Key Points
The prophylactic efficacy of posttransplantation cyclophosphamide (PTCy) against GVHD is dependent on donor CD4+ Foxp3+ Tregs.
PTCy treatment was associated with recovery of epigenetically stable and suppressive donor thymus–derived Tregs in secondary lymphoid organs.
Abstract
Posttransplantation cyclophosphamide (PTCy) is an effective prophylaxis against graft-versus-host disease (GVHD). However, it is unknown whether PTCy works singularly by eliminating alloreactive T cells via DNA alkylation or also by restoring the conventional (Tcon)/regulatory (Treg) T-cell balance. We studied the role of Tregs in PTCy-mediated GVHD prophylaxis in murine models of allogeneic blood or marrow transplantation (alloBMT). In 2 distinct MHC-matched alloBMT models, infusing Treg-depleted allografts abrogated the GVHD-prophylactic activity of PTCy. Using allografts in which Foxp3+ Tregs could be selectively depleted in vivo, either pre- or post-PTCy ablation of donor thymus–derived Tregs (tTregs) abolished PTCy protection against GVHD. PTCy treatment was associated with relative preservation of donor Tregs. Experiments using combinations of Foxp3– Tcons and Foxp3+ Tregs sorted from different Foxp3 reporter mice indicated that donor Treg persistence after PTCy treatment was predominantly caused by survival of functional tTregs that retained Treg-specific demethylation and also induction of peripherally derived Tregs. Finally, adoptive transfer of tTregs retrieved from PTCy-treated chimeras rescued PTCy-treated, Treg-depleted recipients from lethal GVHD. Our findings indicate that PTCy-mediated protection against GVHD is not singularly dependent on depletion of donor alloreactive T cells but also requires rapidly recovering donor Tregs to initiate and maintain alloimmune regulation.
Introduction
Allogeneic blood or bone marrow transplantation (alloBMT) is a life-saving intervention for many malignant and nonmalignant hematologic diseases.1,2 The therapeutic benefit of alloBMT is offset by graft-versus-host disease (GVHD), which often requires prolonged immunosuppressive prophylaxis. To reduce the incidence and severity of GVHD and shorten the duration of posttransplant immunosuppression, and based on antecedent studies in mouse models,3 posttransplantation cyclophosphamide (PTCy) was developed as a novel GVHD prophylaxis after human allografting.4 In the clinic, PTCy facilitates engraftment with a low incidence of severe GVHD after partially HLA-mismatched alloBMT and, as a single-agent administered for only 2 days, also prevents GVHD after HLA-matched alloBMT.5,6 The cellular mechanisms by which PTCy prevents GVHD remain unclear, particularly whether PTCy works singularly by eliminating alloreactive T cells or whether PTCy has other immunoregulatory effects contributing to its clinical efficacy. Additional insight into these mechanisms would allow the refinement of this novel approach clinically and the optimized integration of other immunosuppressants along with PTCy in the HLA-mismatched setting.4,7
T regulatory cells (Tregs) play an important role in the induction and maintenance of immunologic tolerance.8,9 Activation of CD4+Foxp3+ Tregs is one of the earliest events during the initial phase of an immune response.10 The appropriate balance between Tregs and effector T cells is crucial for the maintenance of self-tolerance and of functional immune responses in vivo. In murine alloBMT models, depletion of CD25+ T cells from donor inocula increases GVHD severity, whereas co-administration of CD25+ T cells at a higher ratio protects recipient mice from alloimmune injury caused by lethal doses of conventional T cells (Tcons).11-13 There are at least 2 different Treg subsets characterized by their expression of the master regulatory transcription factor Foxp3,14 namely thymically-derived natural Tregs (tTregs) and those that are situationally induced in the periphery from Tcons (pTregs).15 Furthermore, recent studies suggest that Foxp3 expression alone is insufficient in the generation, maintenance, and function of Tregs and needs to be complemented by Treg-specific epigenetic changes.15 However, the role that Tregs play in promoting immunologic tolerance in alloBMT using PTCy as GVHD prophylaxis is not well understood.
To address these principal outstanding issues, we sought to determine whether Tregs were required for modulation of alloreactivity by PTCy in clinically relevant mouse models of GVHD. Using donor transgenic strains enabling selective ablation of CD4+Foxp3+ T cells and tracking of Foxp3+ Tregs by fluorescent reporters, we demonstrate that preexisting donor tTregs are indispensable for the GVHD prophylactic efficacy of PTCy in MHC-matched models of alloBMT in which both CD4+ and CD8+ T cells are included in the donor inocula. We also show that PTCy treatment promotes rapid reconstitution of donor-derived tTregs without affecting their epigenetic profile or functionality.
Methods
In all experiments, gender-matched mice of 8 to 12 weeks of age were used. BALB.B (H-2Kb), C57BL/6 (H-2Kb; CD45.2+), C3H.SW (H-2Kb/SnJ), and C57BL/6-Foxp3tm1Flv/J (C57BL/6.Foxp3RFP; CD45.2+ reporter)16 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6-Ly5.2/Cr (H-2Kb; CD45.1+) mice were purchased from the National Cancer Institute (Frederick, MD). C57BL/6.Foxp3GFP and C57BL/6.Foxp3DTR mice were gifts from Alexander Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY).17,18 C57BL/6.luc+ (CD45.1+) mice were provided by Robert Negrin (Stanford University, Stanford, CA) and were intercrossed with C57BL/6.Foxp3DTR (CD45.2+) mice for >5 generations to generate C57BL/6.luc+.Foxp3DTR (CD45.2+) mice. All animals were housed in specific pathogen-free barrier facilities at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center or University of Miami following protocols approved by the respective Institutional Animal Care and Use Committees.
Bone marrow transplantation, GVHD monitoring, flow cytometry, sorting, in vitro suppression assays, in vivo bioluminescent imaging (BLI), histopathology and statistical analyses were performed as previously described19,20 and are detailed in the supplemental Methods (available on the Blood Web site). PTCy was administered IP in previously published doses,21-23 as described in the figure legends. The differences in the dosing and timing of PTCy in various transplantation models were dependent on the differential sensitivity of various mouse strains to cyclophosphamide, particularly when administered after myeloablative conditioning.
Results
Absence of donor Tregs abrogates PTCy activity
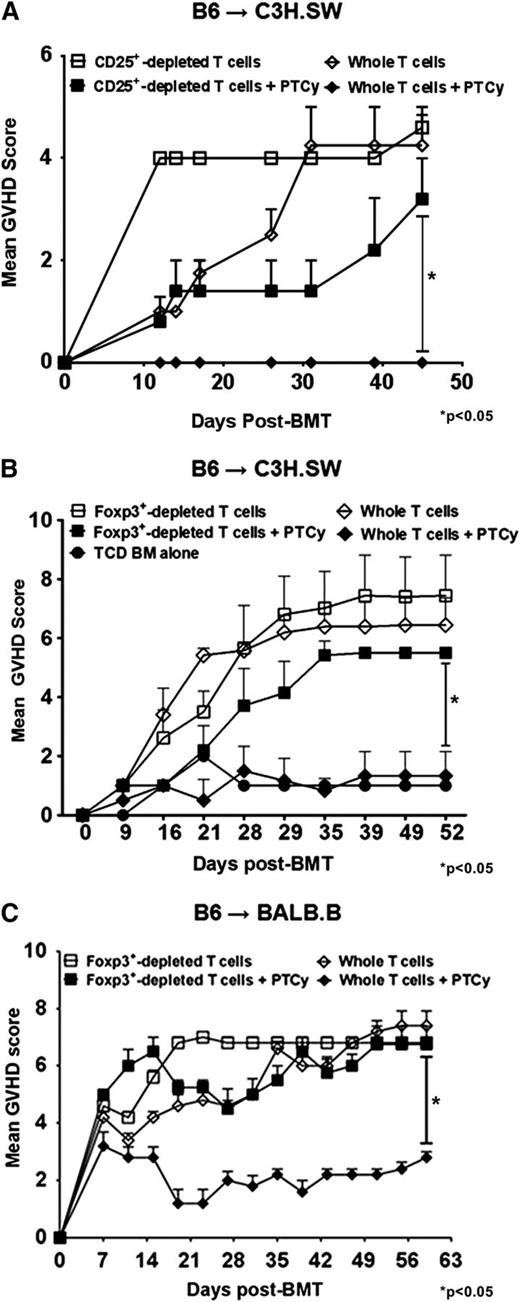
Based on murine data in skin allografting models,24 the dominant mechanism by which PTCy has been thought to mediate tolerance induction is through clonal deletion of alloreactive T cells stimulated early post-transplant. Indeed, when we utilized a widely used CD8+ T cell–dependent GVHD model (C3H.SW→B6)25,26 in which CD8+ T cells are the only infused T-cell subset, PTCy effectively ameliorated GVHD (supplemental Figure 1A). However, when a more physiologic allograft was given of either whole T cells (data not shown) or CD8+ T cells and CD4+CD25– T cells together, we were unable to induce potent GVHD in this model as assessed clinically (supplemental Figure 1B). These results are consistent with prior studies27,28 and occurred despite using the same total numbers of CD8+ T cells in the donor inocula when given with or without CD4+ T cells. Of note, in the reverse B6→C3H.SW model, lethal GVHD could only be induced using physiologic allografts wherein both CD4+ and CD8+ T cells were given (supplemental Figure 1C).
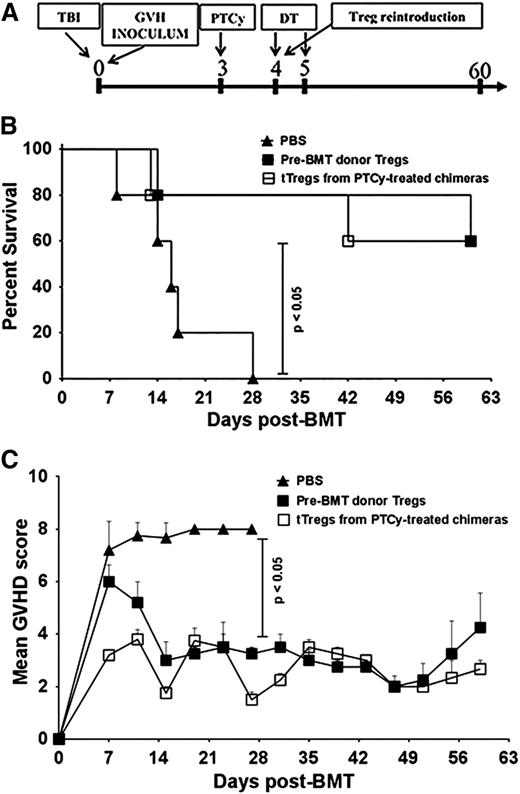
In further experiments using the B6→C3H.SW model, lethally irradiated C3H.SW recipients were infused with TCD-BM alone or TCD-BM supplemented with whole or CD4+CD25+-depleted donor T cells. Consistent with prior murine and human studies in MHC-matched allografting,6,21,22 PTCy protected mice receiving whole T-cell grafts against GVHD and led to excellent survival (Figure 1A-B). In contrast, mice administered grafts depleted of CD4+CD25+ T cells had markedly worse GVHD even when treated with PTCy (Figure 1A). These identical effects were also seen when using CD4+ Foxp3+–depleted donor T cells sorted from B6.Foxp3RFP donors (Figure 1B and supplemental Figure 2A).
Absence of Tregs in donor inocula abrogates the GVHD protective activity of PTCy. (A-B) C3H.SW recipients were lethally conditioned (1050 cGy TBI) before injection of T cell–depleted (TCD) B6 CD45.1+ bone marrow and GVH inocula from either B6 or B6.Foxp3RFP CD45.2+ donors. PTCy was administered on days +3 and +4 at a dose of 33 mg/kg intraperitoneally (IP) as previously described.21 All animals were monitored twice weekly for GVHD and daily for survival until day +60, when the experiments were terminated. After the death of the last mouse in a particular group, GVHD scores at that time were carried forward in the graph depictions until the end of the experiment. (A) Mean GVHD scores of B6→C3H.SW chimeras that received GVH inocula composed of 2.2 × 106 CD4+ and CD8+ T cells from B6 CD45.2+ donors with or without CD4+ CD25hi cells. (B) Mean GVHD scores of chimeras that received GVH inocula comprised of 2.2 × 106 B6 CD4+ and CD8+ T cells from Foxp3RFP donors, with or without CD4+ Foxp3+ T cells (depleted populations contained <1.0% RFP+ cells). (n = 5-7/group). (C) Mean GVHD scores of B6→BALB/B chimeras. Lethally irradiated (775 cGy) BALB.B mice were transplanted with 107 TCD BM cells from B6 CD45.1+ donors and 12 × 106 splenocytes and cutaneous lymph node (CLN) cells from B6.Foxp3GFP (CD45.2+) donors that were replete or depleted of Foxp3GFP+ T cells via sorting. PTCy (200 mg/kg IP) was administered on day +3 post-alloBMT. Mice were scored on day +7 post-alloBMT and every 4 days thereafter until the termination of the experiments at day +60. The data represent 2 independent experiments with a total of 10 animals per group.
Absence of Tregs in donor inocula abrogates the GVHD protective activity of PTCy. (A-B) C3H.SW recipients were lethally conditioned (1050 cGy TBI) before injection of T cell–depleted (TCD) B6 CD45.1+ bone marrow and GVH inocula from either B6 or B6.Foxp3RFP CD45.2+ donors. PTCy was administered on days +3 and +4 at a dose of 33 mg/kg intraperitoneally (IP) as previously described.21 All animals were monitored twice weekly for GVHD and daily for survival until day +60, when the experiments were terminated. After the death of the last mouse in a particular group, GVHD scores at that time were carried forward in the graph depictions until the end of the experiment. (A) Mean GVHD scores of B6→C3H.SW chimeras that received GVH inocula composed of 2.2 × 106 CD4+ and CD8+ T cells from B6 CD45.2+ donors with or without CD4+ CD25hi cells. (B) Mean GVHD scores of chimeras that received GVH inocula comprised of 2.2 × 106 B6 CD4+ and CD8+ T cells from Foxp3RFP donors, with or without CD4+ Foxp3+ T cells (depleted populations contained <1.0% RFP+ cells). (n = 5-7/group). (C) Mean GVHD scores of B6→BALB/B chimeras. Lethally irradiated (775 cGy) BALB.B mice were transplanted with 107 TCD BM cells from B6 CD45.1+ donors and 12 × 106 splenocytes and cutaneous lymph node (CLN) cells from B6.Foxp3GFP (CD45.2+) donors that were replete or depleted of Foxp3GFP+ T cells via sorting. PTCy (200 mg/kg IP) was administered on day +3 post-alloBMT. Mice were scored on day +7 post-alloBMT and every 4 days thereafter until the termination of the experiments at day +60. The data represent 2 independent experiments with a total of 10 animals per group.
In a second MHC-matched, minor MHC antigen–mismatched model (B6→BALB.B), lethally irradiated BALB.B recipients were transplanted with TCD-BM and donor T cells from B6.Foxp3GFP donors. Similar to that seen in the B6→C3H.SW model, PTCy was effective in preventing GVHD and maintaining survival in mice receiving whole T-cell grafts but not in mice receiving T-cell grafts selectively depleted of Foxp3+ cells (Figure 1C and supplemental Figure 2B). Notably, in both models, the GVHD scores of mice receiving Treg-depleted grafts before PTCy were slightly lower but statistically similar to mice receiving Treg-depleted grafts without PTCy. Overall, these results suggest a dependence on Tregs for the clinical efficacy of PTCy in these models. Based on similar effects observed in both models and the clinically relevant requirement for both hematopoietic and nonhematopoietic expression of miHAs for GVHD elicitation in the B6→BALB.B model,29 all subsequent experiments were performed using the B6→BALB.B strain combination.
Inducible depletion of donor Foxp3+ T cells before PTCy treatment accelerates GVHD
To further elucidate the contribution of donor Foxp3+ Tregs to PTCy’s clinical activity, we used as donors B6.Foxp3DTR mice that express the diphtheria toxin receptor (DTR) under the control of the Foxp3 promoter.18 In constructing chimeras (Figure 2A) to distinguish the origin of donor T cells, we relied on the differential expression of CD45 alleles. A near complete depletion (≥98%) of Foxp3+ cells was attained in the donor inocula by treating recipients with two intraperitoneal injections of diphtheria toxin (DT; 50 μg/kg) on days 0 and +1 post-alloBMT (data not shown). DT-treated recipients of donor inocula in which the Foxp3+ T cells were wild-type (Foxp3WT) showed similar GVHD clinical scores and survival as control chimeras receiving Foxp3DTR inocula but no DT (Figure 2B), confirming that DT specifically depleted Tregs and did not in itself exacerbate peritransplant toxicity. Similar to our prior results, B6.Foxp3DTR inocula recipients treated with PTCy remained alive and free of GVHD (Figure 2B). However, ablation of Foxp3+ T cells by DT resulted in clinically severe GVHD and increased mortality even when those mice were also administered PTCy (Figure 2B), again consistent with a dependence on the presence of Tregs for the clinical activity of PTCy.
In vivo ablation of donor Foxp3+ T cells abolishes PTCy protection against GVHD. (A) Experimental schema. BALB/B recipients after lethal conditioning (775 cGy) received 107 TCD BM (B6 CD45.1+) and GVH inocula of 12 × 106 splenocytes and CLN cells from Foxp3DTR or Foxp3WT B6 CD45.2+ donors. Designated groups received DT (50 ng/g IP) or PBS on days 0 and +1. PTCy (200 mg/kg IP) was administered to designated groups on day +3 post-alloBMT. All animals were monitored daily for survival and scored twice weekly for GVHD on day +7 post-alloBMT and every 4 days thereafter until day +60, when experiments were terminated. (B) Mean GVHD scores and survival. Combined results of 3 independent experiments are shown. (C) Frequency of cytokine-producing, inocula-derived CD4+CD45.2+ T cells in spleens of surviving chimeras on day +28. (D) Frequency (left panel) and total numbers (right panel) of CD4+CD45.2+Foxp3+ T cells in the spleens of surviving chimeras on day +28. Data are representative of 3 independent experiments, with a minimum of 4 animals per group. The results are presented as the mean ± standard error of the mean.
In vivo ablation of donor Foxp3+ T cells abolishes PTCy protection against GVHD. (A) Experimental schema. BALB/B recipients after lethal conditioning (775 cGy) received 107 TCD BM (B6 CD45.1+) and GVH inocula of 12 × 106 splenocytes and CLN cells from Foxp3DTR or Foxp3WT B6 CD45.2+ donors. Designated groups received DT (50 ng/g IP) or PBS on days 0 and +1. PTCy (200 mg/kg IP) was administered to designated groups on day +3 post-alloBMT. All animals were monitored daily for survival and scored twice weekly for GVHD on day +7 post-alloBMT and every 4 days thereafter until day +60, when experiments were terminated. (B) Mean GVHD scores and survival. Combined results of 3 independent experiments are shown. (C) Frequency of cytokine-producing, inocula-derived CD4+CD45.2+ T cells in spleens of surviving chimeras on day +28. (D) Frequency (left panel) and total numbers (right panel) of CD4+CD45.2+Foxp3+ T cells in the spleens of surviving chimeras on day +28. Data are representative of 3 independent experiments, with a minimum of 4 animals per group. The results are presented as the mean ± standard error of the mean.
PTCy treatment led to lower percentages of interferon-γ (IFNγ)- or IL-17A–producing CD4+ T cells and higher percentages and total numbers of Tregs compared with control mice when measured on day +28 post-BMT (Figure 2C-D), resulting in a low Teff/Treg ratio (supplemental Figure 3A). Interestingly, DT treatment before PTCy led to relatively high percentages of Tregs at day +28 post-BMT, but total numbers of these Tregs were much lower than was seen in mice receiving PTCy alone. Furthermore, mice treated with DT before PTCy had higher percentages of IFNγ-producing CD4+ T cells and a Teff/Treg ratio that was twice that seen in mice receiving PTCy in the absence of DT (Figure 2C-D and supplemental Figure 3A).
Histopathologic analysis of GVHD target tissues on days +7 to +14 post-BMT revealed high-grade tissue injury in control and DT-depleted B6.Foxp3DTR chimeras (supplemental Figure 3C). Importantly, recipients of Foxp3 wild-type inocula administered DT alone or DT followed by PTCy had baseline transaminase levels, demonstrating that liver injury was related to depletion of donor Tregs and not toxicity from DT itself (supplemental Figure 3D). Although mice receiving B6.Foxp3DTR inocula who were treated with DT before PTCy had higher colon and skin GVHD histopathologic scores than mice treated with PTCy alone, these differences were not significant (supplemental Figure 3C). Despite lower GVHD histopathologic scores in the liver, mice receiving B6.Foxp3DTR inocula and DT before PTCy also had significantly higher transminases consistent overall with higher multiorgan tissue injury than was seen after PTCy alone (supplemental Figure 3D). This intermediate phenotype of alloBMT recipient mice who were donor Treg–depleted before PTCy, which was manifested clinically (GVHD scores; Figures 1 and 2), phenotypically (Teff/Treg ratio, Figure 2 and supplemental Figure 3A) and histopathologically (supplemental Figure 3C), likely reflects the multifaceted impact of PTCy on various immune subsets (including depletion of alloreactive T cells) and also could indicate induction of pTregs in these donor tTreg–depleted mice.
PTCy promotes tTreg and pTreg persistence and expansion in lymphoid organs
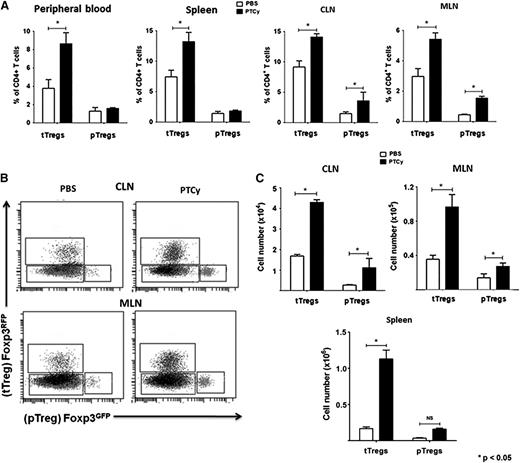
Because PTCy treatment was associated with an elevated frequency of donor CD4+Foxp3+ Tregs in lymphoid organs, even after donor Foxp3 ablation, it was important to determine the origin of Tregs persisting after PTCy, particularly because previous studies have suggested that de novo generation of pTregs during GVHD after alloBMT is negligible.30 Because tTregs and pTregs are indistinguishable by surface markers, we used donor T-cell inocula comprised of Tcons and Tregs sorted from 2 distinct B6 Foxp3 reporter strains to trace the origin of donor Tregs. Irradiated BALB.B mice were transplanted with donor T cells containing Foxp3GFP-depleted Tcons and Foxp3RFP tTregs or Foxp3RFP-depleted Tcons and Foxp3GFP tTregs (see supplemental Methods) and treated with either PTCy or phosphate-buffered saline (PBS) on day +3. On day +28 post-alloBMT, PTCy treatment was associated with expansion of tTregs in the peripheral blood, spleen, mesenteric lymph nodes (MLNs), and CLNs, as well as significant induction and expansion of pTregs in both MLNs and CLNs (Figure 3). However, tTregs were more abundant than pTregs in all organs in PTCy-treated chimeras. This induction of pTregs in PTCy-treated mice also may help explain the partial but ineffective GVHD protection seen with PTCy after DT treatment.
tTregs accumulate in all lymphoid organs of PTCy-treated chimeras, whereas generation of pTregs is most prominent in lymph nodes. Chimeras were constructed as in Figure 2, except that the GVH inocula contained 1.8 × 106 sorted CD4+ Foxp3GFP/RFP– cells (Tcons), supplemented with 2 × 105 CD4+Foxp3RFP/GFP+ (Tregs). Designated groups received PBS or PTCy (200 mg/kg IP) on day +3 after alloBMT. Animals were euthanized on day +28 and blood and organs processed and analyzed by multicolor flow cytometry. (A) Percentages of tTregs and pTregs in peripheral blood, spleen, CLNs, and mesenteric lymph nodes (MLNs). (B) Representative flow data plots gated on CD45.2+ CD3+CD4+ lymphocytes from CLNs and MLNs of a control and a PTCy-treated mouse depict proportions of Foxp3+ tTregs and pTregs. (C) Total counts of tTregs and pTregs in the CLNs, MLNs, and spleens of control and PTCy-treated chimeras. Data shown here represent 2 independent experiments with at least 10 animals per group.
tTregs accumulate in all lymphoid organs of PTCy-treated chimeras, whereas generation of pTregs is most prominent in lymph nodes. Chimeras were constructed as in Figure 2, except that the GVH inocula contained 1.8 × 106 sorted CD4+ Foxp3GFP/RFP– cells (Tcons), supplemented with 2 × 105 CD4+Foxp3RFP/GFP+ (Tregs). Designated groups received PBS or PTCy (200 mg/kg IP) on day +3 after alloBMT. Animals were euthanized on day +28 and blood and organs processed and analyzed by multicolor flow cytometry. (A) Percentages of tTregs and pTregs in peripheral blood, spleen, CLNs, and mesenteric lymph nodes (MLNs). (B) Representative flow data plots gated on CD45.2+ CD3+CD4+ lymphocytes from CLNs and MLNs of a control and a PTCy-treated mouse depict proportions of Foxp3+ tTregs and pTregs. (C) Total counts of tTregs and pTregs in the CLNs, MLNs, and spleens of control and PTCy-treated chimeras. Data shown here represent 2 independent experiments with at least 10 animals per group.
Because Vβ11 TCR+ CD4+ T cells have been implicated in GVHD pathogenesis, particularly with gastrointestinal involvement in the B6→BALB.B model,31,32 we analyzed Vβ11 expression in Tcons, tTregs, and pTregs from different lymphoid organs (Figure 4A). Percentages and total numbers of Vβ11-expressing Tcons were comparable between PBS- and PTCy-treated chimeras (data not shown). However, total numbers of both Vβ11+ tTregs and pTregs were higher in the MLNs and CLNs of PTCy-treated chimeras (data not shown), resulting in significantly lower Tcon/tTreg and Tcon/pTreg ratios in the MLNs and CLNs of PTCy recipients (Figure 4B). The increased Vβ11+ Tcon/tTreg ratio in PBS vs PTCy-treated chimeras was particularly prominent in the MLNs of control chimeras, consistent with the known gastrointestinal predilection of Vβ11+ Tcons (Figure 4B, left panel).
Higher frequency and ratios of Vβ11+ tTregs to Tcons in lymphoid organs of PTCy-treated chimeras. (A) Representative flow plots of Vβ11+ Tcons, tTregs, and pTregs from the MLNs of control and PTCy-treated chimeras. (B) Ratio of Vβ11+ Tcons to Vβ11+ tTregs (left panel) and pTregs (right panel) in spleens, CLNs, and MLNs of control and PTCy-treated chimeras. Data shown are representative of 2 independent experiments with at least 5 animals per group and were analyzed on day +28 post-alloBMT. *P < .05.
Higher frequency and ratios of Vβ11+ tTregs to Tcons in lymphoid organs of PTCy-treated chimeras. (A) Representative flow plots of Vβ11+ Tcons, tTregs, and pTregs from the MLNs of control and PTCy-treated chimeras. (B) Ratio of Vβ11+ Tcons to Vβ11+ tTregs (left panel) and pTregs (right panel) in spleens, CLNs, and MLNs of control and PTCy-treated chimeras. Data shown are representative of 2 independent experiments with at least 5 animals per group and were analyzed on day +28 post-alloBMT. *P < .05.
tTregs and pTregs retrieved from control and PTCy-treated chimeras differ in their suppressive capability and TSDR demethylation profile
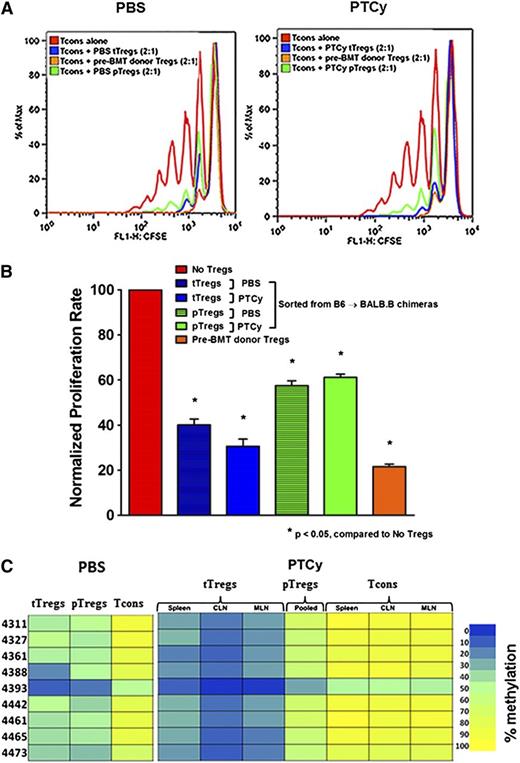
After confirming that PTCy promotes recovery of donor Tregs, we sought to evaluate their functional contribution to GVHD-specific protection. First, we investigated whether Tregs retrieved from PTCy-treated chimeras could suppress T-cell proliferation in vitro. We found that tTregs reisolated from PTCy-treated chimeras were as suppressive as tTregs from controls or pre-alloBMT Tregs from donors (Figure 5A-B). In contrast, pTregs retrieved from either PTCy-treated or control chimeras were only moderately suppressive (Figure 5A-B).
PTCy-treated chimeras have equally suppressive tTregs that are differentially demethylated compared with controls. (A-B) In vitro suppression. Sorted Foxp3– Tcons (CD4+ and CD8+; 1 × 105) were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured with irradiated CD3– splenocytes (1 × 105) plus anti-CD3 (1 μg/mL) alone or with titrating numbers of sorted Tregs (CD4+Foxp3RFP+) for 96 hours. CFSE dilution was measured by flow cytometry. The overlay depicts proliferation of Tcons alone or cocultured with posttransplant (day +28) pTregs, posttransplant (day +28) tTregs, or pretransplant Tregs in a 2:1 ratio. (C) Treg-specific demethylated region (TSDR) methylation profiles. CD4+ Foxp3– Tcons, pTregs, and tTregs of different lymphoid organs from PBS- (pooled from 3 organs) and PTCy-treated alloBMT recipients (day +28) were sorted and genomic DNA isolated for bisulfite sequencing and pyrosequencing of the TSDR. Residue numbers denote individual CpG motifs in reference to the transcription initiation site of Foxp3. Bar colors represent 0% to 100% methylated regions. Data are representative of 2 independent experiments with 3 to 5 chimeras per group.
PTCy-treated chimeras have equally suppressive tTregs that are differentially demethylated compared with controls. (A-B) In vitro suppression. Sorted Foxp3– Tcons (CD4+ and CD8+; 1 × 105) were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured with irradiated CD3– splenocytes (1 × 105) plus anti-CD3 (1 μg/mL) alone or with titrating numbers of sorted Tregs (CD4+Foxp3RFP+) for 96 hours. CFSE dilution was measured by flow cytometry. The overlay depicts proliferation of Tcons alone or cocultured with posttransplant (day +28) pTregs, posttransplant (day +28) tTregs, or pretransplant Tregs in a 2:1 ratio. (C) Treg-specific demethylated region (TSDR) methylation profiles. CD4+ Foxp3– Tcons, pTregs, and tTregs of different lymphoid organs from PBS- (pooled from 3 organs) and PTCy-treated alloBMT recipients (day +28) were sorted and genomic DNA isolated for bisulfite sequencing and pyrosequencing of the TSDR. Residue numbers denote individual CpG motifs in reference to the transcription initiation site of Foxp3. Bar colors represent 0% to 100% methylated regions. Data are representative of 2 independent experiments with 3 to 5 chimeras per group.
To evaluate the stability of the Treg phenotype, we performed epigenetic analysis of the Treg-specific demethylated region (TSDR) in Tcons, tTregs, and pTregs retrieved from control and PTCy-treated chimeras and compared them with CD4+Foxp3+ Tregs from untransplanted donors. The Foxp3 TSDR of tTregs is known to be highly demethylated, whereas Foxp3– Tcons and pTregs have methylated and partially methylated TSDRs, respectively.33 Analysis of steady-state tTregs isolated from donor mice (B6.Foxp3GFP or B6.Foxp3RFP) revealed 0% to 10% methylated TSDRs (supplemental Figure 4). Interestingly, organ-specific or pooled (data not shown) tTregs from PTCy-treated chimeras were minimally methylated (0%-20% methylation) compared with tTregs from PBS-treated controls (10%-50% methylation; Figure 5C). Notably, pooled pTregs from both PTCy recipients and controls were partially methylated (20%-70% methylation), whereas Tcons from either group were fully methylated (Figure 5C). Thus, the post-BMT inflammatory milieu culminating in GVHD appears to have a substantial impact on CpG demethylation stability in the TSDR locus, but PTCy treatment promotes persistence of demethylated tTregs. The observed differences in suppressive ability and epigenetic stability of induced pTregs may help explain the insufficient GVHD protection seen in mice treated with DT before PTCy despite the high circulating percentages of pTregs.
In vivo donor CD4+Foxp3+ T-cell ablation after PTCy treatment worsens GVHD
Because both the absence and inducible depletion of donor CD4+Foxp3+ T cells before PTCy administration abrogated its GVHD prophylactic ability, we next investigated whether donor Foxp3+ Treg ablation after PTCy would also have the same deleterious effects (Figure 6A). In addition, to quantify and visualize the impact of PTCy and DT treatment on GVH effector proliferation, we used B6.luc+.Foxp3DTR donors. These mice express firefly luciferase under the control of the constitutively expressed β-actin promoter,34 permitting noninvasive monitoring of donor non-Tregs after DT-mediated Treg depletion.
Donor CD4+Foxp3+ Treg depletion after PTCy treatment accelerates GVHD. (A) Experimental design. Chimeras were constructed as in Figure 2, except that the GVH inocula were obtained from B6.luc+.Foxp3DTR donors. PTCy (200 mg/kg IP) was administered on day +3 after alloBMT. DT (50 ng/g) or PBS was injected on days +4 and +5. Luciferase expression by luc+ cells in the GVH inocula was quantified in vivo by injection of luciferin and acquisition of bioluminescent signal. All animals were monitored daily for survival and scored twice weekly for GVHD until day +60, when the experiments were terminated. (B) GVHD scores (left panel) and survival (right panel) of chimeras (n = 10 per group) that were monitored until day +60 in 2 independent experiments. (C) Kinetics of BLI after PTCy-induced CD4+Foxp3+ donor Treg depletion. (Left panel) BLI imaging of luciferase-expressing cell distribution at serial time points after alloBMT. Color bars represent the signal intensity scale. ×, weak subject succumbed to anesthesia; †, subjects died before imaging time point. (Right panel) Expansion of luciferase-expressing T cells as quantified by total emitted photons per mouse and group at serial time points after alloBMT. Displayed are pooled results from 2 independent experiments (n = 10 per group), distinct from those shown in (B). On day +28 post-BMT, spleens and CLNs were harvested for flow cytometric analysis of intracellular cytokines and Foxp3 expression. (D) Percentages of cytokine-producing or Foxp3-expressing GVH inocula–derived (CD45.2+) CD4+ T cells. (E) Total numbers of CD4+CD45.2+Foxp3+ Tregs in the spleens and CLNs. Data shown are from day +28 survivors of 2 independent experiments (n = 10 [PTCy]; n = 5 [PTCy+DT]).
Donor CD4+Foxp3+ Treg depletion after PTCy treatment accelerates GVHD. (A) Experimental design. Chimeras were constructed as in Figure 2, except that the GVH inocula were obtained from B6.luc+.Foxp3DTR donors. PTCy (200 mg/kg IP) was administered on day +3 after alloBMT. DT (50 ng/g) or PBS was injected on days +4 and +5. Luciferase expression by luc+ cells in the GVH inocula was quantified in vivo by injection of luciferin and acquisition of bioluminescent signal. All animals were monitored daily for survival and scored twice weekly for GVHD until day +60, when the experiments were terminated. (B) GVHD scores (left panel) and survival (right panel) of chimeras (n = 10 per group) that were monitored until day +60 in 2 independent experiments. (C) Kinetics of BLI after PTCy-induced CD4+Foxp3+ donor Treg depletion. (Left panel) BLI imaging of luciferase-expressing cell distribution at serial time points after alloBMT. Color bars represent the signal intensity scale. ×, weak subject succumbed to anesthesia; †, subjects died before imaging time point. (Right panel) Expansion of luciferase-expressing T cells as quantified by total emitted photons per mouse and group at serial time points after alloBMT. Displayed are pooled results from 2 independent experiments (n = 10 per group), distinct from those shown in (B). On day +28 post-BMT, spleens and CLNs were harvested for flow cytometric analysis of intracellular cytokines and Foxp3 expression. (D) Percentages of cytokine-producing or Foxp3-expressing GVH inocula–derived (CD45.2+) CD4+ T cells. (E) Total numbers of CD4+CD45.2+Foxp3+ Tregs in the spleens and CLNs. Data shown are from day +28 survivors of 2 independent experiments (n = 10 [PTCy]; n = 5 [PTCy+DT]).
Mice depleted of Tregs after PTCy had significantly worse GVHD reflected in higher clinical GVHD scores, higher grade histopathological scores, and overall greater mortality compared with mice treated with PTCy but not DT (Figure 6B and supplemental Figure 5A). These PTCy- and DT-treated mice also had a significant increase in BLI signal intensity, detected first on day +14, indicative of increased donor-derived Teff proliferation (Figure 6C). Similar to that seen with DT treatment before PTCy, at day +28 post-BMT we observed a greater frequency of IFN-γ+ and IL-17A+ CD4+ T cells in lymphoid organs of surviving mice who were Treg-depleted after PTCy (Figure 6D). Also similar to mice who underwent Treg depletion before PTCy, mice depleted of Tregs after PTCy had a rebound in CD4+Foxp3+ T cells to percentages higher than those seen in mice treated with PTCy but not DT. However, total Treg numbers were again much lower than mice treated with PTCy alone and were associated with a higher ratio of IFNγ-producing Teffs to Tregs (Figure 6E and supplemental Figure 5B).
Donor tTregs retrieved from PTCy-treated chimeras rescue PTCy-treated Treg-depleted secondary recipients from GVHD
Next we evaluated the in vivo functionality of Tregs retrieved from PTCy-treated chimeras and also sought to further demonstrate their necessity in preventing GVHD in mice treated with PTCy. To do so, we constructed chimeras as described (Figure 6) in which DT depleted Tregs after PTCy treatment, but also adoptively transferred DT-insensitive tTregs at the same time (Figure 7A). The adoptively transferred tTregs were B6.Foxp3RFP T cells obtained either from untransplanted donors (pre-BMT donor Tregs) or from PTCy-treated B6→BALB.B chimeras at 4 weeks post-BMT (PTCy-tTregs). To ensure that the post-BMT tTregs used for adoptive transfer were indeed tTregs, Foxp3RFP+ CD4+ T cells were obtained from mice that had been transplanted with B6.Foxp3RFP Tregs and B6.Foxp3GFP– Tcons. In these studies, tTregs were used exclusively because the pTreg yield from as many as 20 PTCy recipients was insufficient for adoptive transfer (1.6 × 105) in pilot experiments, where we also determined that a Treg dose of 2 × 105 per mouse was essential for protection (data not shown).
Reconstitution of PTCy-treated Treg-ablated recipients with DT-insensitive Tregs rescues chimeras from lethal GVHD. (A) Experimental design. Chimeras were constructed as in Figure 2 and PTCy was administered on day +3 post-alloBMT. DT (50 ng/g) was given on days +4 and +5. On day +4 post-alloBMT, groups received PBS (controls) or 2 × 105 tTregs purified from day +28 PTCy-treated chimeras as described in Figure 3 or pre-transplant Tregs from B6.Foxp3RFP donors. (B) Survival was monitored twice weekly until day +60, when the experiment was terminated. (C) Mice were scored for GVHD on day +7 and every +4 days subsequently until day +60. Data shown represent 2 independent experiments with at least 10 animals per group.
Reconstitution of PTCy-treated Treg-ablated recipients with DT-insensitive Tregs rescues chimeras from lethal GVHD. (A) Experimental design. Chimeras were constructed as in Figure 2 and PTCy was administered on day +3 post-alloBMT. DT (50 ng/g) was given on days +4 and +5. On day +4 post-alloBMT, groups received PBS (controls) or 2 × 105 tTregs purified from day +28 PTCy-treated chimeras as described in Figure 3 or pre-transplant Tregs from B6.Foxp3RFP donors. (B) Survival was monitored twice weekly until day +60, when the experiment was terminated. (C) Mice were scored for GVHD on day +7 and every +4 days subsequently until day +60. Data shown represent 2 independent experiments with at least 10 animals per group.
Consistent with our previous findings (Figure 6), when donor Foxp3+ Tregs in the donor inocula were DT-depleted after PTCy treatment, chimeras receiving PTCy and DT but no Tregs developed severe GVHD, resulting in 80% mortality as early as day +17 post-alloBMT (Figure 7B). Infusion of DT-insensitive Foxp3RFP+ PTCy-tTregs or pre-BMT Tregs from donors halted the GVHD progression associated with post-PTCy Treg depletion, resulting in significantly better survival and clinical GVHD scores (Figure 7B-C). These results indicate that tTregs recovered from PTCy-treated chimeras were functionally similar to pretransplant donor Tregs. Furthermore, these results confirmed the necessity of tTregs for the prevention of GVHD in mice treated with PTCy.
Murine Tregs express high levels of aldehyde dehydrogenase-2, which mediates their resistance to the Cy analog mafosfamide
Consistent with our murine BMT studies and our recent observations in humans,35 murine Foxp3+ Tregs were more resistant than murine Tcons to mafosfamide, an in vitro active cyclophosphamide analog (supplemental Figure 6A). Given the findings that human Foxp3+ T cells upregulate aldehyde dehydrogenase (ALDH)-1A1 expression during allogeneic stimulation, implicating this pathway in human Treg resistance to cyclophosphamide,35 we used quantitative reverse-transcriptase polymerase chain reaction to examine its relevance in the murine system. Interestingly, ALDH1A1 was undetectable in murine mixed lymphocyte reactions (MLR); however, expression of ALDH2 was 4.4-fold higher in Tregs than in Tcons on day 3 of MLR (supplemental Figure 6B). This lack of ALDH1A1 involvement and dominance of the ALDH2 isoenzyme in mice is consistent with their differential roles in mouse and human hematopoietic stem cells.36,37
The markedly higher ALDH2 expression by Tregs on MLR day 3 is consistent with the relative resistance of Tregs to mafosfamide compared with Tcons and may reflect differences in activation kinetics between these different subsets.10 Indeed, by MLR day 7, ALDH2 expression had become similar between Tregs and Tcons in untreated cells. However, mafosfamide treatment led to persistently higher ALDH2 expression by Tregs compared with Tcons at day 7 (supplemental Figure 6B). Furthermore, ALDH2 inhibition with the ALDH inhibitors DEAB38 or Benomyl39,40 led to markedly worse Treg survival after mafosfamide treatment (supplemental Figure 6C), thus linking ALDH2 expression to mafosfamide resistance in Tregs. Importantly, ALDH2 inhibition alone had no effect on Tcon viability, while Tcons in mafosfamide-treated cultures had significantly reduced viability, regardless of ALDH2 inhibition (supplemental Figure 6C). Taken together, our findings suggest that Tregs may be preferentially resistant to PTCy by virtue of their high expression of ALDH2.
Discussion
Although PTCy is being increasingly used for GVHD prophylaxis, the cellular mechanisms behind its ability to limit donor T-cell alloreactivity are not fully understood. The prevailing model for the clinical efficacy of PTCy postulates that it “simply” depletes alloreactive T cells.3,41 In this model, the presence of Tregs would be entirely inconsequential because all alloreactive T cells would be eliminated, thus obviating any need for immunoregulatory elements. Although this mechanism undoubtedly is a necessary and important element in how PTCy works, the present study demonstrates that donor CD4+Foxp3+ Tregs are essential for GVHD prevention by PTCy in MHC-matched models in which T-cell allografts containing both CD4+ and CD8+ cells are transplanted. CD4+Foxp3+ Tregs are also essential for PTCy’s clinical activity in the MHC-mismatched setting (supplemental Figure 7). Furthermore, donor Tregs are required both before and after PTCy treatment because their inducible depletion at both time points accelerates GVHD. PTCy promotes recovery of functional tTregs, which maintain similar suppressive capability and demethylation features as Tregs found in nontransplanted mice and are able to rescue PTCy-treated Treg-depleted secondary recipients from GVHD. Thus, our findings indicate that Tregs are an indispensable and nonredundant component of PTCy-mediated control of alloreactivity, adding to the complexity of the known immunoregulatory activities of PTCy.
Cyclophosphamide has effects on different immune cell populations in a dose-, time-, and context-dependent manner.42 It is well established that cyclophosphamide can promote homeostatic expansion of antigen-specific T cells,43 an increase in the CD44hi effector T-cell pool,44 divergence of Th1/Th2 cytokine responses,45 and downregulation of suppressor cell activity.46 In the nontransplant setting, where expression of ALDH2 was similar between Tregs and Tcons (supplemental Figure 6B) and Tregs are a naturally more proliferative subset, there is evidence that low doses of cyclophosphamide preferentially deplete tumor-infiltrating Tregs.47-49 However, in the allogeneic setting, ALDH2 expression is greatly increased within Tregs relative to Tcons, likely accounting for the reason Tregs become a more resistant subset in this context. Overall, these findings are in agreement with our recent study in humans suggesting that Cy relatively spares CD4+Foxp3+ fractions after allogeneic stimulation both in vitro and in vivo, and that the Tregs that survive Cy exhibit suppressive capability and maintain low demethylation of the Foxp3 TSDR suggestive of their thymic origin.35
Beyond persistence of tTregs that are resistant to PTCy treatment, our studies here suggest that PTCy actually may promote Treg expansion, including pTregs in lymphoid organs. Although pTregs constitute a small minority of Tregs in non–Treg-depleted mice, these pTregs appear to be less functionally active than the dominant tTregs surviving PTCy, consistent with previous reports of their limited generation and stability during GVHD.50,51 Interestingly, PTCy seems to favor induction of pTregs even in Treg-depleted chimeras. However, this rebound in pTregs was not associated with lower cytokine production by donor Tcons, resulting in only a partial attenuation of histopathologic changes in tissues, and it ultimately was insufficient to protect mice from GVHD-associated lethality. This may be a result of the demonstrated lower functionality of these pTregs compared with tTregs (Figure 5) or may reflect an initial loss of alloimmune regulation that is not reversed by the modest resurgence of pTregs. Thus, the ensuing inflammation resulting from initial Treg depletion may outpace Treg recovery functionally and/or numerically and overall dominate the immune milieu, accounting for GVHD-related mortality. However, what drives the generation of pTregs from Tcons in PTCy-treated mice requires further investigation.
In the B6→BALB.B model of GVHD, the pathology of the intestinal epithelium is primarily mediated by 2 CD4+ T-cell families (Vβ2 and Vβ11).32,52 In our study, Vβ11 TCR+ tTregs were present in much higher proportions and numbers in the mesenteric lymph nodes of PTCy-treated chimeras compared with controls. Historically, Tregs have been defined by the expression of the master transcription factor Foxp3.15 However, Foxp3 expression alone is not adequate for Treg stability and requires coordinate demethylation of Foxp3.15 Fully demethylated CpG motifs, as well as histone modifications within the TSDR, are defining epigenetic features of tTregs, with partial demethylation and full methylation being characteristic of pTregs and Tcons, respectively.33 In our study, tTregs from lymphoid organs of PTCy-treated mice had TSDR demethylation patterns comparable with pretransplant Tregs whereas those from control GVHD chimeras were relatively more methylated. However, pTregs, induced from transplanted Foxp3– Tcons, in both groups exhibited comparable TSDR methylation. These findings suggest that PTCy promoted maintenance of Treg-specific epigenetic hallmarks that were partially lost by tTregs in untreated mice. Indeed, tTregs retrieved from PTCy-treated mice had robust suppressive ability in vivo, which was comparable with that of pre-BMT donor Tregs. In contrast, pTregs from both GVHD controls and PTCy-treated recipients had diminished in vitro suppressive ability. Further studies of pTregs in this model are needed, but we were limited by an inability to obtain optimal numbers for more exhaustive functional studies.
Taken together, we conclude that, beyond elimination of alloreactive T cells, the PTCy GVHD prophylactic strategy also preferentially promotes persistence of donor tTregs. These Tregs not only persist but in fact expand in lymphoid organs, where they maintain allospecific regulation. Furthermore, administration of PTCy is associated with the emergence of pTregs. However, whether tTregs and pTregs have mutually exclusive roles or act in a complementary manner to restrain alloreactivity after PTCy treatment requires further investigation. The role of other immune subsets, particularly specialized antigen-presenting cells such as dendritic cells, in the GVHD protective activity of PTCy remains to be elucidated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ada Tam and Lee Blosser at the Johns Hopkins Sidney Kimmel Cancer Center Flow Cytometry Core for excellent technical assistance with cell sorting.
This work was supported by National Institutes of Health grants from the National Cancer Institute (R01CA122779), the National Heart, Lung, and Blood Institute (R01HL110907 to L.L.; R01HL56067 to B.R.B), and the National Institute of Allergy and Infectious Diseases (R01AI34495 to B.R.B.; R01AI46689 to R.B.L.).
Authorship
Contribution: S.G. designed and performed research, analyzed the data, designed the figures, and wrote the paper; D.B.R. designed and performed research, analyzed the data, and edited the paper; A.P.-M. contributed data and edited the paper; C.G.K., B.R.B., and R.B.L. designed research and edited the paper; and L.L. developed the concept, designed research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leo Luznik, Cancer Research Building I, Room 2M88, 1650 Orleans St, Baltimore, MD 21287; e-mail: luznile@jhmi.edu.



![Figure 6. Donor CD4+Foxp3+ Treg depletion after PTCy treatment accelerates GVHD. (A) Experimental design. Chimeras were constructed as in Figure 2, except that the GVH inocula were obtained from B6.luc+.Foxp3DTR donors. PTCy (200 mg/kg IP) was administered on day +3 after alloBMT. DT (50 ng/g) or PBS was injected on days +4 and +5. Luciferase expression by luc+ cells in the GVH inocula was quantified in vivo by injection of luciferin and acquisition of bioluminescent signal. All animals were monitored daily for survival and scored twice weekly for GVHD until day +60, when the experiments were terminated. (B) GVHD scores (left panel) and survival (right panel) of chimeras (n = 10 per group) that were monitored until day +60 in 2 independent experiments. (C) Kinetics of BLI after PTCy-induced CD4+Foxp3+ donor Treg depletion. (Left panel) BLI imaging of luciferase-expressing cell distribution at serial time points after alloBMT. Color bars represent the signal intensity scale. ×, weak subject succumbed to anesthesia; †, subjects died before imaging time point. (Right panel) Expansion of luciferase-expressing T cells as quantified by total emitted photons per mouse and group at serial time points after alloBMT. Displayed are pooled results from 2 independent experiments (n = 10 per group), distinct from those shown in (B). On day +28 post-BMT, spleens and CLNs were harvested for flow cytometric analysis of intracellular cytokines and Foxp3 expression. (D) Percentages of cytokine-producing or Foxp3-expressing GVH inocula–derived (CD45.2+) CD4+ T cells. (E) Total numbers of CD4+CD45.2+Foxp3+ Tregs in the spleens and CLNs. Data shown are from day +28 survivors of 2 independent experiments (n = 10 [PTCy]; n = 5 [PTCy+DT]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/13/10.1182_blood-2013-10-525873/4/m_2131f6.jpeg?Expires=1768656281&Signature=XGHMEQAdBALRR9y6XfShT65Psgq4tEIWyHT-g4Z4RNkXogdU~11XA~wzBHai5naqIOfHvRwxjRs1xIpe~WdQ56YH98wkgBEB8YW-F3QLfFIzLv04iyjQr9WlYsd9e8Z8PHCgq7Nl9pwmNelbBa8JOu5BssXDc~rH7BudqpOsQeg~6HeANyOI1EtAcKBcfPfx~bhDEgUJB2POrQ1wr~Lvo8XpdtJoAy~q6VyccdDIJfQKRAnwAHfcESLKAn2HNpWjzThzm6Lmk7xlZx3ZJBMl3ogrTFpgWP6jjoP8Ek93pWuMbJMYaIgEDQd-V6rHybD2B7aWU6dT4~qOBflPzxoJlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)