Key Points
C2 confers increased susceptibility to childhood B-ALL.
C2 is associated with increased risk of late relapse in childhood B-ALL.
Abstract
A role for HLA class I polymorphism in childhood acute lymphoblastic leukemia (ALL) has been suggested for many years, but unambiguous associations have not been found. Here, we show that the HLA-C–encoded supertypic epitope C2, which constitutes a high-affinity ligand for the inhibitory natural killer (NK) cell receptor KIR2DL1, is significantly increased in ALL patients (n = 320; P = .005). Stratification for ethnicity and disease subtype revealed a strong association of C2 with B-ALL in German cases (P = .0004). The effect was independent of KIR2DS1 and KIR2DL1 allelic polymorphism and copy number. Analysis of clinical outcome revealed a higher incidence of late relapse (>2.5 years) with increasing number of C2 alleles (P = .014). Our data establish C2 as novel risk factor and homozygosity for C1 as protective for childhood B-ALL supporting a model in which NK cells are involved in immunosurveillance of pediatric B-ALL via interaction of KIR with HLA-C ligands.
Introduction
Natural killer (NK) cells serve important functions in immunosurveillance of hematologic malignancies. Inhibitory killer cell immunoglobulin-like receptors (KIR) recognizing the HLA-C–encoded ligands C1 and C2 are central to this process. C2 is recognized by the inhibitory KIR2DL1 with high affinity and specificity as well as by the stimulatory KIR2DS1 receptor. KIR2DL2/3 interact with C1 ligands with less affinity and specificity, ie, KIR2DL2 and to a lesser extent KIR2DL3 show some cross-reactivity with C2 ligands.1 In the setting of hematologic stem cell transplantation, the presence of C2 ligands was already identified as risk factor in patients with myeloid leukemia.2-4 In lymphoblastic leukemia, recent studies suggest that KIR or KIR ligands may play a role.5,6 Here, we asked the question if the C1/C2 dimorphism constitutes a susceptibility locus for childhood acute lymphoblastic leukemia (ALL).
Study design
Patients and controls
We analyzed a cohort of 320 pediatric ALL cases treated at the University Hospital Düsseldorf between 1988 and 2013. Patient characteristics and inclusion and exclusion criteria are given in supplemental Table 1 and supplemental Figure 1, available on the Blood Web site. The healthy control cohort was taken from the Düsseldorf Bone Marrow Registry as part of a prospective HLA class I typing project involving randomly selected donors (n = 1515). Donors did not undergo any specific medical examination or selection according to health status, were not related to any of the patients, and were not previously selected for stem cell donation for an unrelated leukemic patient. German ethnicity was assigned to donors with first and last name of the German-speaking area and residency in Germany.
KIR and HLA genotyping
KIR genotyping was performed by polymerase chain reaction with sequence-specific primers (PCR-SSP) as previously described.7 A total of 10% of samples were randomly retyped by an independent KIR typing method with full concordance.8 Subtyping for KIR2DL1 alleles (KIR2DL1*001-004) was done as previously described.9 Additional SSPs were used for KIR2DL1*004 (forward-CATCTTCTTCCAGGTAACCCCC, reverse-TTTTGTTGGAGCACCAGCA). KIR2DL1 copy-number variation was analyzed by SYBR green–based real-time PCR (StepOnePlus PCR system; Applied Biosystems). Results were normalized to KIR3DL2, which served as internal 2-copy control gene (gene frequency in whites of 99.7%).10 HLA class I typing was performed by Luminex technology (One Λ). Confirmatory typing for C1/C2 epitopes was performed by PCR-SSP as previously described.11
Statistics
Two-tailed Fisher’s exact test was used to compare categorized groups. Cumulative incidences of relapse and late relapse (defined as relapse occurring ≥30 months from remission12 ) were analyzed after adjusting for the competing risk of nonrelapse mortality. Interactions of different covariates on the analytical end point relapse were evaluated by stepwise proportional hazards general linear model (PHGLM) analysis using Cox regression models analyzing time to relapse with nonrelapse mortality as competing risk. Differences between time-to-relapse distributions were compared by a log-rank test.
Results and discussion
In order to examine the influence of HLA-C–encoded KIR ligands, a cohort of pediatric ALL patients (n = 320) was stratified into 3 groups according to the C1/C2 dimorphism. As shown in Table 1, the frequency of patients with 2 C1 alleles was significantly decreased, whereas the corresponding carrier frequency for C2 was significantly increased in the ALL cohort (P = .0051). Furthermore, allele frequencies were significantly biased toward C2 in ALL patients (P = .0037). This observation was true for B-cell ALL (B-ALL) (n = 273), whereas in T-cell ALL (T-ALL) (n = 43), the distribution was similar to controls (data not shown). Because C1 and C2 frequencies vary greatly between different ethnicities even within Europe, further analysis was restricted to B-ALL patients of German origin, which constituted the largest disease- and ethnically matched subgroup in our patient cohort (n = 204). C2 allele frequencies of the German control cohort (n = 1020, 36.2%) were comparable to those from populations of the same area retrieved from a public database,13 confirming that the control cohort indeed is representative for German ethnicity (supplemental Figure 2). Similar to the previous analysis comprising all cases, homozygosity for C1 was significantly decreased in B-ALL patients of German ethnicity (Table 1). Of note, no significant difference was seen for C1 carrier frequency (C1/C1 + C1/C2) between cases and controls (81.9% vs 86.6%). In contrast, C2 carrier frequency (C1/C2 + C2/C2) was significantly increased in the German B-ALL cohort (72.1% vs 58.8%, P = .0004). Together with the fact that the odds ratios (ORs) are similar for C1/C2 and C2/C2 individuals (OR = 1.44) in B-ALL German cases (Table 1), this clearly suggests that presence of C2 constitutes a risk factor in B-ALL, whereas homozygosity for C1 confers an equally strong protective effect (Table 1).
It was next examined if frequencies of C2-specific KIR genes KIR2DL1 and KIR2DS1 were also affected. In our previous study of B-ALL patients (cases included in the present larger cohort), no significant deviations from control values were seen for any KIR gene.14 However, epistatic interaction of the C2-specific KIR2DL1 gene with C2 might be difficult to detect given the high carrier frequency of KIR2DL1 in whites (>95%).10,15 We thus attempted to detect a possible epistatic interaction by analyzing allelic and copy-number variation (CNV). KIR2DL1 CNV of German B-ALL cases was comparable to German controls except cases with 0 copies, which were found more frequently in B-ALL patients (Table 1 and supplemental Table 2). However, following adjustment for multiple testing, significance was lost. We also considered allelic variation of KIR2DL1 as possible contributing factor. Analysis of allele-specific expression in NK cells of healthy controls by flow cytometry revealed a significantly decreased expression of KIR2DL1*004, an allele that was previously shown to have impaired signal-transduction capacity (supplemental Table 3).16 However, PCR-SSP subtyping for the most common alleles (covering >98% of KIR2DL1 allotypes in whites)15 including KIR2DL1*004 did not reveal significant differences (Table 1 and supplemental Table 2). Similarly, no significant changes in frequency and CNV were found for the C2-specific stimulatory KIR2DS1 (Table 1 and supplemental Table 2). Of note, KIR2DS1 exhibits only minor allelic polymorphism (2DS1*002 present in >97% of whites).15 Thus, our thorough analysis of KIR2DL1 polymorphism did not reveal any overt epistatic interaction with C2 in B-ALL.
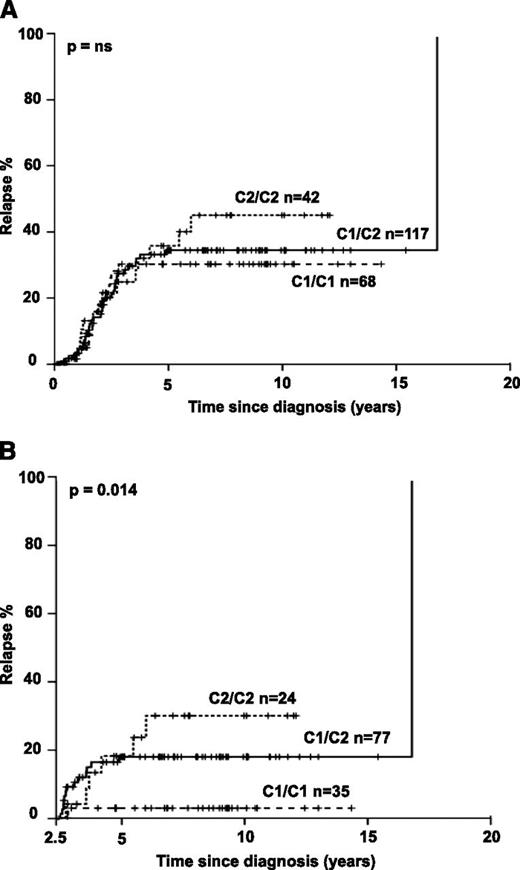
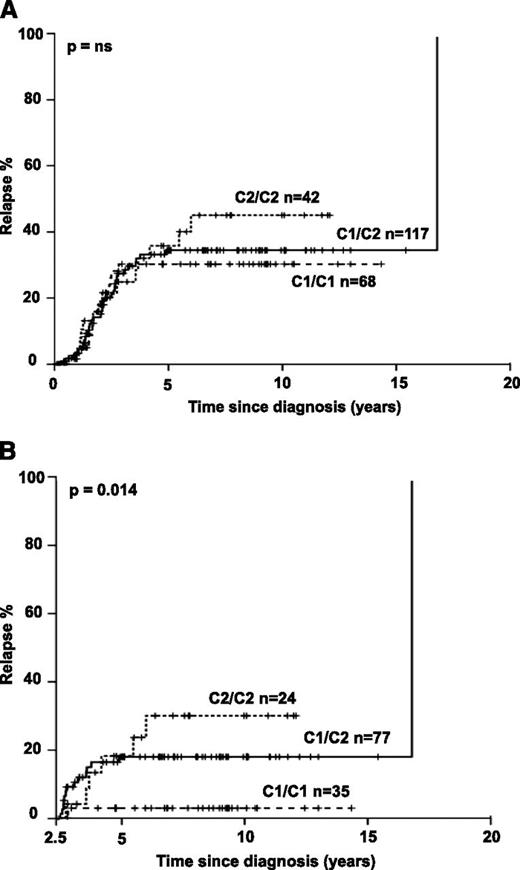
We next asked if HLA-C–encoded KIR ligands would also impact clinical outcome. Indeed, B-ALL patients homozygous for C2 exhibited a nonsignificant increase in relapse frequency (Figure 1A). Importantly, time-dependent analysis revealed that C1/C1 patients exhibited a very low frequency of late relapse and that the number of C2 alleles was significantly associated with increasing risk for late relapse (Figure 1B). Distribution of known confounding factors (age, sex, initial leukocyte count, and immunophenotype) was comparable among the 3 groups (supplemental Table 1). Multivariate analysis including the above-mentioned confounding factors and considering the number of C2 ligands as covariate confirmed a significantly increased risk for relapse in B-ALL with C2 ligands (P = .013; OR = 2.32; 95% confidence interval [CI], 1.18-4.57). A significant association of C2 ligands with relapse was also seen when National Cancer Institute criteria for pediatric high-risk leukemia (white blood cell count ≥50 000/μL and/or age <1 year or >10 years) were applied for multivariate analysis (P = .046; OR = 1.21; 95% CI, 1.00-1.46). We next evaluated if the C2 effect was sensitive to the changes in treatment protocols that were implemented in the last 2 decades. Splitting the cases into an “early” (1988-2003) and a “late” cohort (2004-2010) of similar size revealed a stable C2 effect, suggesting that evolution of treatment regimen did not confound analyses (supplemental Figure 3). Notably, no significant association with leukemia-free survival and overall survival was found (supplemental Figure 4).
Increased frequency of late relapse in B-ALL patients with HLA-C2. Kaplan Meier analysis was performed for all B-ALL patients with available clinical and follow-up information. Patients not achieving remission during induction chemotherapy and patients undergoing hematopoietic stem cell transplantation without prior relapse were excluded from this analysis. Cases were divided into 3 groups based on C1/C1, C1/C2, and C2/C2 genotypes. (A) Kaplan-Meier estimates are shown for all patients for the full observation time starting at the time of diagnosis. (B) Only patients who achieved complete remission, finished standard treatment, and remained event free for at least 2.5 years were analyzed (qualifying for late relapse ≥30 months after diagnosis). P values were calculated by 2-sided log-rank test; A: P = .391).
Increased frequency of late relapse in B-ALL patients with HLA-C2. Kaplan Meier analysis was performed for all B-ALL patients with available clinical and follow-up information. Patients not achieving remission during induction chemotherapy and patients undergoing hematopoietic stem cell transplantation without prior relapse were excluded from this analysis. Cases were divided into 3 groups based on C1/C1, C1/C2, and C2/C2 genotypes. (A) Kaplan-Meier estimates are shown for all patients for the full observation time starting at the time of diagnosis. (B) Only patients who achieved complete remission, finished standard treatment, and remained event free for at least 2.5 years were analyzed (qualifying for late relapse ≥30 months after diagnosis). P values were calculated by 2-sided log-rank test; A: P = .391).
The present study shows that HLA-C2 ligands not only are associated with increased susceptibility to B-ALL but also constitute a risk factor for late relapse. These observations are compatible with a model in which C2 ligands impair NK cell–mediated surveillance for leukemic cells at both the stage of leukemia initiation and in remission following completion of chemotherapy. Mechanistically, the inhibitory KIR2DL1 binds to C2 with higher affinity than KIR2DL2/3 to C1 and KIR2DL2 to C2. The exceptional specificity and affinity of the KIR2DL1-C2 interaction might lead to stronger inhibition of NK cells, which could compromise the efficiency of leukemia surveillance. The fact that we could not detect a major influence of KIR2DL1 allelic variation or CNV is not necessarily at odds with this affinity model. Firstly, KIR CNV is associated with increased expression frequency but not with increased mean expression on the cell surface and does not majorly influence target cell recognition.17 Thus, CNV effects on leukemia recognition might be quite subtle. Secondly, significant allele-specific expression differences were restricted to KIR2DL1*004, which is a relatively rare variant in whites. Our study might thus be underpowered to detect a significant allele-specific effect.
Notably, we did not find any association of the KIR ligand Bw4 with B-ALL (Table 1). It is currently unclear how these results compare with a recent study describing an association with Bw4 in non-Hispanic white ALL patients.6 Notably, in both studies, high-affinity inhibitory KIR ligands seem to be associated with susceptibility to pediatric ALL.
This is the first study of childhood B-ALL reporting an association of KIR ligands with clinical outcome in a nontransplant setting. If confirmed in independent studies, these observations might have relevance for adjustment of therapy (eg, intensity, duration) to the increased risk of late relapse in B-ALL patients with C2 ligands, particularly those with homozygous C2 ligands.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all parents who gave their consent to use the biological material from their minors and all volunteering adult blood donors.
This work was supported by the Deutsche Krebshilfe e.V. (M.U., R.M., and A.B.) (project 110351), the Forschungskommission of the Medical Faculty of the Heinrich-Heine-Universität Düsseldorf (F.B.), and the Deutsche Forschungsgemeinschaft (M.U.) (UH 91/7-1).
Authorship
Contribution: F.B. and A.R.M. contributed equally to this work; F.B., A.R.M., and M.U. designed the project, performed the experiments, and wrote the paper; N.S. performed the experiments; J.C.F. and J.E. contributed samples and performed statistical analysis; O.C. and A.M. performed analyses and revised the manuscript; A.B. and R.M. contributed clinical samples, treated patients according to the current therapy protocol, and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Uhrberg, Institute for Transplantation Diagnostics and Cell Therapeutics, Heinrich Heine University, Moorenstr 5, 40225 Düsseldorf, Germany; e-mail: uhrberg@itz.uni-duesseldorf.de.
References
Author notes
F.B. and A.R.M. contributed equally to this study.