In this issue of Blood, Meyer et al report that deleting Jak2 selectively in megakaryocytes and platelets results in an unexpected thrombocytosis phenotype. Their results demonstrate that Jak2 is dispensable for megakaryocyte differentiation and platelet formation but is required for suppressing circulating thrombopoietin (Tpo).1
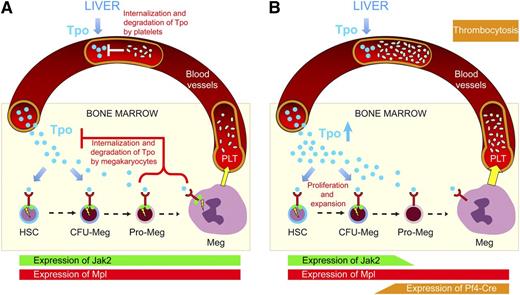
Model for the regulation of megakaryopoiesis and platelet numbers by Tpo. (A) Normal steady-state situation. Tpo (blue circles) is produced in the liver at a constant rate and reaches the bone marrow via the blood stream. Tpo enters the bone marrow microenvironment and binds to its receptor, Mpl (drawn in red), that is expressed on HSCs and megakaryocytic progenitors. Signaling requires the presence of Jak2 (green circles) and results in expansion of the HSCs and megakaryocytic progenitor pool. The megakaryocytic differentiation and polyploidization begins at the stage of promegakaryoblasts (pro-Meg) and ends with fully differentiated megakaryocytes (Meg), which deliver platelets (PLT) to the lumen of the blood vessels (yellow arrow). The bone marrow cells that express Mpl and platelets bind, internalize, and degrade Tpo, thereby lowering the available Tpo. (B) Megakaryocyte and platelet-specific knockout of Jak2. Expression of Cre-recombinase driven by the Pf4 regulatory elements begins in late megakaryocytic progenitors and deletes Jak2 in megakaryocytes and platelets. Mpl expression remains normal throughout megakaryopoiesis, but Tpo cannot signal in late megakaryopoetic cells or platelets. Mpl without Jak2 cannot efficiently remove and degrade Tpo. As a consequence, more Tpo is available in the bone marrow, leading to an expansion of HSCs, early megakaryocyte-biased progenitors, and colony-forming unit Meg. Thrombocytosis is observed in the peripheral blood.
Model for the regulation of megakaryopoiesis and platelet numbers by Tpo. (A) Normal steady-state situation. Tpo (blue circles) is produced in the liver at a constant rate and reaches the bone marrow via the blood stream. Tpo enters the bone marrow microenvironment and binds to its receptor, Mpl (drawn in red), that is expressed on HSCs and megakaryocytic progenitors. Signaling requires the presence of Jak2 (green circles) and results in expansion of the HSCs and megakaryocytic progenitor pool. The megakaryocytic differentiation and polyploidization begins at the stage of promegakaryoblasts (pro-Meg) and ends with fully differentiated megakaryocytes (Meg), which deliver platelets (PLT) to the lumen of the blood vessels (yellow arrow). The bone marrow cells that express Mpl and platelets bind, internalize, and degrade Tpo, thereby lowering the available Tpo. (B) Megakaryocyte and platelet-specific knockout of Jak2. Expression of Cre-recombinase driven by the Pf4 regulatory elements begins in late megakaryocytic progenitors and deletes Jak2 in megakaryocytes and platelets. Mpl expression remains normal throughout megakaryopoiesis, but Tpo cannot signal in late megakaryopoetic cells or platelets. Mpl without Jak2 cannot efficiently remove and degrade Tpo. As a consequence, more Tpo is available in the bone marrow, leading to an expansion of HSCs, early megakaryocyte-biased progenitors, and colony-forming unit Meg. Thrombocytosis is observed in the peripheral blood.
Jak2 is an intracellular tyrosine kinase that associates with hematopoietic cytokine receptors and is essential for mediating signaling by Tpo, erythropoietin, and in part also granulocyte colony-stimulating factor. Jak2 inhibitors can be used to suppress excess hematopoiesis in patients with myeloproliferative neoplasms, although at higher doses anemia and thrombocytopenia are frequently observed. Jak2 knockout mice die during embryogenesis due to absence of definitive erythropoiesis.2 Similarly, induced conditional knockout of Jak2 in adult hematopoiesis was lethal due to severe anemia and thrombocytopenia.3,4 Therefore, at first sight, thrombocytosis in mice lacking Jak2 in megakaryocytes may seem paradoxical. However, mice selectively lacking the Tpo receptor, Mpl, in megakaryocytes and platelets also displayed thrombocytosis.5 The genetic and functional data presented by Meyer et al and the recent report on Mpl-deficient mice by Ng et al5 shed new light into a delicately balanced system that keeps the levels of circulating platelets constant. Together these data suggest that the primary function of Jak2 and Mpl in late megakaryopoiesis and platelets is to reduce the availability of Tpo rather than to promote terminal megakaryocyte differentiation and platelet production. Because Mpl is dependent on Jak2 for signaling, the 2 stories are intricately connected.
Although Jak2 expression is ubiquitous, Mpl is expressed in hematopoietic stem cells (HSCs) and early progenitors and at later stages becomes limited to the megakaryocytic lineage (panel A). The circulating Tpo concentration is directly regulated by a negative feedback mechanism exerted by platelets and megakaryocytes.6 Mpl protein expressed on the surface of platelets and megakaryocytes binds Tpo, which is then internalized and degraded.7,8 Consequently, Mpl-deficient mice are thrombocytopenic with very high Tpo serum levels. However, thrombocytosis was observed in 2 mouse models with decreased Mpl expression on differentiated megakaryocytes and platelets as a first hint that the main function of Mpl on megakaryocytes and platelets could be to down-regulate Tpo availability.9,10 The studies by Ng et al and Meyer et al both use Pf4-Cre mice, which express the Cre-recombinase primarily in megakaryocytes and platelets, allowing lineage-specific deletion of conditional alleles during late megakaryocyte maturation but preserving expression in HSCs and progenitors. Both studies now firmly demonstrate that the absence of Mpl and Jak2 in megakaryocytes and platelets does not interfere with terminal differentiation and platelet formation. Thus, with hindsight, it was good to abandon the initially proposed alternative name for Tpo: megakaryocyte growth and development factor. Moreover, both studies found that the HSC and progenitor pool was expanded, suggesting that the stimulatory effects of Tpo mediated by Mpl and Jak2 are due to increased numbers of committed megakaryocytic progenitors (panel B). Although it is easy to see how loss of Mpl expression on megakaryocytes and platelets reduces Tpo binding and internalization, the loss of Jak2 in late megakaryopoiesis could equally well result in normal platelet numbers. So why do the Pf4-Cre Jak2-deficient mice display such a pronounced thrombocytosis?
One hypothetical scenario is that Jak2 deficiency reduces Mpl surface expression, eg, because Jak2 protein may be needed as a chaperone to guide Mpl from the endoplasmatic reticulum through the Golgi to reach the cell surface. However, surface expression of Mpl on megakaryocytes and platelets in Pf4-Cre Jak2-deficient mice was comparable with wild-type controls (see Figure 2J in Meyer et al). The Tpo serum levels in Pf4-Cre Jak2-deficient mice were equal to controls, but given that the platelets numbers were fivefold increased, the Tpo levels in these mice are expected to be lower than in the controls. This suggests that the Jak2-deficient megakaryocytes and platelets are less capable of binding and removing Tpo, as indeed shown by Meyer et al (see Figure 2K in Meyer et al). The mechanism of how Jak2 is involved in this process remains to be determined.
Together, the results of the 2 studies by Meyer et al and Ng et al demonstrate that Mpl and Jak2 are dispensable for terminal megakaryocyte differentiation and platelet formation and suggest that the key regulatory step controlled by Tpo and Mpl is to stimulate a megakaryocyte-biased stem and progenitor cells and thereby to determine the number of committed megakaryocytic progenitors and undifferentiated megakaryocytic precursors.
Conflict-of-interest disclosure: The author declares no competing financial interests.