 STAT1−/− (H-2b) mice were purchased from Taconic Farms (Hudson, NY). B6 interferon regulatory factor-9 (IRF9)−/− mice (H-2b) were generated by Tadatsugu Taniguchi18 and were purchased from RIKEN BioResource Center (Ibaraki, Japan). B6 (H-2b) and C3H.HeNCr (H-2k) were purchased from the National Cancer Institute (NCI) Animal Production Program (Frederick, MD). Strain 129 mice with a floxed region flanking the STAT1 gene (STAT1flox/flox) were generated as previously described.19 S100A9−/− mice (H-2b) were a gift from Dr Dmitry Gabrilovich (H. Lee Moffitt Cancer Center, Tampa, FL). Wild-type (WT)-1 T-cell receptor transgenic mice (H-2b) were generated as described in the supplemental Methods (available on the Blood Web site). Mice were age-matched and were used between 4 and 8 weeks of age. All animals were housed in a pathogen-free facility throughout the study. The Animal Care and Use Committee at the National Institutes of Health approved all protocols.
T-cell–depleted (TCD) BM transplantation
Donor mice were euthanized by CO2 asphyxiation following National Institutes of Health Animal Care and Use Committee guidelines. Bone marrow (BM) cells were harvested from the tibias and fibulas by crushing with mortar and pestle in 10% complete mouse media (CMM).6 BM cells were passed through a 70-μm nylon filter, and were depleted of erythrocytes using ACK Lysing Buffer (Lonza, Walkersville, MD). Single-cell suspensions were then labeled with CD3 microbeads (Miltenyi Biotec, Auburn CA), followed by a magnetic separation using the AutoMACS Pro separation system (Miltenyi Biotec). Lethally irradiated (10 Gy) recipient mice were injected intravenously through the tail vein on day +0 with 4 × 106 (10 × 106 was used for C3H.HeNCr recipients) CD3-depleted BM cells in serum-free RPMI (Invitrogen). Mice were weighed individually biweekly, and the mean weight of each treatment group was calculated at each time point and was compared with the day +0 weight. GVHD was monitored using a clinical scoring system.20 Examination for moribund mice was performed by a veterinarian and veterinary technicians who were blinded to the experimental groups, and they assessed the mice daily in accordance with approved institutional protocols.
DLIs
GVHD was induced using a DLI administered either on day +10 or +14 after transplantation. In some experiments a second DLI was infused on day +28. DLIs were generated from single-cell suspensions of pooled cervical, inguinal, and axillary lymph nodes obtained from WT mice matched to the BM donor. Lymph node cells were resuspended in serum-free RPMI and were passed through a 70-μm nylon filter before injection, and then they were administered intravenously through the tail vein when indicated.
Pharmacologic inhibition of STAT1
Off-target inhibition of STAT1 was achieved by injecting donor mice intraperitoneally with (24 nM) 2.5 ug of Exenatide (Sigma-Aldrich) dissolved in 0.2 mL phosphate-buffered saline (K-D Medical, Columbia, MD) 2 days before BM harvest. Recipient mice continued to receive intraperitoneal injections after TCD alloHSCT once daily for 10 days.
Cytokine analyses
Spleen and BM cells were cultured at 1 × 106 cells/mL in CMM at 37°C with 1 ug/mL CpG oligodeoxynucleotides (gift from Dr Dennis Klinman; NCI, Frederick, MD) for 3 hours in CMM. Supernatants were harvested and analyzed by enzyme-linked immunosorbent assays (ELISA) for murine IFNα (PBL Interferon Source, Piscataway, NJ) and interleukin (IL)-10 (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. ELISA plates were read on a VersaMAX Microplate Reader at 450 nm and were analyzed using SoftMAX Pro 5 reader (Molecular Devices, Sunnyvale, CA). cDNA was synthesized from the cultured cells, and quantitative RT-PCR was performed using primers specific for IFNα and IL-12 on the StepOne Plus Real-Time PCR System (Applied Biosystems, Foster City, CA). Gene expression was calculated using the ΔΔCt method.
Flow cytometric analysis
In brief, 1 × 106 freshly isolated, erythrocyte-depleted splenocytes, lymph node, or BM cells were treated with anti-FcγIII/II receptor monoclonal antibody (moAb) clone 2.4G2 and then were stained at 4°C for 20 minutes with an moAb cocktail containing either CD11c or NK1.1-FITC, B220-PE, CD4 or CD45.2-PerCP Cy5.5, CD8 or CD45.1-APC, CD11b-Pacific Blue (BD Biosciences), CD9-APC, or Siglec H-PerCP Cy5.5 (eBioscience, San Diego, CA), and then they were washed in fluorescence-activated cell-sorting buffer (phosphate-buffered salt solution with 0.2% fetal calf serum and 0.1% sodium azide). To examine for the presence of Tregs, surface staining for CD25-FITC and CD4-PerCP Cy5.5 were performed, followed by Foxp3-PE intracellular staining according to the manufacturer’s instructions (eBioscience).
In vivo pDC depletion
Depletion of pDCs was achieved using a moAb against murine PDCA-1 (Miltenyi Biotec) resuspended in a phosphate-buffered saline/2 mM EDTA solution (both from K-D Medical). Recipients of a TCD alloHSCT were injected intraperitoneally 2 days before their DLI with 250 ug of PDCA-1 or IgG2b control (Sigma-Aldrich). Mice continued to receive injections every other day for a total of 5 injections. Depletion of pDCs was verified by flow cytometric analysis of representative spleens from each group 24 hours after their final intraperitoneal injection.
MB49 tumor challenge
The MB49 tumor cell line is derived from a chemically induced urothelial carcinoma in a male B6 mouse and it expresses the male-specific miHA H-Y.6 MB49 cells were maintained in culture at 37°C in 5% CO2 in CMM. Exponentially growing tumor cells were prepared as a single-cell suspension in serum-free media and were injected into the subcutaneous fat of the flank at a dose of 2 × 106 tumor cells on day 42 after BMT. Tumors were measured in 2 dimensions (length × width) 2× per week by digital caliper. Mice were euthanized with CO2 when tumor diameters reached 2 cm, in accordance with animal protocols. If a mouse was found dead, the previously recorded tumor measurement was carried for the rest of the experiment.
Statistical analysis
Statistics were performed using GraphPad Prism version 4.0c for the Macintosh OS (GraphPad Software, San Diego, CA). Significant differences when comparing 2 groups were determined by the 2-tailed Mann-Whitney test. The Kruskal-Wallis with Dunn’s multiple-comparison posttest was used to assess statistical differences among 3 or more groups. A P value <.05 was considered statistically significant.
Results
Transplantation of STAT1−/− BM or pharmacologic STAT1 inhibition reduces GVHD severity mediated by delayed donor T-cell infusion with preserved immune competence
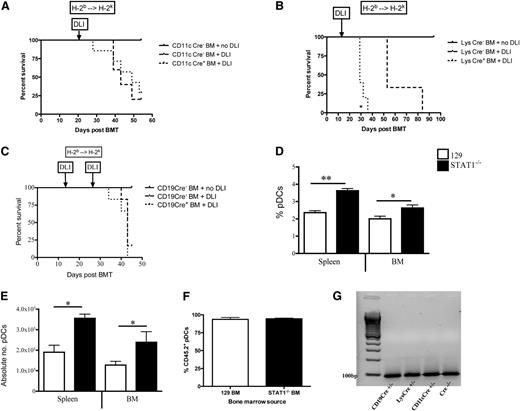
Based on our previous observation that the lack of IFNγ receptor on donor non-T cells prevented GVHD,6 we first wanted to determine whether a similar effect occurred by interfering with STAT1, the main component of the canonical signaling pathways of both type I and II interferons, in donor hematopoietic cells. Using a MHC-matched, miHA-mismatched alloHSCT model (129 → C3H.SW) we observed that when STAT1−/− TCD BM was followed by delayed infusion of donor-derived STAT1+/+ T cells as a DLI administered on day +14, there were improved clinical GVHD scores (Figure 1A) as a result of the prevention of GVHD-associated weight loss (supplemental Figure 1A), with improved B-cell reconstitution (Figure 1B) and expansion of Tregs (Figure 1C). The GVHD protection from STAT1−/− BM was T-cell dose-dependent (Figure 1D), as doubling the DLI dose from 20 to 40 × 106 suppressed Treg expansion (Figure 1E) and caused weight loss (Figure 1D and supplemental Figure 1B). GVHD-associated, DLI-induced weight loss was also prevented in a different miHA-mismatched alloHSCT model (129 → B6 × C3H.SW, supplemental Figure 1C). Interestingly, in the setting of T-cell replete (TCR) BM grafts, use of STAT1−/− BM did not impact GVHD (supplemental Figure 1D). We hypothesized that T cells in the BM graft would encounter persisting recipient STAT1+/+ APCs following TCR transplant whereas delayed DLI would occur in the context of STAT1−/− APCs. Indeed, we found that total spleen (supplemental Figure 1E) and splenic dendritic cells (DCs) (supplemental Figure 1F) were mainly donor derived by 14 days post-alloHSCT irrespective of the BM source.
TCD STAT1−/− BM prevents DLI-induced GVHD. All recipients received a miHA-mismatched alloHSCT from either STAT1+/+ or STAT1−/− BM (129 → C3H.SW). Lethally irradiated C3H.SW recipients were transplanted with 4 × 106 TCD STAT1+/+ BM or STAT1−/− BM on day +0. On day +14, 20 × 106 DLI was administered to induce GVHD. Some recipients did not receive a DLI as a negative GVHD control. All mice were followed for (A) clinical GVHD scores. On day +28, all groups were euthanized and immune reconstitution of (B) B cells and (C) Tregs in the spleen was ascertained by flow cytometry. (D) Lethally irradiated C3H.SW recipients were transplanted with 4 × 106 STAT1−/− BM on day +0. On day +14, 0, 10, 20, or 40 × 106 DLI was administered to induce GVHD. Mice were followed for GVHD-associated weight loss. On Day +40, weight loss was compared, and (E) Treg expansion in the spleen was ascertained by flow cytometry. (F) Lethally irradiated recipients received a miHA-mismatched alloHSCT from STAT1+/+ BM (B6 → C3H.SW) treated with vehicle (phosphate-buffered saline [PBS]) or Exenatide (Ex4) for 3 days before alloHSCT. BM recipients continued to receive vehicle or Exenatide for 10 days after alloHSCT. 20 × 106 DLI was administered on day +14 to induce GVHD, and recipients were followed for clinical GVHD scores, and (G) % change in weight was compared at day +40. N = 3 to 7 mice/group, data are representative from 1 of 2 similar experiments. *P < .05, **P < .01, ***P < .001.
TCD STAT1−/− BM prevents DLI-induced GVHD. All recipients received a miHA-mismatched alloHSCT from either STAT1+/+ or STAT1−/− BM (129 → C3H.SW). Lethally irradiated C3H.SW recipients were transplanted with 4 × 106 TCD STAT1+/+ BM or STAT1−/− BM on day +0. On day +14, 20 × 106 DLI was administered to induce GVHD. Some recipients did not receive a DLI as a negative GVHD control. All mice were followed for (A) clinical GVHD scores. On day +28, all groups were euthanized and immune reconstitution of (B) B cells and (C) Tregs in the spleen was ascertained by flow cytometry. (D) Lethally irradiated C3H.SW recipients were transplanted with 4 × 106 STAT1−/− BM on day +0. On day +14, 0, 10, 20, or 40 × 106 DLI was administered to induce GVHD. Mice were followed for GVHD-associated weight loss. On Day +40, weight loss was compared, and (E) Treg expansion in the spleen was ascertained by flow cytometry. (F) Lethally irradiated recipients received a miHA-mismatched alloHSCT from STAT1+/+ BM (B6 → C3H.SW) treated with vehicle (phosphate-buffered saline [PBS]) or Exenatide (Ex4) for 3 days before alloHSCT. BM recipients continued to receive vehicle or Exenatide for 10 days after alloHSCT. 20 × 106 DLI was administered on day +14 to induce GVHD, and recipients were followed for clinical GVHD scores, and (G) % change in weight was compared at day +40. N = 3 to 7 mice/group, data are representative from 1 of 2 similar experiments. *P < .05, **P < .01, ***P < .001.
To determine if pharmacologic inhibition would replicate the results with BM genetically deficient in STAT1, we treated mice in the peritransplant period with Exenatide, a glucagon-like peptide approved for treatment of type II diabetes and shown to inhibit of STAT1 in vitro and in vivo (supplemental Figure 1G).22,23 WT B6 mice were treated with Exenatide and were used as BM donors for transplantation into C3H.SW recipients, which also received Exenatide after transplant but before DLI, to mimic STAT1 deficiency during engraftment. Indeed, similar to transplantation of STAT1-deficient BM, we found that Exenatide decreased GVHD scores (Figure 1F) and attenuated weight loss (Figure 1G).
We next tested whether recipients of female STAT1−/− BM and female T cells could respond to a male vaccine at the time of DLI as measured by resistance to HY-expressing tumor on day +42 (Figure 2A). T cells were required for tumor control, as recipients of no DLI experienced faster onset of large tumors. Although there was a slight delay in tumor growth after DLI and vaccination against HY in recipients of STAT1+/+ BM, ultimately recipients succumbed to large tumors associated with the development of GVHD. In contrast, recipients of STAT1−/− BM, DLI, and HY vaccination controlled tumor growth (Figure 2B), resulting in improved overall survival (Figure 2C) likely from improved GVHD.
TCD STAT1−/− BM preserves GVT activity in relation to tumor growth but enhances overall survival after DLI and DC vaccination. (A) Lethally irradiated female recipients received TCD miHA-mismatched BM from female STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14 received 20 × 106 DLI from female STAT1+/+ donors as well as 1 × 105 DCs from male STAT1+/+ donors. On day +28, a second dose of male DC vaccine was administered. Some recipients did not receive a DLI and a DC vaccine as a negative control for tumor protection. On day +42, 2 × 106 MB49 (which expresses the male antigen H-Y) was inoculated onto the right flank. (B) Tumors were measured in 2 dimensions twice weekly, and (C) alloHSCT recipients were followed for overall survival after tumor challenge. N = 5 mice/group, data are representative from 1 of 2 similar experiments. *P < .05.
TCD STAT1−/− BM preserves GVT activity in relation to tumor growth but enhances overall survival after DLI and DC vaccination. (A) Lethally irradiated female recipients received TCD miHA-mismatched BM from female STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14 received 20 × 106 DLI from female STAT1+/+ donors as well as 1 × 105 DCs from male STAT1+/+ donors. On day +28, a second dose of male DC vaccine was administered. Some recipients did not receive a DLI and a DC vaccine as a negative control for tumor protection. On day +42, 2 × 106 MB49 (which expresses the male antigen H-Y) was inoculated onto the right flank. (B) Tumors were measured in 2 dimensions twice weekly, and (C) alloHSCT recipients were followed for overall survival after tumor challenge. N = 5 mice/group, data are representative from 1 of 2 similar experiments. *P < .05.
Selective STAT1 deficiency in donor-derived CD19+, CD11chi, or Lys-expressing cells does not protect against GVHD
Because TCD STAT1−/− BM was sufficient to protect against GVHD, we hypothesized that a donor-derived APC population was responsible. Using STAT1flox/flox mice expressing Cre recombinase under the control of promoters active in APCs (CD11c [DCs], Lys [macrophages and neutrophils], or CD19 [B cells]), we examined the role of specific STAT1-deficient APC subsets in modulating GVHD. However, selective STAT1 deficiency in donor-derived CD19+ cells, CD11c+ cells or Lys-expressing cells did not protect against GVHD after MHC-matched BMT (CD11c Cre+ BM [B6 → C3H.SW, supplemental Figure 2A], Lys Cre+ BM [B6.129 → C3H.SW, supplemental Figure 2B], CD19 Cre+ BM [B6.129 → C3H.SW, supplemental Figure 2C]). Similar results were observed after lethal MHC-mismatched BMT (H-2b → H-2k) for TCD CD11c-Cre+ (B6 → C3H.HeNCr, Figure 3A) and TCD CD19-Cre+ BM (B6.129 → C3H.HeNCr, Figure 3C). Interestingly, transplantation of TCD Lys Cre+ BM exacerbated DLI-induced GVHD lethality (B6.129 → C3H.HeNCr, Figure 3B), potentially reflecting modulation of suppressive BM-derived myeloid cells.24
Selective deletion of STAT1 in donor APCs does not prevent GVHD lethality, but global STAT1 deficiency expands pDCs. Lethally irradiated recipients received TCD MHC-mismatched BM from (A) CD11c Cre+ or Cre− littermates followed by 1 × 106 DLI from Cre− donors on day +21 (B6 → C3H.HeNCr), (B) Lys Cre+ or Cre− littermates followed by 1 × 106 DLI from Cre− donors on day +14 (B6.129 → C3H.HeNCr) or (C) CD19 Cre+ or Cre− littermates followed by 1 × 106 DLI from Cre− donors on day +14 (B6.129 → C3H.HeNCr). All groups were followed for survival. (D) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14 immune reconstitution of CD11b−CD11cintB220+ pDCs were enumerated by flow cytometry. (E) Comparison of the absolute numbers of pDCs from the BM and spleen of alloHSCT recipients by flow cytometry were calculated from total cell counts of each organ. (F) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (CD45.2+ 129 → CD45.1+ B6), and on day +14 donor chimerism of pDCs were enumerated by flow cytometry. (G) pDCs were isolated from CD19 Cre+/− × STAT1flox/flox, Lys Cre+/− × STAT1flox/flox, CD11c Cre+/− × STAT1flox/flox, and a Cre−/− × STAT1flox/flox control to assess for expression of STAT1 RNA (100 bp) by RT-PCR. N = 5 to 7 mice/group, data are representative from 1 of 2 similar experiments. *P < .05, **P < .01.
Selective deletion of STAT1 in donor APCs does not prevent GVHD lethality, but global STAT1 deficiency expands pDCs. Lethally irradiated recipients received TCD MHC-mismatched BM from (A) CD11c Cre+ or Cre− littermates followed by 1 × 106 DLI from Cre− donors on day +21 (B6 → C3H.HeNCr), (B) Lys Cre+ or Cre− littermates followed by 1 × 106 DLI from Cre− donors on day +14 (B6.129 → C3H.HeNCr) or (C) CD19 Cre+ or Cre− littermates followed by 1 × 106 DLI from Cre− donors on day +14 (B6.129 → C3H.HeNCr). All groups were followed for survival. (D) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14 immune reconstitution of CD11b−CD11cintB220+ pDCs were enumerated by flow cytometry. (E) Comparison of the absolute numbers of pDCs from the BM and spleen of alloHSCT recipients by flow cytometry were calculated from total cell counts of each organ. (F) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (CD45.2+ 129 → CD45.1+ B6), and on day +14 donor chimerism of pDCs were enumerated by flow cytometry. (G) pDCs were isolated from CD19 Cre+/− × STAT1flox/flox, Lys Cre+/− × STAT1flox/flox, CD11c Cre+/− × STAT1flox/flox, and a Cre−/− × STAT1flox/flox control to assess for expression of STAT1 RNA (100 bp) by RT-PCR. N = 5 to 7 mice/group, data are representative from 1 of 2 similar experiments. *P < .05, **P < .01.
Transplantation of STAT1−/− BM results in expansion of pDCs
We next looked at recipients of TCD whole STAT−/− BM to determine if immune reconstitution of a particular cell subset could indicate the specific STAT1-deficient donor cell mediating GVHD protection. At the time of DLI on day +14, there were no quantitative differences in total splenocytes, BM cells, DCs, NK cells, Gr-1+ cells, CD4+ cells, or CD8+ cells (supplemental Figure 3A-D). However, the percentage (Figure 3D) and absolute number (Figure 3E) of plasmacytoid DCs (pDCs) in the spleen and BM was significantly increased in recipients of TCD STAT1−/− BM. Chimerism of pDCs from either WT or STAT1−/− BM was equivalent and showed donor-derivation by Day +14, at the time of DLI (Figure 3F). Furthermore, isolated pDCs from all 3 of the cre recombinase STAT1flox/flox mice used as BM donors in Figure 3A-C expressed STAT1 (Figure 3G), suggesting that intermediate expression of CD11c in pDCs was not sufficient to delete STAT1 in STAT1flox/flox mice.
pDCs from recipients of STAT1−/− BM produce less α interferon and IL-12 during reconstitution and display a tolerogenic phenotype
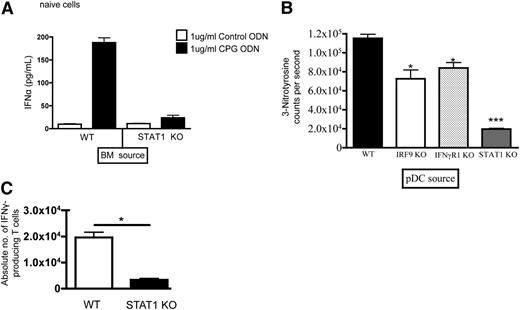
We hypothesized that pDCs reconstituted from STAT1−/− BM and unable to respond to interferons would be resistant to activation following alloHSCT. Indeed, the proportion of STAT1−/− pDCs producing IFNα, the predominant cytokine produced by activated pDCs, was reduced in the spleen on day +14 post-alloHSCT in recipients of STAT1−/− BM (Figure 4A-B). This was confirmed by reduced IFNα gene expression (Figure 4C) and by reduced ex-vivo IFNα production in sorted pDCs (Figure 4D) from BM following alloHSCT consistent with compartment-specific effects. Interestingly, there was no difference in gene expression or IFNα production by splenic pDCs. Importantly, there was no reduction in IFNα production by pDCs isolated from the BM of naïve STAT1−/− mice, suggesting that the difference in pDCs reconstituted from STAT1−/− donors reflected altered response to interferons in the post-alloHSCT environment. Furthermore, we observed decreased IL-12 (Figure 4E) and increased IL-10 (Figure 4F) by STAT1−/− pDCs.
Transplantation of allogeneic TCD STAT1−/− BM expands CD9−Siglec Hhi pDCs in recipient spleens and BM that show poor IFNα and IL-12 production, but enhanced IL-10 production. (A) CpG stimulation of pDCs isolated from the BM or spleen of nontransplanted (naïve) STAT1+/+ and STAT1−/− mice were compared with pDCs isolated 14 days after lethally irradiated C3H.SW recipients were transplanted with TCD STAT1+/+ and STAT1−/− BM. All pDCs were analyzed by flow cytometry, by first gating on CD11b−CD11cint cells, and then by gating on B220+ and intracellular IFNα. A representative mouse is shown for each group. (B) The percentage of IFNα+ pDCs were multiplied by the splenic or BM count to calculate absolute number of cells. (C) pDCs isolated from each organ from nontransplanted (naïve) and alloHSCT recipients were also incubated with CpG and were analyzed for fold change compared with unstimulated controls for IFNα gene expression by quantitative RT-PCR, and (D) IFNα protein secretion by ELISA of cell supernatants from ex vivo cultures. In addition, pDCs isolated 14 days after alloHSCT were activated overnight ex vivo with CpG and were analyzed for (E) IL-12 gene expression and (F) IL-10 production by ELISA. (G) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14 pDCs from BM and spleen were analyzed for expression of CD9 and Siglec H, then the ratio of CD9−Siglec Hhi (tolerogenic) to CD9+Siglec Hlo (stimulatory) pDCs was calculated in each organ. N = 5 to 6 mice/group, data are representative from 1 of 2 similar experiments. *P < .05.
Transplantation of allogeneic TCD STAT1−/− BM expands CD9−Siglec Hhi pDCs in recipient spleens and BM that show poor IFNα and IL-12 production, but enhanced IL-10 production. (A) CpG stimulation of pDCs isolated from the BM or spleen of nontransplanted (naïve) STAT1+/+ and STAT1−/− mice were compared with pDCs isolated 14 days after lethally irradiated C3H.SW recipients were transplanted with TCD STAT1+/+ and STAT1−/− BM. All pDCs were analyzed by flow cytometry, by first gating on CD11b−CD11cint cells, and then by gating on B220+ and intracellular IFNα. A representative mouse is shown for each group. (B) The percentage of IFNα+ pDCs were multiplied by the splenic or BM count to calculate absolute number of cells. (C) pDCs isolated from each organ from nontransplanted (naïve) and alloHSCT recipients were also incubated with CpG and were analyzed for fold change compared with unstimulated controls for IFNα gene expression by quantitative RT-PCR, and (D) IFNα protein secretion by ELISA of cell supernatants from ex vivo cultures. In addition, pDCs isolated 14 days after alloHSCT were activated overnight ex vivo with CpG and were analyzed for (E) IL-12 gene expression and (F) IL-10 production by ELISA. (G) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14 pDCs from BM and spleen were analyzed for expression of CD9 and Siglec H, then the ratio of CD9−Siglec Hhi (tolerogenic) to CD9+Siglec Hlo (stimulatory) pDCs was calculated in each organ. N = 5 to 6 mice/group, data are representative from 1 of 2 similar experiments. *P < .05.
Data suggest that pDCs can be subclassified into stimulatory or tolerogenic populations based on CD9 and Siglec H expression.25 Indeed, we found an increased ratio of tolerogenic (CD9−Siglec Hhi) to stimulatory (CD9+Siglec Hlo) pDCs in the BM of STAT1−/− BM recipients (Figure 4G and supplemental Figure 4A). Taken together, these results demonstrate that increased pDCs in the BM STAT1−/− BM recipients reflect a specific expansion of a CD9−SiglecHhi pDC subset with reduced IFNα and IL-12-production and increased IL-10 production.
pDCs reconstituted from STAT1−/− donors generate reduced free radicals and produce less IFNα on CpG-ODN stimulation
Because the reduced IFNα production and tolerogenic phenotype could be a secondary phenomenon related to reduced inflammatory cytokines from decreased GVHD, we next assessed the functional capacity of pDCs recovered from untransplanted STAT1+/+ vs STAT1−/− mice in vitro. Bacterial-derived CpG-rich oligonucleotides have been shown to activate potently pDCs via TLR9. As shown in Figure 5A, the amount of IFNα produced by CpG ODN-stimulated pDCs recovered from STAT1−/− mice was markedly reduced compared with pDCs from STAT1+/+ mice. GVHD causes substantial inflammation that activates DCs, leading to free-radical production that potentially contributes to DC function specifically downstream of STAT1.21 Thus, we next measured peroxynitrite, as measured by levels of 3-nitrotyrosine, in cell supernatants from expanded pDCs generated from TCD WT STAT1+/+, IRF9−/−, IFNγR1−/−, and STAT1−/− BM. Although pDCs expanded from IRF9−/− BM or IFNγR1−/− BM produced decreased levels of nitrotyrosine compared with STAT1+/+ BM, pDCs expanded from STAT1−/− BM produced the least amount of nitrotyrosine, suggesting some interference with free-radical generation and, potentially, GVHD-induced tissue injury (Figure 5B).26 We next assessed the capacity of STAT1−/− pDCs recovered post-alloHSCT to stimulate T cells in vitro. Indeed, pulsed pDCs isolated from STAT1−/− BM recipients pulsed with Db126, the immunodominant peptide derived from WT-1, induced less IFNγ production by WT-1–specific T cells when compared with those stimulated by pDCs from STAT1+/+ BM recipients (Figure 5C). Interestingly, there were no differences in proliferation by TCR Tg T cells (supplemental Figure 4B).
STAT1−/− mice have BM-derived pDCs that produce low levels of IFNα and nitrogen free radicals, which decrease activation of antigen-specific T cells after alloHSCT. (A) pDCs were isolated from the BM of nontransplanted (naïve) STAT1+/+ and STAT1−/− mice, stimulated with control ODN vs CpG ODN and analyzed for IFNα production by ELISA. (B) Splenic pDCs were isolated from irradiated recipients of WT, IRF9−/−, IFNγR1−/−, or STAT1−/− BM on day +14, and then were analyzed for 3-nitrotyrosine production in culture ex vivo. (C) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14 pDCs were isolated from BM, and were pulsed with WT-1 peptide, and then were activated by CpG. T cells from WT-1 TCR transgenic mice were cocultured with the activated, WT-1 expressing pDCs and intracellular IFNγ expression was enumerated by flow cytometry. N = 5 mice/group, data are representative from 1 of 2 similar experiments. *P < .05, ***P < .001.
STAT1−/− mice have BM-derived pDCs that produce low levels of IFNα and nitrogen free radicals, which decrease activation of antigen-specific T cells after alloHSCT. (A) pDCs were isolated from the BM of nontransplanted (naïve) STAT1+/+ and STAT1−/− mice, stimulated with control ODN vs CpG ODN and analyzed for IFNα production by ELISA. (B) Splenic pDCs were isolated from irradiated recipients of WT, IRF9−/−, IFNγR1−/−, or STAT1−/− BM on day +14, and then were analyzed for 3-nitrotyrosine production in culture ex vivo. (C) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14 pDCs were isolated from BM, and were pulsed with WT-1 peptide, and then were activated by CpG. T cells from WT-1 TCR transgenic mice were cocultured with the activated, WT-1 expressing pDCs and intracellular IFNγ expression was enumerated by flow cytometry. N = 5 mice/group, data are representative from 1 of 2 similar experiments. *P < .05, ***P < .001.
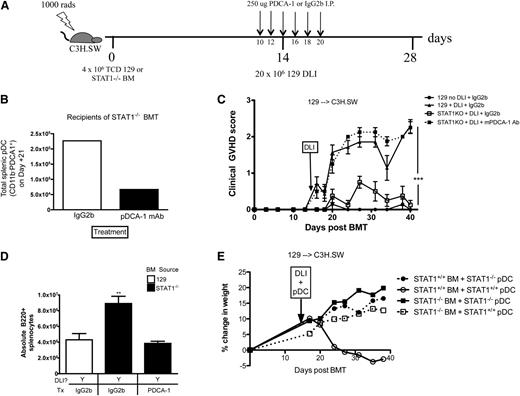
pDCs are required for GVHD protection in recipient of STAT1−/− BM
We next established whether the expanded pDC population we identified in recipients of STAT1−/− BM was necessary for GVHD prevention. After MHC-matched, miHA-mismatched TCD alloHSCT (129 → C3H.SW), recipients were treated with a moAb against PDCA-1 to deplete pDCs during the period around DLI (Figure 6A) resulting in a decrease in splenic pDCs (Figure 6B). Clinical GVHD scores were significantly increased in recipients of STAT1−/− BM after pDC depletion (Figure 6C), with a decrease in B220+ cells in the spleen (Figure 6D) demonstrating that pDCs are critical for full GVHD protection mediated by STAT1−/− BM grafts. In fact, we observed that adoptive transfer of isolated STAT1−/− pDCs to alloHSCT recipients of STAT1+/+ BM prevents GVHD. In contrast, adoptive transfer of STAT1+/+ pDCs to alloHSCT recipients of STAT1−/− BM does not exacerbate GVHD, implying the GVHD-mediated protection by STAT1−/− pDCs is a dominant effect (Figure 6E).
PDC depletion around the time of DLI exacerbates GVHD after alloHSCT of TCD STAT1−/− BM. (A) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14, 20 × 106 DLI was administered to induce GVHD. Some recipients did not receive a DLI as a negative GVHD control. All groups were treated with either IgG2b or PDCA-1 moAb on day +12, 14, 16, 18, and 20 to deplete pDCs. (B) Some recipients were sacrificed on day +21 for enumeration of pDCs in the spleen. Treatment with PDCA moAb depleted about 71% of pDCs in the spleen as determined by flow cytometry. (C) All alloHSCT recipients were followed for clinical GVHD scores. (D) On day +40, splenic B220+ counts were enumerated by flow cytometry. (E) Lethally irradiated recipients were transplanted with STAT1+/+ or STAT1−/− BM on Day +0, then on day +14 they were given a DLI with concurrent 5 × 105 STAT1+/+ pDCs or STAT1−/− pDCs and were followed for GVHD-associated weight loss. N = 5 to 8 mice/group, data are representative from 1 of 2 similar experiments. **P < .01, ***P < .001.
PDC depletion around the time of DLI exacerbates GVHD after alloHSCT of TCD STAT1−/− BM. (A) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → C3H.SW), and on day +14, 20 × 106 DLI was administered to induce GVHD. Some recipients did not receive a DLI as a negative GVHD control. All groups were treated with either IgG2b or PDCA-1 moAb on day +12, 14, 16, 18, and 20 to deplete pDCs. (B) Some recipients were sacrificed on day +21 for enumeration of pDCs in the spleen. Treatment with PDCA moAb depleted about 71% of pDCs in the spleen as determined by flow cytometry. (C) All alloHSCT recipients were followed for clinical GVHD scores. (D) On day +40, splenic B220+ counts were enumerated by flow cytometry. (E) Lethally irradiated recipients were transplanted with STAT1+/+ or STAT1−/− BM on Day +0, then on day +14 they were given a DLI with concurrent 5 × 105 STAT1+/+ pDCs or STAT1−/− pDCs and were followed for GVHD-associated weight loss. N = 5 to 8 mice/group, data are representative from 1 of 2 similar experiments. **P < .01, ***P < .001.
pDCs recovered from recipients of STAT1−/− BM express S100A8, S100A9, and STAT3
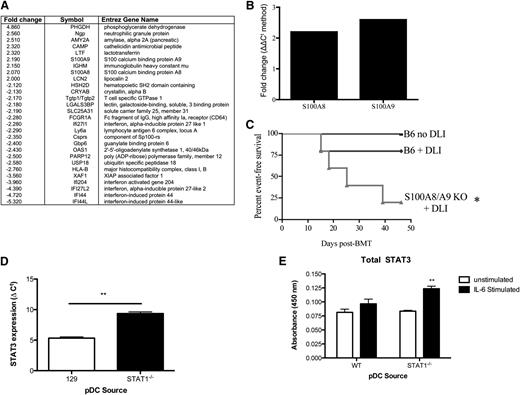
The number of interferon-stimulated genes activated by STAT1 is estimated to be between 600 and 2000.27 Thus, to begin to identify relevant downstream targets of STAT1 involved in GVHD protection, we isolated pDCs generated from TCD STAT1+/+ and STAT1−/− BM alloHSCT recipients and performed microarray analysis on cDNA prepared from these cells. The data discussed in this publication have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus and are accessible through Gene Expression Omnibus Series accession number: GSE59731. Ingenuity Pathway Analysis identified a twofold increase in the expression of S100A8 and S100A9 (Figure 7A), which are molecules previously associated with myeloid-derived suppressor cells.28 Using quantitative RT-PCR, we verified a 2- to 2.5-fold increased gene expression of S100A8/A9 in pDCs generated from recipients of TCD STAT1−/−BM as compared with recipients of TCD STAT1+/+ BM (Figure 7B). Correspondingly, TCD S100A9−/− BM exacerbated GVHD-free survival after DLI (Figure 7C), with worse clinical scores at the time of takedown (supplemental Figure 4C). Finally, because induction of these molecules has been associated with STAT3,28 a transcription factor important for immune tolerance,29 we wanted to determine whether the absence of STAT1 led to unopposed STAT3 levels in pDCs. Indeed, isolated pDCs from the BM, spleen, and lymph nodes of chimeras generated from TCD STAT1−/− BM demonstrated increased total STAT3 gene expression (Figure 7D) and STAT3 protein from pDC lysates (Figure 7E).
PDCs generated after alloHSCT with TCD STAT1−/− BM have features of suppressor cells. (A) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → B6 × C3H.SW), and on day +14 pDCs were isolated from spleen by magnetic cell selection and were pooled together from 12 recipients/group. cDNA was generated and hybridized to an Affymetrix genechip mouse genome 430 2.0 array. Samples were normalized by RMA algorithm to a log2 intensity value and were analyzed by Ingenuity Pathway Analysis v8.5 software. Genes that showed at least twofold increased or decreased expression are shown after analysis using the Benjamini-Hochberg method of multiple testing correction, P < .01. Microarray was performed once from pDCs pooled from 12 mice/group. (B) Splenic pDCs from recipients of TCD STAT1−/− BM were isolated by negative selection using Miltenyi microbeads, and total RNA was isolated. cDNA was reverse transcribed using an Invitrogen RT-PCR kit. Quantitative RT-PCR was performed using TaqMan probes designed by Applied Biosystems for S100A8 and S100A9. Samples were normalized to a GAPDH housekeeping gene, and expression of S100A8 or S100A9 by STAT1−/− pDCs was compared with STAT1+/+ pDCs. Fold change was calculated from triplicates using the 2|ΔΔCt| method. (C) Lethally irradiated recipients received TCD miHA-mismatched BM from S100A9+/+ or S100A9−/− donors (B6 → C3H.SW), and on day +14 some groups were treated with 20 × 106 DLI to induce GVHD. Some recipients did not receive a DLI as a negative GVHD control. Mice who developed clinical GVHD scores >0 were scored as an event. (D) pDCs pooled from the spleen and BM of recipients of TCD STAT1+/+ (WT) or STAT1−/− BM were isolated and activated with IL-6, then assessed for STAT3 expression by quantitative RT-PCR and (E) ELISA. N = 3 to 7 mice/group, data are representative from 1 of 2 similar experiments. * = P < .05, ** = P < .01.
PDCs generated after alloHSCT with TCD STAT1−/− BM have features of suppressor cells. (A) Lethally irradiated recipients received TCD miHA-mismatched BM from STAT1+/+ or STAT1−/− donors (129 → B6 × C3H.SW), and on day +14 pDCs were isolated from spleen by magnetic cell selection and were pooled together from 12 recipients/group. cDNA was generated and hybridized to an Affymetrix genechip mouse genome 430 2.0 array. Samples were normalized by RMA algorithm to a log2 intensity value and were analyzed by Ingenuity Pathway Analysis v8.5 software. Genes that showed at least twofold increased or decreased expression are shown after analysis using the Benjamini-Hochberg method of multiple testing correction, P < .01. Microarray was performed once from pDCs pooled from 12 mice/group. (B) Splenic pDCs from recipients of TCD STAT1−/− BM were isolated by negative selection using Miltenyi microbeads, and total RNA was isolated. cDNA was reverse transcribed using an Invitrogen RT-PCR kit. Quantitative RT-PCR was performed using TaqMan probes designed by Applied Biosystems for S100A8 and S100A9. Samples were normalized to a GAPDH housekeeping gene, and expression of S100A8 or S100A9 by STAT1−/− pDCs was compared with STAT1+/+ pDCs. Fold change was calculated from triplicates using the 2|ΔΔCt| method. (C) Lethally irradiated recipients received TCD miHA-mismatched BM from S100A9+/+ or S100A9−/− donors (B6 → C3H.SW), and on day +14 some groups were treated with 20 × 106 DLI to induce GVHD. Some recipients did not receive a DLI as a negative GVHD control. Mice who developed clinical GVHD scores >0 were scored as an event. (D) pDCs pooled from the spleen and BM of recipients of TCD STAT1+/+ (WT) or STAT1−/− BM were isolated and activated with IL-6, then assessed for STAT3 expression by quantitative RT-PCR and (E) ELISA. N = 3 to 7 mice/group, data are representative from 1 of 2 similar experiments. * = P < .05, ** = P < .01.
Discussion
Preventative GVHD strategies that preserve T-cell function and immunocompetence are appealing for protection against relapse and infections, but they remain elusive. Using MHC-matched, miHA-mismatched alloHSCT models, we found that non-T cells reconstituted from a STAT1-deficient allograft prevent GVHD induced by delayed add-back of high doses of fully competent T cells as a DLI that can respond to DC vaccination resulting in enhanced tumor protection. Furthermore, we demonstrate that pharmacologic off-target inhibition of STAT1 using Exenatide22,23 can also protect against GVHD. This is consistent with previous observations that inhibition of STAT1 can abrogate inflammation in models of solid organ transplantation.30,31 Although targeting of STAT1 has been tested in models of GVHD, these studies relied on the manipulation of STAT1 in donor lymphocytes.12 Although effective in preventing GVHD, targeting STAT1 solely in donor T cells may unintentionally impair overall immunity because deficiency of type I7 and type II6 interferon signaling in donor T cells attenuates their ability to cause GVT. Based on our earlier observation that TCD donor BM deficient in IFNγR1 could prevent GVHD, we took a novel approach by targeting STAT1 in donor non-T cells. Using this model, we identified an expanded STAT1-deficient pDC population that was activation deficient and was required for GVHD protection.
Previous studies have shown that treatment with suberonylanilide hydroxamic acid, a histone deacetylation inhibitor, after MHC-mismatched alloHSCT led to decreased GVHD mediated by inhibition of STAT1.32 One possible strategy to translate our findings is based on the effectiveness of the restricted timing of pharmacologic STAT1 inhibition as demonstrated in the Exenatide experiments. Using our approach, the patient would only need to be treated transiently until DLI, and it is hoped that this approach would preclude the long-term risks immunosuppression. In addition to suberonylanilide hydroxamic acid and Exenatide, the use of JAK1 inhibitors8,33 and/or the development of rationale STAT1 inhibitors such as H2-234 may also be promising in GVHD.
pDCs have been shown to mediate tolerance to cardiac allografts,35 and they contribute to oral tolerance.36 Whether pDCs exacerbate or diminish GVHD after alloHSCT has been controversial in preclinical models. Alloantigen expression on pDCs alone is sufficient to prime alloreactive T cells and to cause GVHD when activated by total body irradiation.37 However, depletion of pDCs from BM grafts accelerates GVHD mortality, and GVHD prevents maturation of pDCs leading to generation of a suppressive CD11clo120G8+ precursor pDC.38 Transplanting donor pre-pDCs, or adding pDCs to donor BM grafts, can also attenuate GVHD and augment GVT.39,40 Furthermore, CCR9+ pDCs can induce Tregs and can inhibit antigen-specific T-cell responses, suppressing GVHD.41 However, depletion of host-derived pDCs is not sufficient to prevent GVHD.42 Interestingly in humans, decreased levels of pDCs in the circulation have been associated with an increased incidence of acute and chronic GVHD43,44 ; children without GVHD have high absolute numbers of pDCs,45 and absence of GVHD has been associated with improved pDC recovery and improved overall survival.46 Collectively, these studies suggest that pDCs, depending on whether they are donor or host-derived, may be able to modulate GVHD, but they can also be activated in the posttransplant setting to support alloreactivity.
Our studies provide a novel strategy potentially to generate a tolerogenic pDC by targeted manipulation of STAT1 in the context of alloHSCT. We demonstrate that this results in expansion of immature CD9−SiglecHhi STAT1−/− pDCs in the BM and supports the fact that STAT1 is required for DC maturation.47 It is possible that pDC expansion is also explained, in part, by the fact that the background strain was 129, which has a higher frequency of pDCs.48 However, given that STAT1−/− mice have normal homeostatic levels of pDCs, but an inability to expand pDC numbers above baseline via IFNα,48 our observations that pDCs are expanded after alloHSCT suggests that other factors present in the post-alloHSCT are responsible. CD9−SiglecHhi pDCs have been previously described to be tolerogenic.25 Indeed, we found that reconstituting STAT1−/− pDCs were poor producers of peroxynitrite, IFNα, and IL-12, but were increased producers of IL-10. Interestingly, STAT1−/− macrophages are also poor producers of superoxide-free radicals.49
We also found that pDCs reconstituted from STAT1-deficient BM also expressed increased levels of S100A8/A9 as well as STAT3. Although expression of S100A8/A9 has been associated with the suppressive activity of myeloid cells,28 our observations suggest that S100A8/A9 may also be important in tolerogenic pDCs. Indeed, we found that absence of these molecules from donor BM exacerbates GVHD. STAT1 and STAT3 have been described to oppose one another in other models and through a number of mechanisms such as suppressors of cytokine signaling.50,51 Furthermore, increased expression of STAT3 has been associated with immune tolerance.29 Thus, it is interesting that we observed increased STAT3 in pDCs derived from a STAT1-deficient allograft. Additional studies will be necessary to confirm whether the increased STAT3 is required for GVHD protection. Finally, we observed that recipients of STAT1−/− BM showed an expansion of Tregs, and we hypothesized that the pDCs are driving Treg generation. Expansion of activated human pDCs has been previously associated with Treg generation in vitro,52 and further studies should demonstrate whether the Tregs are required for the prevention of GVHD in this model.
In conclusion, inhibition of STAT1 in donor allografts may be a viable approach for protecting patients from GVHD and may provide a platform for infusing high doses of T cells as a delayed DLI. Importantly, we show that STAT1 inhibition is only needed for a short period after engraftment until T-cell infusion, potentially providing opportunities for restricted timing of STAT1 inhibitors and preventing long-term immunosuppression. Indeed, intact vaccine-mediated tumor protection observed in our studies suggests that STAT1 inhibition may allow for the incorporation of emerging immunotherapeutic approaches that are being developed outside of the allogeneic setting.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Crystal Mackall for her valuable input and John Buckley for his technical expertise.
This work was supported by grants from the National Institutes of Health/NCI K08 CA174750, Hyundai Hope on Wheels, the Midwest Athletes Against Childhood Cancer Fund, Lisa’s Heart Kids Cancer Research Fund (C.M.C.), the Children’s Cancer Foundation (T.J.F.), and the Intramural Research Program at the National Institutes of Health (J.K., L.H., T.J.F.).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authorship
Contribution: C.M.C. and N.M.N. designed and performed research; collected, analyzed, and interpreted data; performed statistical analysis; and drafted the manuscript; C.D.C. performed research and collected, analyzed, and interpreted data; S.M.L. and H.Q. performed research; collected, analyzed, and interpreted data; and performed statistical analysis; P.J.K. and L.H. provided STAT1flox/flox mice, assisted with breeding and genotyping of mice, and revised the manuscript; Y.K.S. and J.K. performed microarray, analyzed and interpreted data, and revised the manuscript; T.J.F. designed and supervised research, analyzed and interpreted data, performed statistical analysis, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian M. Capitini, University of Wisconsin, 1111 Highland Ave, WIMR 4137, Madison, WI 53705; e-mail: ccapitini@pediatrics.wisc.edu.
References
Author notes
C.M.C. and N.M.N. contributed equally to this study.

![Figure 1. TCD STAT1−/− BM prevents DLI-induced GVHD. All recipients received a miHA-mismatched alloHSCT from either STAT1+/+ or STAT1−/− BM (129 → C3H.SW). Lethally irradiated C3H.SW recipients were transplanted with 4 × 106 TCD STAT1+/+ BM or STAT1−/− BM on day +0. On day +14, 20 × 106 DLI was administered to induce GVHD. Some recipients did not receive a DLI as a negative GVHD control. All mice were followed for (A) clinical GVHD scores. On day +28, all groups were euthanized and immune reconstitution of (B) B cells and (C) Tregs in the spleen was ascertained by flow cytometry. (D) Lethally irradiated C3H.SW recipients were transplanted with 4 × 106 STAT1−/− BM on day +0. On day +14, 0, 10, 20, or 40 × 106 DLI was administered to induce GVHD. Mice were followed for GVHD-associated weight loss. On Day +40, weight loss was compared, and (E) Treg expansion in the spleen was ascertained by flow cytometry. (F) Lethally irradiated recipients received a miHA-mismatched alloHSCT from STAT1+/+ BM (B6 → C3H.SW) treated with vehicle (phosphate-buffered saline [PBS]) or Exenatide (Ex4) for 3 days before alloHSCT. BM recipients continued to receive vehicle or Exenatide for 10 days after alloHSCT. 20 × 106 DLI was administered on day +14 to induce GVHD, and recipients were followed for clinical GVHD scores, and (G) % change in weight was compared at day +40. N = 3 to 7 mice/group, data are representative from 1 of 2 similar experiments. *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/12/10.1182_blood-2013-05-500876/4/m_1976f1.jpeg?Expires=1769207318&Signature=Se9wdTddPjbKBCCju-nlWJ-a41OKgd9PUMPxwo7~GOToi892-MinBf5eSpoV3MBEnRbrRxWy5E~xdR~6VyfjmbMkdRQprK781S4MDQia0v-~mHFMTDOoYteJX2rv8kdsRRqz9bm9JfLxx~NJrOPBJ3xCaVuNSQpkE8PBhT2xy5kOI2B8Xv7lMf8HlKSvlhiC3-7S1KJDgrzXdQKHhD4VeMGao4NKItBcYAD~Ilza~k6-Xa-Dw8s9OF6spSVox1BomFpk5ppm4WgP7JjN~OHlHlwMs9CEc3MAtnf6KozJWnnUfWHM1vSYGJ1c~lKhr8yDKAuwx~2Wu0hmpRCQyaFFAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal