Key Points
Endothelial PI3Kβ is not required in the quiescent vasculature, but PI3Kβ loss confers sensitivity for thrombotic microangiopathy.
PI3Kβ activity is required for endothelial angiogenic differentiation and microvascular repair.
Abstract
Thrombotic microangiopathy (TMA) commonly involves injury of kidney glomerular endothelial cells (ECs) and fibrin occlusion of the capillaries. The mechanisms underlying repair of the microvasculature and recovery of kidney function are poorly defined. In the developing vasculature, the phosphoinositide 3-kinase (PI3K) α isoform integrates many growth factor cues. However, the role of individual isoforms in repair of the established vasculature is unclear. We found that postnatal endothelial deletion of PI3Kβ sensitizes mice to lethal acute kidney failure after TMA injury. In vitro, PI3Kβ-deficient ECs show reduced angiogenic invasion of fibrin matrix with unaltered sensitivity to proapoptotic stress compared with wild-type ECs. This correlates with decreased expression of the EC tip cell markers apelin and Dll4 and is associated with a reduction in migration and proliferation. In vivo, PI3Kβ-knockdown ECs are deficient in assembly of microvessel-like structures. These data identify a critical role for endothelial PI3Kβ in microvascular repair following injury.
Introduction
Endothelial damage causing thrombotic microangiopathy (TMA) commonly results from exposure to Shiga-like toxin associated with epidemic Escherichia coli infections, mutations of complement regulatory proteins, drug toxicity, and acute and chronic immune responses against allogeneic vascular endothelium in the context of bone marrow or solid-organ transplantation.1-3 These diseases often follow a fulminant course leading to rapid failure of affected organs.4 In particular, thrombosis involving the kidney glomerular capillaries results in acute kidney failure and is a major cause of morbidity.2,5,6 Nevertheless, despite catastrophic glomerular endothelial damage requiring kidney-replacement therapy, many individuals with acute injury to a healthy microvasculature recover kidney function.7,8
The mechanisms underlying repair of the microvasculature after TMA injury of microvessel endothelial cells (ECs) and resolution of fibrin clot occlusion of the capillaries leading to recovery of organ function are poorly defined. The cues to embryonic vascular development are lead candidates to initiate repair of the established microcirculation. These include factors secreted by parenchymal cells of the tissue such as vascular endothelial growth factor (VEGF), angiopoetin-1, or sphingosine 1-phosphate, which signal to the endothelium through receptor tyrosine kinases (RTKs) and G protein–coupled receptors (GPCRs).9-11
In the endothelium, phosphoinositide 3-kinases (PI3Ks) integrate many growth factor signals in development and hence are candidates to mediate reparative cues.12 However, the function of individual PI3K isoforms and their role in repair of the established microvasculature are unclear. This family of lipid kinases includes 4 catalytic isoforms expressed in ECs (p110α, p110β, p110δ, and p110γ) that associate with a regulatory subunit to couple PI3K activity to receptor activation.13 PI3Ks that associate with the p85 regulatory subunit are grouped into class IA, to couple to RTKs, whereas p110γ, the single class IB isoform, is regulated by p101/p84 and is coupled to GPCRs. PI3Kβ uniquely signals downstream of both RTKs and GPCRs14,15 and is uniquely regulated by rho family guanosine triphosphate–binding proteins.16 The PI3Kα isoform (encoded by the Pik3ca gene) is critically required for embryonic development, because both global and EC-restricted knockout of Pik3ca results in embryonic lethality with features of disordered vascular development.17,18 Conversely, both global19 and endothelial-restricted PI3Kβ loss is tolerated in unstressed mice.18 Consequently, the function of PI3Kβ in the endothelium is unknown.
We observe that postnatal endothelial deletion of PI3Kβ sensitizes mice to lethal acute kidney failure after TMA injury. In vitro, PI3Kβ inactivation inhibits EC invasion of fibrin matrix. This correlates with decreased expression of the angiogenic tip cell markers apelin and Dll4 and is associated with a reduction in EC migration and proliferation. In vivo, PI3Kβ-knockdown ECs are defective in assembly of microvessel-like structures. These data identify an important role for endothelial PI3Kβ in microvascular repair following injury.
Methods
Reagents
The following antibodies were purchased: anti-human DLL4 (R&D Systems), polyclonal anti-human apelin and anti-human ESM1 (Abcam), anti-human CD31 and anti-mouse CD31 (BD Pharmingen), anti-mouse fibrinogen/fibrin (Genway), and anti-apelin-36 (Phoenix Pharmaceuticals). Secondary fluorophore- or horseradish peroxidase (HRP)-conjugated species-specific antibodies were purchased from Jackson Immunoresearch. Marasmius oreades lectin A (EY Laboratories) was conjugated to saporin (Sigma-Aldrich) using a sulfo-LC-SPDP kit as described by the manufacturer (Thermo Scientific), then purified by high-performance liquid chromatography. The PI3Kβ-selective small-molecule inhibitor TGX-221 was obtained from Cayman Chemical and used in vitro at 100 nM. CellTracker green and red were from Invitrogen. VEGF-A and stromal cell–derived growth factor-1 were obtained from R&D Systems.
Animals
Immunodeficient 129S6/SvEvTac-Rag2tm1Fwa (Rag2−/−γc−/−) mice were purchased from Taconic. The generation of p110βflox/flox mice was previously described.15 B6.D2-Tg(Tek-Cre/ERT2)1Arnd (Tie2-CreERT2) mice were supplied by Dr Bernd Arnold20 and bred against p110βflox/flox mice to produce Tie2- CreERT2+/−/p110βflox/flox mice used in these experiments. Activation of the Cre recombinase deletes exons 21 and 22 from Pik3cb, resulting in expression of a messenger RNA (mRNA) that encodes an internally truncated protein that lacks lipid kinase activity.15 However, this protein is absent from tissues for reasons that are unclear at the moment and thus creates the equivalent of a p110β knockout.19 Twelve-week-old mice were treated with 2 mg tamoxifen (Sigma-Aldrich) or vehicle (oil) daily for 5 days via an intraperitoneal injection. Littermate animals were used as controls. The mice were rested for 1 week before experimentation. Expression of the mutant Pik3cbδ21,22 mRNA was determined in kidney cortex RNA via reverse-transcription polymerase chain reaction (PCR). Kidney glomerular TMA microvascular injury was induced as described in detail elsewhere.21 Briefly, mice were treated with 50 μg/kg lipopolysaccharide and 200 μg/kg M oreades lectin A conjugated to the cytotoxin saporin by intra-arterial injection (toxin), retrograde via the left carotid artery. The animals were maintained according to the Canadian Council for Animal Care guidelines under a protocol approved by the Animal Care and Use Committee of the University of Alberta.
Endothelial cells
Late blood-outgrowth human primary endothelial progenitor cells (ECs) were isolated as described previously,22 with modifications. Mononuclear cells were isolated from 120 mL peripheral blood from healthy adult donors or from single-donor leukapheresis. CD34+ cells were isolated using the EasySep human CD34 selection kit (STEMCELL Technologies) according to the manufacturer’s instructions. The CD34+ cells were seeded on fibronectin-coated 6-well tissue culture plates in endothelial growth medium 2 (EGM2; endothelial basal medium-2 [EBM2] + SingleQuots growth supplement + 10% fetal bovine serum; Lonza). After a 24-hour incubation at 37°C in 5% CO2, nonadherent cells were discarded, and the adherent cells were fed every other day with EGM2 until late-outgrowth EC colonies appeared in culture 7 to 21 days after seeding. Mouse cardiac microvascular ECs were purchased from Cedarlane (Burlington, ON, Canada).
Creatinine and blood urea nitrogen assay
The serum creatinine concentration was determined by the mouse creatinine kit (Crystal Chem) as per the manufacturer’s instructions. The creatinine concentration from samples was interpolated using a calibration standard curve. The serum urea concentration was determined using a urea assay kit (BioAssay Systems) as per the manufacturer’s instructions.
Quantitative PCR
Real-time PCR was performed using a model 7500 thermal cycler (Applied Biosystems). Total RNA was isolated from kidney cortex using the RNeasy mini kit (Qiagen) or from fibrin gels in vitro using TRIzol/chloroform. A total of 1 μg of total RNA was reversed transcribed into complementary DNA using qScript cDNA supermix (Quanta). The PCR primers were designed using PrimerExpress 3.0 (Applied Biosystems) software and purchased from Integrated DNA Technology (supplemental Table 1, available at the Blood Web site).
SDS polyacrylamide gel electrophoresis and western blot
Whole-cell lysates were prepared using RIPA buffer, then proteins were resolved on sodium dodecyl sulfate (SDS) gels and transferred onto a nitrocellulose membrane (Bio-Rad). Membranes were immunoblotted using the primary antibodies, followed by HRP-conjugated secondary antibody as described previously.23 The membranes were developed using the ECL prime kit (GE Healthcare) and visualized using MultiImage 2 and FluorChem software (Alpha Innotech).
Apoptosis and proliferation assays
Cell proliferation was evaluated using the Click-iT 5-ethynyl-2′-deoxyuridine flow cytometry assay kit (Invitrogen), as per the manufacturer’s instructions, and quantitated using flow cytometry. In vitro apoptosis was evaluated as described previously.23
siRNA transfection
Three different small interfering RNA (siRNA) sequences against human p110β were used, and all produced similar results: 2 sequences targeted the coding region, and 1 sequence targeted the 3′ untranslated region (SI04436327, SI00085862, and SI02622214; Qiagen). Transfection of 50 nM siRNA was performed using HiPerFect (Qiagen) as described previoulsy.23 The efficiency of gene knockdown was evaluated by western blot and/or real-time PCR after 72 hours (supplemental Figure 3).
Chemotaxis assay
Cell migration was assayed using a 24-well Transwell plate with 8 μm pores (Corning). The ECs (treated with p110β siRNA or control siRNA or left untreated) were seeded at a density of 105 cells in the insert in the presence of EBM2. EGM2 was placed in the receiver chamber. To evaluate chemokinesis, EGM2 was placed in both chambers. The plate was incubated at 37°C for 24 hours. Migrated cells in the lower chamber were labeled with Calcein AM diluted in cell-dissociation solution (Sigma-Aldrich) for 1 hour at 37°C and 5% CO2. The inserts were removed and the plate was read at 485 to 527 nm using a fluorescent plate reader (Fluoroskan; Thermo Scientific).
In vitro 3-dimensional angiogenesis assay
Angiogenic sprouting of ECs (treated with p110β siRNA or control siRNA or left untreated) in vitro was determined as described previously,24 with some modifications. Human ECs were mixed with collagen-coated Cytodex 3 microcarrier beads (Sigma-Aldrich) and incubated at 37°C in 5% CO2 for 4 hours with shaking every 20 minutes. The coated beads were then transferred to T25 tissue culture flasks and incubated for a further 2 hours. The EC-coated beads were washed with phosphate-buffered saline (PBS) X2 and mixed with 2 mg/mL fibrinogen (Sigma-Aldrich) and 0.15 U/mL aprotinin (Sigma-Aldrich). A total of 500 μL of the EC-coated beads and fibrinogen solution was added to 0.625 U/mL thrombin (T4648; Sigma-Aldrich) in a 24-well tissue culture plate and incubated for ∼15 min until the fibrin gelled, EGM2 was added, and sprout formation was analyzed at 18 hours. At least 30 beads from each treatment in each experiment were analyzed.
In vivo vasculogenesis assay
Vasculogenesis was analyzed in vivo as described previously.25 Briefly, human ECs (pretreated with p110β siRNA or control siRNA or left untreated) and human aortic smooth muscle cells (Cell Applications) were mixed at a ratio 75:25 and suspended in growth factor–reduced Matrigel (BD Biosciences). The cell suspension was injected subcutaneously into Rag 2−/−γc−/− mice and retrieved after 1 week. The Matrigel plugs were placed in optimal cutting temperature medium, snap frozen in liquid nitrogen, and then processed for immunofluorescence microscopy.
Histology and immunohistochemical staining
Kidneys were collected from each killed mouse and either placed in optimal cutting temperature and snap frozen in liquid nitrogen or placed in IHC zinc fixative (BD Pharmingen). Zinc-fixed tissues were stained with hematoxylin and eosin. For immunofluorescence staining, 5 μm frozen sections were stained with the primary antibody or a nonspecific immunoglobulin G at 4°C overnight and detected with a fluorescein-labeled or HRP-labeled secondary antibody. The fluorescence signal of at least 10 glomeruli in an equatorial section per mouse was visualized by fluorescence microscopy (Quorum Wave FX-X1; Guelph, ON, Canada), and quantitated using open-source ImageJ software. Microvessels in Matrigel plugs were stained with species-specific anti-human or anti-mouse CD31. The number of microvessel-like tubes formed by human ECs was counted in 20 ×40 fields of view in a blinded fashion.
Semiquantitative analysis of renal injury
Kidney pathology and glomerular injury was evaluated as described elsewhere26 by a renal pathologist (D.C.R.) blinded to the treatment group. Briefly, injury was scored if thrombosed capillary lumens, apoptotic cells, or capillary microaneurysms were detected. Similarly, acute tubular necrosis was scored on a scale per field of view (1 ≤ 25%, 2 ≤ 50%, 3 ≤ 75%, and 4 ≥ 75% of injured tubules) sampling at least 2 fields per glomerulus.
Statistical analysis
Statistical analyses were performed using PRISM software (GraphPad). The data are expressed as mean ± standard error of the mean (SEM) and analyzed using 1-way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis or 2-way ANOVA where applicable. Nonparametric results were analyzed using the Mann-Whitney U test. Kaplan-Meier data were analyzed by log-rank test for trend. A P value <.05 was considered significant.
Results
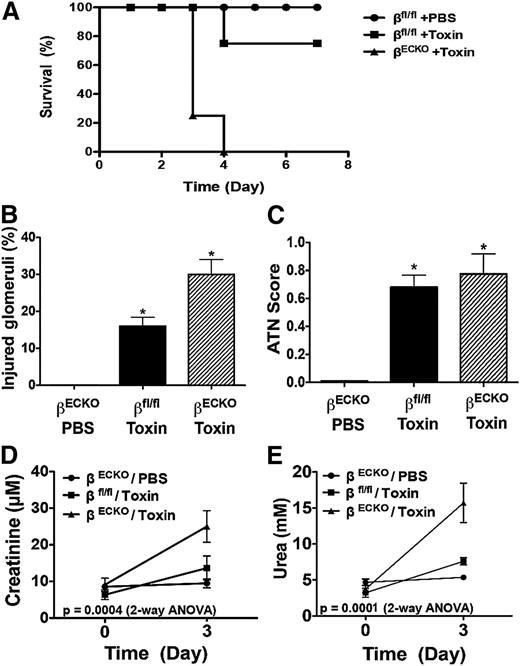
To examine the role of PI3Kβ in the mature microvasculature, we induced Cre-recombinase–mediated postnatal deletion of Pik3cb selectively in ECs (hereafter βECKO). Similar to the impact of embryonic Pik3cb deletion, adult mice tolerated PI3Kβ loss (supplemental Figure 1). Kidney function was comparable in the uninjured βECKO and p110β-wild type (βfl/fl) littermates. A challenge with a sublethal dose of lectin-cytotoxin conjugate to selectively injure the glomerular capillary microvascular endothelium21 was well tolerated by βfl/fl mice. In contrast, the βECKO mice became morbid on day 3 after challenge, and none survived more than 4 days after cytotoxin exposure (Figure 1A).
Endothelial PI3Kβ loss sensitizes mice to TMA. (A) Kaplan-Meier survival plot of Tie2-CreERT2+/−/p110βflox/flox mice treated with vehicle (βfl/fl) or tamoxifen to induce endothelial Cre recombinase activity and excision of Pik3cb (βECKO). As indicated, the animals received PBS (n = 6) or 200 μg/kg lectin-saporin cytotoxin conjugate to selectively injure the glomerular microvascular endothelium (n = 10 mice/group; toxin-treated βECKO vs βfl/fl, P < .01). Kidney glomerular injury and function is worsened by endothelial PI3Kβ loss after TMA injury. (B-C) Quantification of histologic scoring of injured glomeruli (B) and acute tubular necrosis (C) among the groups at day 3 after cytotoxin exposure. (D-E) Quantification of serum creatinine (D) and urea concentration (E) among the groups. The data represent mean ± SEM; n ≥ 5 animals per group; *P < .05, **P < .01, toxin-treated group vs PBS group.
Endothelial PI3Kβ loss sensitizes mice to TMA. (A) Kaplan-Meier survival plot of Tie2-CreERT2+/−/p110βflox/flox mice treated with vehicle (βfl/fl) or tamoxifen to induce endothelial Cre recombinase activity and excision of Pik3cb (βECKO). As indicated, the animals received PBS (n = 6) or 200 μg/kg lectin-saporin cytotoxin conjugate to selectively injure the glomerular microvascular endothelium (n = 10 mice/group; toxin-treated βECKO vs βfl/fl, P < .01). Kidney glomerular injury and function is worsened by endothelial PI3Kβ loss after TMA injury. (B-C) Quantification of histologic scoring of injured glomeruli (B) and acute tubular necrosis (C) among the groups at day 3 after cytotoxin exposure. (D-E) Quantification of serum creatinine (D) and urea concentration (E) among the groups. The data represent mean ± SEM; n ≥ 5 animals per group; *P < .05, **P < .01, toxin-treated group vs PBS group.
Histologic features of TMA injury of glomerular capillaries persisted on day 3 after cytotoxin injury among βECKO mice (supplemental Figure 1b-c). Glomerular injury was more frequent among βECKO mice than controls (Figure 1B), and consistent with the anticipated sequelae of the damaged glomerular microcirculation, histologic features of acute tubular necrosis were evident among both βECKO and control, cytotoxin-treated groups (Figure 1C). Notably, the kidney function of the βECKO mice was markedly worse compared with βfl/fl mice at day 3 after TMA injury (Figure 1D-E).
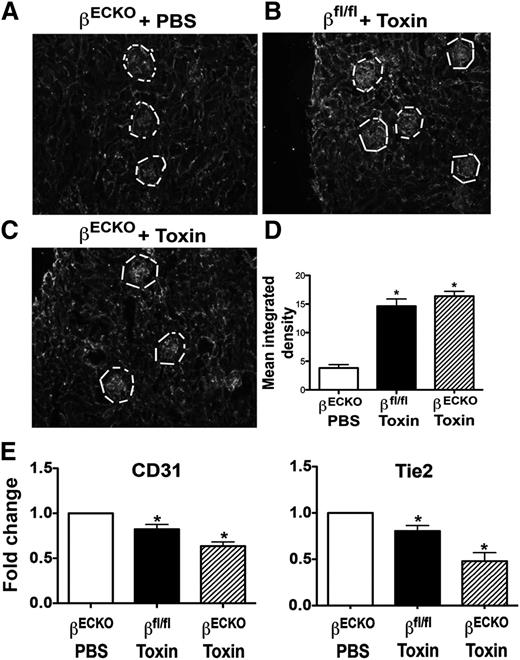
Within the kidney cortex, increased fibrin staining of the glomerulus was evident among both βECKO and βfl/fl mice after cytotoxin exposure vs PBS controls, confirming similar localization of microvascular TMA injury among these groups (Figure 2A-D). To better quantitate the extent of microvascular endothelial injury, the abundance of CD31 and Tie2 mRNAs in kidney cortex was determined (Figure 2E). Expression of each of these endothelial-specific, constitutively expressed genes was decreased in kidney cortex in cytotoxin-treated animals, reflecting EC loss from the kidney microcirculation (Figure 2E). Expression of the tubular epithelial stress genes, KIM-1 and lipocalin 2, was upregulated (data not shown), consistent with the histologic findings of tubular injury. Conversely, no injury was evident in the heart, and heart function was normal in toxin-treated βECKO mice (supplemental Figure 2). Taken together, these data indicate that endothelial p110β loss in the quiescent mature vasculature was tolerated. However, endothelial PI3Kβ was required to maintain organ function and survival after renal microvascular TMA injury.
Cytotoxin injury of glomerular endothelium induces glomerular microvascular injury. (A-C) Mice were treated as in Figure 1, then frozen sections of kidney were stained with anti-mouse fibrinogen/fibrin antibody. The fluorescence intensity among regions of interest drawn around Bowman’s capsule containing the glomerular microvessels (white line) was measured using ImageJ (D). Each bar represents mean ± SEM; n ≥ 5 mice per group; *P < .05, toxin-treated groups vs PBS control group. (E) Quantification of EC transcripts from total RNA extracted from the renal cortex. The real-time PCR data were analyzed using the δCt method. Each bar represents mean ± SEM; n ≥ 5 animals per group; *P < .05, toxin-treated groups vs PBS control group.
Cytotoxin injury of glomerular endothelium induces glomerular microvascular injury. (A-C) Mice were treated as in Figure 1, then frozen sections of kidney were stained with anti-mouse fibrinogen/fibrin antibody. The fluorescence intensity among regions of interest drawn around Bowman’s capsule containing the glomerular microvessels (white line) was measured using ImageJ (D). Each bar represents mean ± SEM; n ≥ 5 mice per group; *P < .05, toxin-treated groups vs PBS control group. (E) Quantification of EC transcripts from total RNA extracted from the renal cortex. The real-time PCR data were analyzed using the δCt method. Each bar represents mean ± SEM; n ≥ 5 animals per group; *P < .05, toxin-treated groups vs PBS control group.
We next evaluated the mechanistic role of endothelial PI3Kβ in vitro. Primary human ECs express all 3 class IA PI3K catalytic subunit isoforms (supplemental Figure 3a-b). Selective knockdown of the p110β catalytic isoform did not alter the expression of the p110α or p110δ isoforms or of the counterregulatory phosphatase, PTEN (supplemental Figure 3a-b).
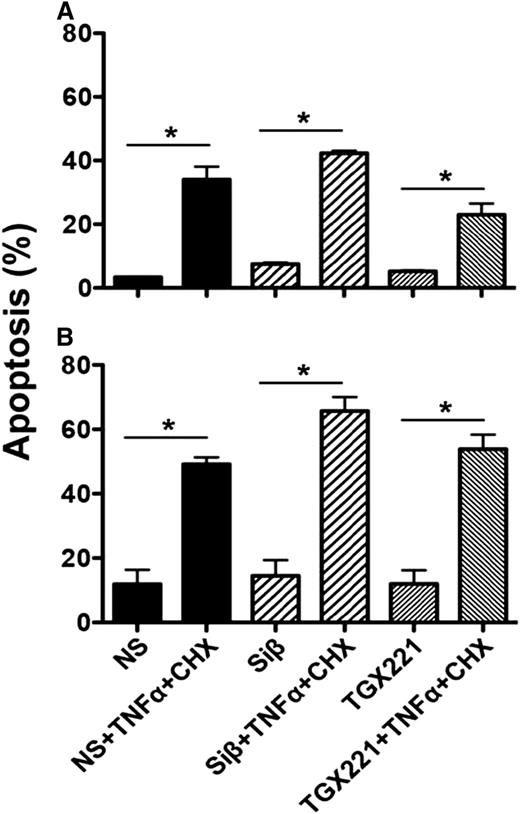
We first tested the effect of p110β loss on apoptosis of ECs. We observed no difference in caspase-3 activation in unstressed mouse or human ECs (Figure 3A-B). Tumor necrosis factor/cycloheximide challenge induced apoptosis in a similar fraction of wild-type, p110β-deficient, and p110β-inhibited (TGX-221–treated) ECs (Figure 3A-B). Moreover, VEGF stimulation elicited similar phosphorylation of Akt and downstream effectors including FoxO1/3 in TGX-221-treated or p110β-deficient ECs vs vehicle-treated ECs (Figure 4). However, GPCR-dependent responses to stromal cell–derived growth factor-1 are blocked, confirming effective disruption of PI3Kβ signaling (supplemental Figure 4). Thus, disrupted p110β signaling does not sensitize ECs to apoptosis or interrupt VEGF-mediated signaling to this important prosurvival pathway.
Effect of p110β inactivation on EC apoptosis. Active caspase-3 in (A) mouse or (B) human ECs was determined as in “Methods” with or without tumor necrosis factor (TNF)/cycloheximide (CHX) challenge. Where indicated, ECs were pretreated with TGX-221 (100 nM) for 60 minutes or treated with siRNA (Siβ) as in supplemental Figure 3 to decrease p110β expression (mean ± SEM; *P < .05 vs the respective unchallenged group; n ≥ 3 independent experiments). NS, nonspecific siRNA.
Effect of p110β inactivation on EC apoptosis. Active caspase-3 in (A) mouse or (B) human ECs was determined as in “Methods” with or without tumor necrosis factor (TNF)/cycloheximide (CHX) challenge. Where indicated, ECs were pretreated with TGX-221 (100 nM) for 60 minutes or treated with siRNA (Siβ) as in supplemental Figure 3 to decrease p110β expression (mean ± SEM; *P < .05 vs the respective unchallenged group; n ≥ 3 independent experiments). NS, nonspecific siRNA.
p110β inactivation does not inhibit VEGF-stimulated Akt phosphorylation. ECs were serum-starved overnight, then (A) pretreated with TGX-221 (100 nM; gray bar) for 60 minutes or (B) treated with Siβ siRNA (gray bar) as in supplemental Figure 3 to decrease p110β expression, before stimulation with VEGF (20 ng/mL). Control cells were treated with carrier or nonspecific siRNA (black bars). Lysates were immunoblotted for phospho-S473 Akt or phospho-T24 FoxO1/3, a target for Akt-mediated phosphorylation. PI3Kβ inactivation did not significantly affect Akt phosphorylation (mean ± SEM; n ≥ 3 independent experiments; P = nonsignificant by ANOVA).
p110β inactivation does not inhibit VEGF-stimulated Akt phosphorylation. ECs were serum-starved overnight, then (A) pretreated with TGX-221 (100 nM; gray bar) for 60 minutes or (B) treated with Siβ siRNA (gray bar) as in supplemental Figure 3 to decrease p110β expression, before stimulation with VEGF (20 ng/mL). Control cells were treated with carrier or nonspecific siRNA (black bars). Lysates were immunoblotted for phospho-S473 Akt or phospho-T24 FoxO1/3, a target for Akt-mediated phosphorylation. PI3Kβ inactivation did not significantly affect Akt phosphorylation (mean ± SEM; n ≥ 3 independent experiments; P = nonsignificant by ANOVA).
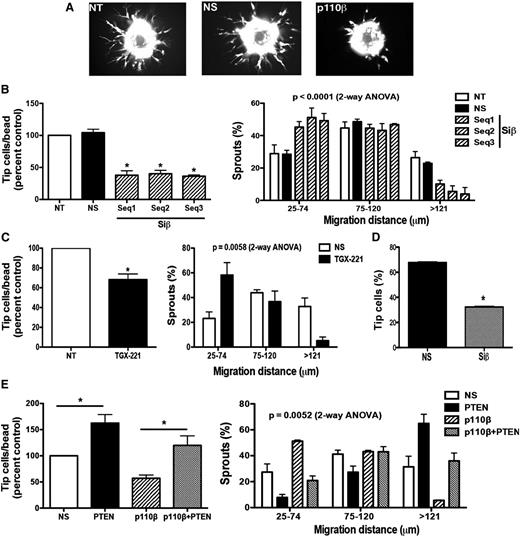
We directed our attention to investigate features of ECs that contribute to microvascular repair. Loss of p110β markedly impaired endothelial invasion into 3-dimensional (3D) fibrin matrices in vitro (Figure 5). The p110β-deficient ECs consistently produced both fewer sprouts and shorter sprout extensions compared with ECs treated with nonspecific siRNA or ECs without siRNA transfection (Figure 5A-B). Similarly, pharmacologic inhibition of PI3Kβ activity using TGX-221 inhibited EC sprouting (Figure 5C). Further, p110β wild-type ECs failed to rescue p110β-deficient EC sprouting in mixed cocultures (Figure 5D and supplemental Figure 5). Similar to p110β-deficient EC cultures, p110β-deficient ECs initiated few sprouts in coculture with wild-type cells. Although the p110β wild-type cells preferentially occupied the tip cell position in the mixed-EC sprouts, p110β-deficient ECs were able to contribute to either tip or stalk position. Interestingly, the angiogenic sprouting defect was rescued by loss of the counterregulatory lipid phosphatase, PTEN, in p110β/ PTEN double-deficient ECs in vitro (Figure 5E). Conversely, combined overexpression of p110β and p85α failed to promote additional EC sprouting (supplemental Figure 6). Hence, endothelial p110β loss impairs EC responses to reparative cues in a cell-autonomous fashion, but PI3 kinase-β activity is insufficient to augment growth factor-stimulated angiogenesis.
Endothelial invasion of fibrin matrix in vitro is decreased by PI3Kβ loss and is rescued by PTEN inactivation. Knockdown of p110β in human ECs was performed as in supplemental Figure 3. Where indicated, nontransfected (NT) ECs or cells transfected with p110β (Siβ) or nonspecific siRNA (NS) were labeled with CellTracker green, cultured on Cytodex beads, embedded in a 3D fibrin gel in EGM2 for 18 hours, then imaged by fluorescence microscopy (A). The number of invading EC tip cells (B left panel) and distance of sprout extension (B, right panel) into the gel among p110β-knockdown vs control ECs is quantitated (mean ± SEM; n > 5 independent experiments; *P < .05, Siβ vs NS). (C) ECs were pretreated with TGX-221 (100 nM) or vehicle for 60 minutes, then evaluated for sprouting (left panel) and length of sprout extension (right panel; *P < .05 vs control). Siβ- or NS-transfected ECs were labeled with CellTracker red or green respectively, then equal numbers were mixed and cultured on Cytodex beads for suspension in fibrin gels. The fraction of sprouts with a leading Siβ or NS EC was quantitated (D; n = 3 independent experiments; *P < .05, Siβ vs NS). (E) ECs were transfected with siRNAs against p110β and/or PTEN, then evaluated for sprout formation (n = 3 independent experiments; * P < .05 vs the comparator group as indicated).
Endothelial invasion of fibrin matrix in vitro is decreased by PI3Kβ loss and is rescued by PTEN inactivation. Knockdown of p110β in human ECs was performed as in supplemental Figure 3. Where indicated, nontransfected (NT) ECs or cells transfected with p110β (Siβ) or nonspecific siRNA (NS) were labeled with CellTracker green, cultured on Cytodex beads, embedded in a 3D fibrin gel in EGM2 for 18 hours, then imaged by fluorescence microscopy (A). The number of invading EC tip cells (B left panel) and distance of sprout extension (B, right panel) into the gel among p110β-knockdown vs control ECs is quantitated (mean ± SEM; n > 5 independent experiments; *P < .05, Siβ vs NS). (C) ECs were pretreated with TGX-221 (100 nM) or vehicle for 60 minutes, then evaluated for sprouting (left panel) and length of sprout extension (right panel; *P < .05 vs control). Siβ- or NS-transfected ECs were labeled with CellTracker red or green respectively, then equal numbers were mixed and cultured on Cytodex beads for suspension in fibrin gels. The fraction of sprouts with a leading Siβ or NS EC was quantitated (D; n = 3 independent experiments; *P < .05, Siβ vs NS). (E) ECs were transfected with siRNAs against p110β and/or PTEN, then evaluated for sprout formation (n = 3 independent experiments; * P < .05 vs the comparator group as indicated).
Next, we evaluated the effect of p110β knockdown on VEGF-stimulated EC proliferation or migration. p110β knockdown results in a modest reduction in the fraction of proliferating ECs (supplemental Figure 7a). In addition, the p110β-deficient ECs showed decreased chemotactic (supplemental Figure 7b) and chemokinetic (supplemental Figure 7c) migration compared with control cells. Similarly, treatment of ECs with TGX-221 decreased migration (supplemental Figure 7B-C). The loss of p110β function therefore modestly decreases EC proliferation and movement that support sprout extension in addition to the defect in sprout initiation.
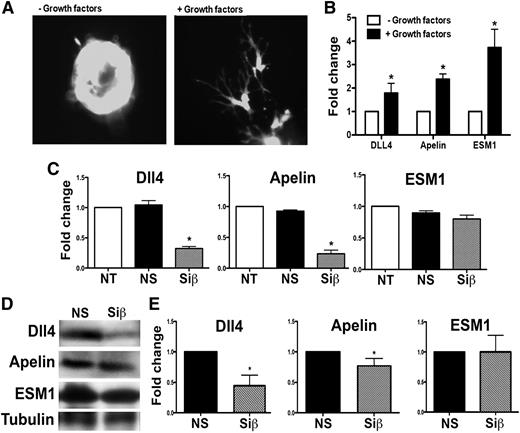
To evaluate the effect of loss of p110β function on EC differentiation that enables invasion of the fibrin matrix, we evaluated the expression of the angiogenic tip cell markers APLN, DLL4, and ESM1.27 Growth factor stimulation of ECs in 3D fibrin gels induced expression of the characteristic tip cell morphology and genes (Figure 6A-B). In contrast, p110β knockdown resulted in a significant decrease in Dll4 and apelin mRNA and protein expression compared with controls (Figure 6C-D). However, induced expression of the tip cell gene, ESM1, was not reduced after p110β knockdown. To determine if EC apelin expression in βECKO mice is altered, we immunostained tissue sections from uninjured and injured mice for apelin expression. The unstressed glomerulus exhibits minimal apelin staining, but apelin expression is induced after microvascular injury among wild-type, but not βECKO, mice (Figure 7). Taken together with defective sprout initiation, these data suggest that loss of p110β impairs full EC differentiation to acquire the tip cell phenotype associated with sprout invasion of fibrin matrices in vitro and in vivo.
PI3Kβ loss alters expression of tip cell markers. Primary human ECs were cultured on beads in fibrin gels as in Figure 5 for 18 hours, stimulated with SingleQuot growth factors or not, then imaged to evaluate tip cell morphology (A). Total RNA was extracted, then expression of characteristic tip cell transcripts was quantified (B). (C) Quantification of gene expression after growth factor stimulation of nontransfected (NT), nonspecific siRNA (NS)-transfected, or p110β (Siβ)-transfected cells at 18 hours of culture in fibrin gels. (D) NS or Siβ ECs were isolated from the fibrin gels by digestion with collagenase type 1 for 1 hour, lysed with RIPA buffer, and proteins were resolved using SDS polyacrylamide gel electrophoresis and quantified (E). The bars represent the mean ± SEM of ≥4 independent experiments; *P < .05, Siβ vs NS.
PI3Kβ loss alters expression of tip cell markers. Primary human ECs were cultured on beads in fibrin gels as in Figure 5 for 18 hours, stimulated with SingleQuot growth factors or not, then imaged to evaluate tip cell morphology (A). Total RNA was extracted, then expression of characteristic tip cell transcripts was quantified (B). (C) Quantification of gene expression after growth factor stimulation of nontransfected (NT), nonspecific siRNA (NS)-transfected, or p110β (Siβ)-transfected cells at 18 hours of culture in fibrin gels. (D) NS or Siβ ECs were isolated from the fibrin gels by digestion with collagenase type 1 for 1 hour, lysed with RIPA buffer, and proteins were resolved using SDS polyacrylamide gel electrophoresis and quantified (E). The bars represent the mean ± SEM of ≥4 independent experiments; *P < .05, Siβ vs NS.
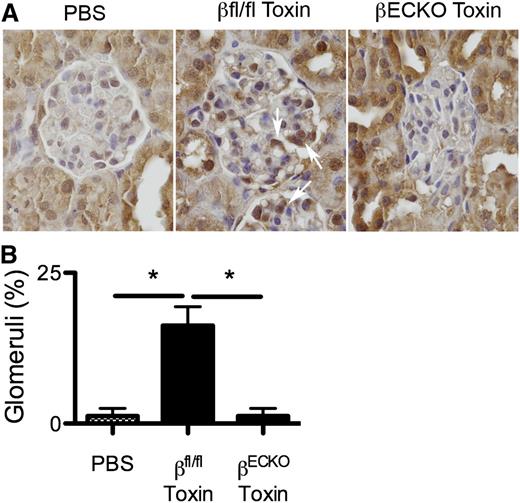
PI3Kβ loss inhibits the induction of apelin expression in vivo. Wild-type littermates or βECKO mice were treated with the lectin-saporin cytotoxin as in Figure 1, then kidney tissue was harvested at day 3 and immunostained for apelin expression (A). Uninjured kidney glomeruli show little apelin staining. Toxin-mediated EC injury induces glomerular apelin expression (middle panel, arrows) in wild-type, but not βECKO, mice. The frequency of glomeruli expressing apelin is quantitated (B) (mean ± SEM; n = 4 mice/group; *P < .05 by ANOVA).
PI3Kβ loss inhibits the induction of apelin expression in vivo. Wild-type littermates or βECKO mice were treated with the lectin-saporin cytotoxin as in Figure 1, then kidney tissue was harvested at day 3 and immunostained for apelin expression (A). Uninjured kidney glomeruli show little apelin staining. Toxin-mediated EC injury induces glomerular apelin expression (middle panel, arrows) in wild-type, but not βECKO, mice. The frequency of glomeruli expressing apelin is quantitated (B) (mean ± SEM; n = 4 mice/group; *P < .05 by ANOVA).
Finally, we investigated the impact of PI3Kβ loss in human EC function and in vivo vasculogenesis. Human ECs were transfected with nonsilencing or p110β siRNA were suspended in Matrigel and injected subcutaneously into immunodeficient Rag2−/−γc−/− mice. Control siRNA-treated ECs in the Matrigel plugs were observed to form microvessel-like structures and to inosculate with the invading host vasculature to form chimeric blood vessels (supplemental Figure 8a). Some of these were perfused with mouse blood. However, p110β-deficient ECs were generally restricted to the center of the Matrigel plug and formed fewer microvessel-like structures, and no chimeric blood vessels were observed (supplemental Figure 8b-c). Similarly, VEGF-stimulated neoangiogenesis into subcutaneous Matrigel plugs is impaired in the βECKO mice (supplemental Figure 9). Taken together, these results indicate that PI3Kβ-deficient ECs, like endogenous mouse glomerular microvascular ECs, are defective in vascular cell remodeling associated with microvascular repair.
Discussion
In this study, we identify an important function of PI3Kβ in the vasculature. This lipid kinase is dispensable for vascular development in the embryo and vascular homeostasis in the adult quiescent vasculature. However, endothelial PI3Kβ confers substantial protection to the adult microvasculature under stress and facilitates recovery of organ function after microvascular injury. Mechanistic studies in vitro characterize defects in repair functions of PI3Kβ-deficient ECs, including invasion of fibrin matrix, motility, and gene transcription associated with differentiation to the invasive angiogenic tip cell phenotype. Human ECs deficient in PI3Kβ also failed to remodel to form vascular structures in vivo.
The study of microvascular development during embryogenesis and tumor neoangiogenesis has discovered specialization of the EC at the tip of an angiogenic sprout.11 In addition to characteristic filopodia extensions, expression of PDGFB and several other endothelial-specific genes have been identified as components of the tip cell phenotype.27-29
This same program may be exploited in repair of the endothelium of the established vasculature. Mechanical injury to the adult endothelium, such as the carotid artery, is repaired by migration of ECs from the edges of the wound. In vitro and in vivo, the lead ECs at the edge of a wound in the monolayer share features of the angiogenic tip cell, such as induced expression of PDGFB, but the lead ECs lack filopodia.30 The role of this EC specialization in repair of injury to the established microvasculature has been reported in a model of vascular regression in response to pharmacologic inhibition of endothelial growth factor receptors. McDonald et al identify both endothelial tip cell morphologic differentiation and invasion of remnant microvessel basement membrane sheaths to pattern microvascular regrowth upon withdrawal of the growth factor receptor inhibitor.31,32 Taken together with these observations, our data suggest a common model for microvascular repair dependent on EC specialization to the tip cell phenotype to re-endothelialize the glomerular microvasculature.
In several models of angiogenesis, VEGF stimulates differentiation of the ECs leading angiogenic sprouts and induces expression of apelin, Dll4, and ESM1.11,27 Reduced expression of both apelin and Dll4, but not ESM1, in sprouting PI3Kβ-deficient ECs stimulated with VEGF indicates that PI3Kβ activity regulates a subset of these genes. Because apelin deficiency in mice confers impaired angiogenesis, apelin loss likely contributes to the defective sprouting and repair among PI3Kβ-deficient ECs.33,34 On the other hand, Dll4 loss is anticipated to have the opposite effect.35-37 However, because sprout initiation by PI3Kβ-deficient ECs could not be rescued by paracrine effects of apelin elaborated by wild-type cells, the dominant effect of PI3Kβ inactivation may also involve other events. For example, we document defective responses to GPCR signaling.
The downstream events controlled by PI3Kβ activity are largely unknown. A subtle defect in ex vivo angiogenesis in response to CXCL12, but not VEGF, was demonstrated in aortic rings after embryonic deletion of PI3Kβ.18 Defective integrin-mediated adhesion contributes to poor platelet aggregation, aggregate stability, and attenuation of thrombosis in vivo after pharmacologic inhibition of PI3Kβ in wild-type animals.38-40 A similar defect in adhesion complex remodeling may explain modest impairment of EC migration and sprout extension in vitro and account, in part, for impaired vascular assembly in vivo. Moreover, rescue of the sprouting defect we observed in PI3Kβ-deficient ECs by concomitant PTEN loss suggests alternate PI3K isoforms may compensate for PI3Kβ loss and may explain the mild phenotype of embryonic PI3Kβ deletion. This points to PTEN inactivation as a means to overcome defective signaling for angiogenesis and repair along this pathway.
Taken together, our data identify endothelial signal transduction integrated through PI3Kβ as a novel pathway in vascular repair. Several growth factors are implicated in the defense of the glomerular endothelium, including mediators secreted by endogenous glomerular cells and both paracrine and autocrine VEGF signaling to the endothelium.26,41-43 Collectively, our data identify the PI3Kβ signaling node as an integration point for these signals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge technical assistance from Qiu-Xia Zhang, Steve Kulak, and Yue Huang.
This work was funded in part by Canadian Institutes for Health Research operating grants MOP 53319 (A.G.M.) and MOP 58998 (G.Y.O.). G.H. was supported by a University of Alberta 75th Anniversary studentship award. P.Z. was supported by a fellowship award from the Alberta Institute of Transplant Sciences.
Authorship
Contribution: G.H. and A.G.M. designed the research; G.H., P.Z., M.F., and L.F.Z. performed the research; B.V., Z.K., and G.Y.O. contributed materials; G.H., P.Z., M.F., D.C.R., and A.G.M. analyzed the data; and G.H., B.V., G.Y.O., and A.G.M. edited the manuscript.
Conflict-of-interest disclosure: B.V. is a consultant to Karus Therapeutics (Oxford, United Kingdom) and Activiomics (London, United Kingdom). The remaining authors declare no competing financial interests.
Correspondence: Allan G. Murray, Room 275 HMRC, University of Alberta, Edmonton, AB T6G 2S2, Canada; e-mail: allan.murray@ualberta.ca.