Key Points
Jak2 deletion in PLTs and MKs leads to thrombocytosis due to dysregulated TPO turnover.
Jak2 loss in PLTs/MKs induces non-autonomous expansion of stem/progenitors, and specifically of MK-primed hematopoietic stem cells (HSCs).
Abstract
JAK inhibitor treatment is limited by the variable development of anemia and thrombocytopenia thought to be due to on-target JAK2 inhibition. We evaluated the impact of Jak2 deletion in platelets (PLTs) and megakaryocytes (MKs) on blood counts, stem/progenitor cells, and Jak-Stat signaling. Pf4-Cre–mediated Jak2 deletion in PLTs and MKs did not compromise PLT formation but caused thrombocytosis, and resulted in expansion of MK progenitors and Lin−Sca1+Kit+ cells. Serum thrombopoietin (TPO) was maintained at normal levels in Pf4-Cre–positive Jak2f/f mice, consistent with reduced internalization/turnover by Jak2-deficient PLTs. These data demonstrate that Jak2 in terminal megakaryopoiesis is not required for PLT production, and that Jak2 loss in PLTs and MKs results in non-autonomous expansion of stem/progenitors and of MKs and PLTs via dysregulated TPO turnover. This suggests that the thrombocytopenia frequently seen with JAK inhibitor treatment is not due to JAK2 inhibition in PLTs and MKs, but rather due to JAK2 inhibition in stem/progenitor cells.
Introduction
Myeloproliferative neoplasms (MPN) are characterized by clonal proliferation of mature myeloid cells. Somatic mutations in the JAK2 pathway, including JAK2V617F,1-4 JAK2 exon 12 mutations,5 and in the thrombopoietin (TPO) receptor MPL6 are observed in MPN patients. JAK kinase inhibitors attenuate splenomegaly and constitutional symptoms in MPN,7,8 but cannot induce molecular responses. JAK inhibitor therapy results in the variable development of anemia and thrombocytopenia preventing dose intensification and maximal target inhibition.9 Cytopenias have been considered on-target effects of JAK2 inhibitors inseparable from therapeutic efficacy. However, the risk of thrombocytopenia varies among JAK2 inhibitors,10 suggesting thrombocytopenia may not be a direct consequence of JAK2 inhibition.
JAK2 mediates cytokine signaling from erythropoietin, TPO, and granulocyte-macrophage (GM)–colony-stimulating factor receptors.11 Germline deletion of Jak2 causes embryonic lethality by deficient definitive erythropoiesis,12,13 which was confirmed by a conditional Jak2 allele and germline Cre-recombinase.14 Conditional Jak2 loss in hematopoietic stem cells (HSCs)/progenitor cells induces anemia and thrombocytopenia,15,16 whereas deletion of Mpl in platelets (PLTs) leads to thrombocytosis.17-19 Although these studies highlight the significance of Jak-Stat signaling for steady state hematopoiesis, the specific requirement for Jak2 in different terminally differentiated myeloid lineages, including PLTs, has not been clarified. We, therefore, investigated the effects of PLT-specific Jak2 loss in vivo.
Study design
Mice
Signaling
PLTs were purified on a sepharose CL-2B column, and stimulated with TPO and lysates probed for Jak2, Stat3/5, Tyk2, β-actin (Cell Signaling), and Mpl (Wei Tong, Philadelphia).
Flow cytometry
Bone marrow was stained for lineage markers, Sca-1, c-Kit, CD41, CD150, CD48, CD16/32, and CD10521 (eBioscience), and PLTs for CD42, CD61, and Mpl, and analyzed/sorted on FACSAria III (BD Biosciences). Megakaryocytes (MKs) were purified by CD41-phycoerythrin-magnetic beads (Stemcell Technologies).18
Quantitative polymerase chain reaction
RNA was isolated in TRIzol (Life Technologies) and reverse transcribed by the Verso cDNA Kit (Thermo) for quantitative polymerase chain reaction with SYBR Green (Applied Biosystems) and β-actin for normalization.
Colony assays
Acetylcholinesterase-positive MK colonies were grown from 0.5 × 105 bone marrow cells or 3000 megakaryocyte-erythroid progenitors (Pre-MegE), MK progenitors (MKPs), or Lin−Sca1+Kit+ (LSK) cells in MegaCult-C (Stemcell Technologies) with TPO, IL-6, and IL-3 (R&D). Myeloid colonies from 4000 Pre-MegE, MKP, or 200 LSK cells were cultured in MethoCult (Stemcell Technologies) with stem cell factor, IL-3, IL-6, and erythropoietin.
Thrombopoietin measurement
TPO was determined by Quantikine enzyme-linked immunosorbent assay (R&D) in serum, or in media22 incubated with purified PLTs.
Results and discussion
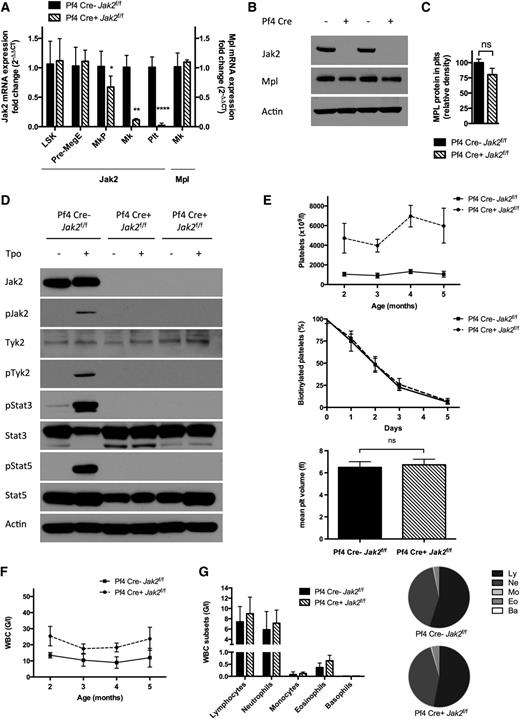
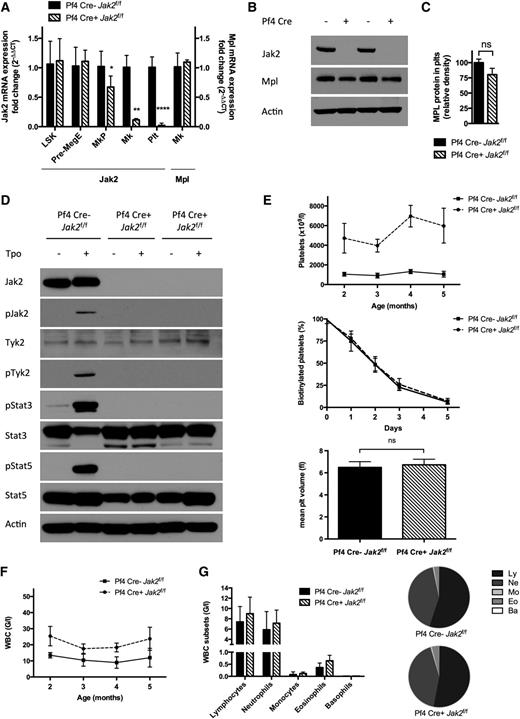
To assess the requirement for Jak2 in PLTs, we deleted Jak2 in PLTs and MKs using Cre-recombinase expressed under the Pf4 promoter.14,19,20,23 Pf4-Cre–positive Jak2f/f mice showed loss of Jak2 messenger RNA (mRNA) expression in PLTs and MKs demonstrating complete excision, and we observed a modest reduction in Jak2 mRNA expression in MKPs (P = .029) (Figure 1A). Jak2 mRNA expression was unaffected in Pre-MegE or LSK cells (n = 3 to 6 mice/group). Western blot analysis revealed loss of Jak2 protein expression in Pf4-Cre–positive Jak2f/f PLTs, despite continued Mpl expression (Figure 1B-C). Jak2 deletion in PLTs abrogated Jak2, Stat3, Stat5, and Tyk2 phosphorylation upon TPO stimulation (Figure 1D). The absence of residual Tyk2 phosphorylation was consistent with cell line studies, suggesting that TPO-mediated activation of Tyk2 requires Jak2.24
Jak2 loss in PLTs and MKs of Pf4-Cre–positive Jak2f/f mice results in a thrombocytosis phenotype. (A) Jak2 mRNA expression is abolished in PLTs and MKs, and reduced in MKPs (mean reduction 33%, P = .029) of Pf4-Cre–positive (Pf4 Cre+) Jak2f/f mice, whereas it is preserved in Pre-MegE and LSK cells, consistent with Jak2 loss in late megakaryo-/thrombopoiesis. Mpl mRNA expression is unchanged in MKs of Pf4-Cre–positive Jak2f/f mice (n = 3 to 6 mice per group and population). (B) Jak2 protein is absent in PLTs of Pf4-Cre–positive Jak2f/f mice and intact in Pf4-Cre–negative Jak2f/f littermate controls, while the TPO receptor Mpl is expressed. (C) Mpl protein expression in PLTs, as assessed by densitometry of Western bands is slightly reduced (P > .05). (D) PLTs devoid of Jak2 in Pf4-Cre–positive Jak2f/f mice are not reactive to stimulation with TPO (100 ng/ml) with inactive Stat3 and Stat5, whereas Jak signaling is activated in Pf4-Cre–negative Jak2f/f littermate controls. Tyk2 remains inactive in the absence of Jak2. (E) Elevated PLTs with unaffected survival and volume in Pf4-Cre–positive Jak2f/f mice, as compared with littermate controls demonstrate the dispensability of PLT Jak2 signaling for the production of PLTs (n = 5 to 17 mice per group and time-point). (F) WBC count is slightly elevated in Pf4-Cre–positive Jak2f/f mice. (G) Elevated WBCs are due to a proportionate expansion of all leukocyte subsets with a subtle relative disadvantage of lymphocytes in Pf4-Cre–positive Jak2f/f mice. Results are representative of at least 2 independent experiments and shown as mean ± SEM. *P < .05; **P < .01; ****P < .0001.
Jak2 loss in PLTs and MKs of Pf4-Cre–positive Jak2f/f mice results in a thrombocytosis phenotype. (A) Jak2 mRNA expression is abolished in PLTs and MKs, and reduced in MKPs (mean reduction 33%, P = .029) of Pf4-Cre–positive (Pf4 Cre+) Jak2f/f mice, whereas it is preserved in Pre-MegE and LSK cells, consistent with Jak2 loss in late megakaryo-/thrombopoiesis. Mpl mRNA expression is unchanged in MKs of Pf4-Cre–positive Jak2f/f mice (n = 3 to 6 mice per group and population). (B) Jak2 protein is absent in PLTs of Pf4-Cre–positive Jak2f/f mice and intact in Pf4-Cre–negative Jak2f/f littermate controls, while the TPO receptor Mpl is expressed. (C) Mpl protein expression in PLTs, as assessed by densitometry of Western bands is slightly reduced (P > .05). (D) PLTs devoid of Jak2 in Pf4-Cre–positive Jak2f/f mice are not reactive to stimulation with TPO (100 ng/ml) with inactive Stat3 and Stat5, whereas Jak signaling is activated in Pf4-Cre–negative Jak2f/f littermate controls. Tyk2 remains inactive in the absence of Jak2. (E) Elevated PLTs with unaffected survival and volume in Pf4-Cre–positive Jak2f/f mice, as compared with littermate controls demonstrate the dispensability of PLT Jak2 signaling for the production of PLTs (n = 5 to 17 mice per group and time-point). (F) WBC count is slightly elevated in Pf4-Cre–positive Jak2f/f mice. (G) Elevated WBCs are due to a proportionate expansion of all leukocyte subsets with a subtle relative disadvantage of lymphocytes in Pf4-Cre–positive Jak2f/f mice. Results are representative of at least 2 independent experiments and shown as mean ± SEM. *P < .05; **P < .01; ****P < .0001.
We next investigated the impact of PLT-specific Jak2 deletion in vivo. Pf4-Cre–positive Jak2f/f mice developed thrombocytosis with average PLTs of 4720 G/l (range 3436 to 6460 G/l, P < .0001) at 2 months and 5966 G/l (3204 to 7892 G/l, P < .0001) at 5 months of age (n = 5 to 17 mice/group; Figure 1E). PLT survival assessed by in vivo biotinylation and PLT volume were normal in Pf4-Cre–positive Jak2f/f mice compared with littermate controls (Figure 1E). PLT-specific Jak2 loss resulted in leukocytosis with a mean white blood cell (WBC) count of 23.7 G/l (vs 11.9 G/l in controls, P = .005) with proportionate expansion of all leukocyte subsets (Figure 1F-G). Hematocrit levels were not significantly affected. PLT-specific Jak2 haploinsufficiency had no impact on blood counts compared with controls. These data demonstrate that Jak2 expression and activity in PLTs are not required for terminal PLT production.
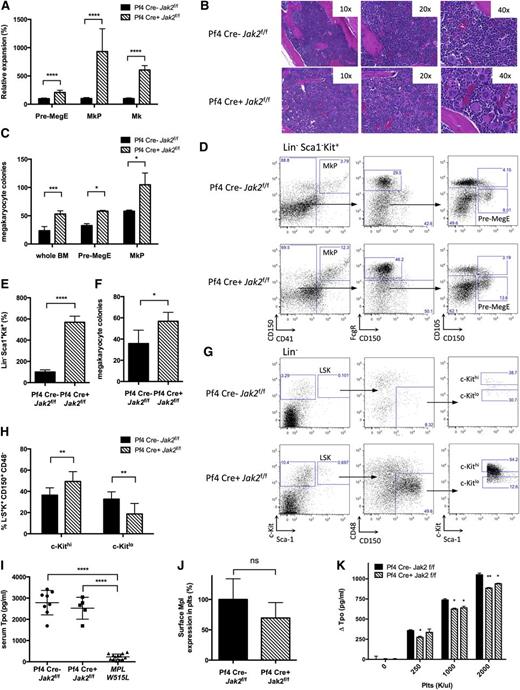
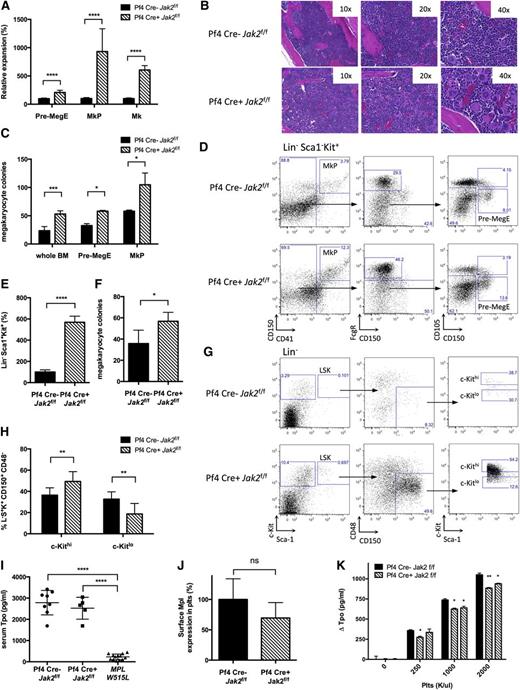
The thrombocytosis observed in Pf4-Cre–positive Jak2f/f mice suggested PLT/MK-specific Jak2 deletion led to non-autonomous expansion of Jak2-expressing stem/progenitor cells. Pf4-Cre–positive Jak2f/f mice had expansion of Pre-MegE (209% of controls, P < .0001), MKP (932% of controls, P = .0001), and MKs (606% of controls, P < .0001) (n = 3 to 12 mice/group; Figure 2A,D). Histopathologic analysis confirmed bone marrow megakaryocytosis (Figure 2B). MK colony formation from Pf4-Cre–positive Jak2f/f bone marrow and sorted Pre-MegE or MKP was enhanced (Figure 2C). We observed an increase in colony-forming unit (CFU)-GEMM and CFU-GM colonies but not BFU-E colonies from Pf4-Cre–positive Jak2f/f mice (see supplemental Figure 1A-B, available on the Blood Web site). These data demonstrate that PLT/MK-specific Jak2 loss induces expansion of megakaryopoiesis in vivo.
Cell non-autonomous expansion of stem/progenitor cells and LSK bias toward megakaryopoiesis in Pf4-Cre–positive Jak2f/f mice. (A) MKs, MKPs, and Pre-MegE are expanded in Pf4-Cre–positive (Pf4 Cre+) Jak2f/f mice as assessed by flow cytometry according to Pronk et al21 (n = 3 to 12 mice per group and population). (B) Megakaryocytic expansion is highlighted by hematoxylin and eosin stained bone marrow. (C) Formation of MK colonies from 0.5 × 105 whole bone marrow cells, 3000 sorted Pre-MegE or 3000 sorted MKP is increased in Pf4-Cre–positive Jak2f/f mice. (D) Flow cytometry gating scheme for megakaryocyte progenitors according to Pronk et al.21 (E) LSK cells are expanded in Pf4-Cre–positive Jak2f/f bone marrow analogous to Pre-MegE and MKP (n = 5 to 11 mice per group). (F) Formation of MK colonies from 3000 sorted LSK cells is increased in Pf4-Cre–positive Jak2f/f mice. (G) Flow cytometry gating scheme for MK-primed LSK subsets based on c-Kit surface expression analogous to Shin et al.26 (H) LSK CD150+CD48− HSCs are skewed toward MK-primed HSCs (c-Kithi) in Pf4-Cre–positive Jak2f/f mice. (I) Serum TPO is maintained at high steady state levels despite thrombocytosis in Pf4-Cre–positive Jak2f/f mice, while serum TPO is reduced in thrombocythemic mice with the MPLW515L allele and intact Jak229 (n = 5 to 13 mice per group). (J) Surface Mpl expression in PLTs as shown by flow cytometry is slightly reduced (P > .05, n = 4 to 10 mice per group). (K) TPO uptake by PLTs of Pf4-Cre–positive Jak2f/f mice, as reflected by TPO depletion of media incubated with purified PLTs, is compromised as compared with littermate controls. Results are representative of at least 2 independent experiments and shown as mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Cell non-autonomous expansion of stem/progenitor cells and LSK bias toward megakaryopoiesis in Pf4-Cre–positive Jak2f/f mice. (A) MKs, MKPs, and Pre-MegE are expanded in Pf4-Cre–positive (Pf4 Cre+) Jak2f/f mice as assessed by flow cytometry according to Pronk et al21 (n = 3 to 12 mice per group and population). (B) Megakaryocytic expansion is highlighted by hematoxylin and eosin stained bone marrow. (C) Formation of MK colonies from 0.5 × 105 whole bone marrow cells, 3000 sorted Pre-MegE or 3000 sorted MKP is increased in Pf4-Cre–positive Jak2f/f mice. (D) Flow cytometry gating scheme for megakaryocyte progenitors according to Pronk et al.21 (E) LSK cells are expanded in Pf4-Cre–positive Jak2f/f bone marrow analogous to Pre-MegE and MKP (n = 5 to 11 mice per group). (F) Formation of MK colonies from 3000 sorted LSK cells is increased in Pf4-Cre–positive Jak2f/f mice. (G) Flow cytometry gating scheme for MK-primed LSK subsets based on c-Kit surface expression analogous to Shin et al.26 (H) LSK CD150+CD48− HSCs are skewed toward MK-primed HSCs (c-Kithi) in Pf4-Cre–positive Jak2f/f mice. (I) Serum TPO is maintained at high steady state levels despite thrombocytosis in Pf4-Cre–positive Jak2f/f mice, while serum TPO is reduced in thrombocythemic mice with the MPLW515L allele and intact Jak229 (n = 5 to 13 mice per group). (J) Surface Mpl expression in PLTs as shown by flow cytometry is slightly reduced (P > .05, n = 4 to 10 mice per group). (K) TPO uptake by PLTs of Pf4-Cre–positive Jak2f/f mice, as reflected by TPO depletion of media incubated with purified PLTs, is compromised as compared with littermate controls. Results are representative of at least 2 independent experiments and shown as mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
We next assessed the impact of PLT-specific Jak2 loss on HSC/progenitor cells in vivo. We observed a significant expansion in LSK number (567% of controls, P < .0001), (n = 5 to 11 mice/group; Figure 2E). LSK cells from Pf4-Cre–positive Jak2f/f mice showed enhanced CFU-GEMM and CFU-GM colony formation (supplemental Figure 1C) and marked expansion in megakaryocytic colony formation on a per-cell basis (Figure 2F). This suggested the possibility that Pf4-Cre–positive Jak2f/f mice had selective expansion of MK-“primed” stem cells.25 Consistent with recent studies showing MK bias of c-Kithi HSC,26 Pf4-Cre–positive Jak2f/f mice had significant expansion of c-KithiLin−Sca1+Kit+CD150+CD48− HSC (Figure 2G-H). These data demonstrate PLT-specific Jak2 loss induces expansion of MK-biased stem/progenitors.
Previous studies have shown that PLTs and MKs regulate serum TPO through internalization attenuating TPO levels in thrombocytosis.27,28 We hypothesized that Jak2 loss in PLTs and MKs would compromise TPO internalization and maintain high steady state TPO despite thrombocytosis. We assessed TPO levels in Pf4-Cre–positive Jak2f/f mice, controls, and mice expressing MPLW515L,29 which develop a similar extent of thrombocytosis and MK expansion as Pf4-Cre–positive Jak2f/f mice. Serum TPO was markedly reduced in MPLW515L mice (TPO 230+/− 37 pg/mL in MPLW515L vs 2787+/− 204 pg/mL in controls, P < .0001), but not in Pf4-Cre–positive Jak2f/f mice (TPO 2524+/− 230 pg/mL, P < .0001) (n = 8 to 13 mice/group; Figure 2I). Jak2-deficient PLTs internalized less TPO than PLTs from Pf4-Cre–negative Jak2f/f controls (ΔTPO 884 pg/mL vs 1051 pg/mL, P < .01; Figure 2K). Mpl mRNA expression was unchanged in MKs of Pf4-Cre–positive Jak2f/f mice (Figure 1A), while Mpl total and surface protein was modestly reduced in Jak2-deficient PLTs (Figures 1C and 2J). These data suggest that TPO levels are maintained at an inappropriately high rheostat in Pf4-Cre–positive Jak2f/f mice due to reduced TPO internalization by Jak2-deficient PLTs.
In conclusion, our data demonstrate that Jak2 is dispensable for the production of MKs and PLTs. This finding suggests that thrombocytopenia observed with certain JAK2 inhibitors is due to JAK2 inhibition in more proximal stem/progenitor cells consistent with conditional Jak2 knockout studies in hematopoiesis,15,16 or potentially due to inhibition of other targets. Analogous to genetic Jak2 loss, Jak2 inhibition in PLTs/MKs may actually enhance PLT production,30 but may be overruled by the impact of Jak2 inhibition on stem/progenitors. Complementary to previous studies on the role of Mpl in PLTs,17-19 our results demonstrate an important role for PLT-specific Jak2 signaling in regulating TPO turnover, and show that PLT Jak2 signaling negatively regulates stem/progenitor cells, including MK-primed HSCs. Notably, HSCs biased toward megakaryopoiesis have been shown to express higher levels of MK-specific genes, including Mpl.25,26 Increased Mpl in MK-primed HSCs might enhance susceptibility to TPO in Pf4-Cre–positive Jak2f/f mice and contribute to thrombocytosis. Recent studies further suggest a role for MKs in regulating HSCs and have shown that PLT secretory factors can impact HSC numbers.31 Taken together, these data underscore the dynamic interaction between PLTs, MKPs, and stem/progenitors, and suggest that signaling in mature myeloid lineages can alter stem/progenitor function in vivo.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Levine Laboratory, Omar Abdel-Wahab, Christopher Park, Barry Coller, and Lorena Buitrago for helpful comments and discussion. The authors also thank P. Jan Hendrikx, Tom Baumgartner, Christi O’Donnell, and Paul Byrne from the Flow Cytometry Core Facility of Memorial Sloan Kettering Cancer Center for their support in sorting megakaryocyte progenitors, as well as Wei Tong (The Children’s Hospital of Philadelphia) for the kind gift of anti-Mpl antibody, and Kay-Uwe Wagner for Jak2f/f mice.
This study was supported by grants from the National Institutes of Health, National Cancer Institute (R01 CA151949-05) (R.L.L.), the Swiss National Science Foundation, the Swiss Cancer League, and the Huggenberger-Bischoff Foundation for Cancer Research (S.C.M.). R.L.L. is a Leukemia and Lymphoma Society Scholar.
Authorship
Contribution: S.C.M., M.D.K., B.A.W., L.M.L., L.B., M.K., N.B., and S.M. performed experiments; and S.C.M. and R.L.L. designed research, analyzed data, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ross L. Levine, Human Oncology and Pathogenesis Program, Leukemia Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 20, New York, NY 10065; e-mail: leviner@mskcc.org.
References
Author notes
M.D.K. and B.A.W. contributed equally to this study.