Key Points
Single cell lineage tracing shows early separation of blood-endothelial precursors in the mouse embryo.
Hemogenic endothelium in the YS generates the blood-endothelial common lineage and produces definitive precursors.
Abstract
The first blood and endothelial cells of amniote embryos appear in close association in the blood islands of the yolk sac (YS). This association and in vitro lineage analyses have suggested a common origin from mesodermal precursors called hemangioblasts, specified in the primitive streak during gastrulation. Fate mapping and chimera studies, however, failed to provide strong evidence for a common origin in the early mouse YS. Additional in vitro studies suggest instead that mesodermal precursors first generate hemogenic endothelium, which then generate blood cells in a linear sequence. We conducted an in vivo clonal analysis to determine the potential of individual cells in the mouse epiblast, primitive streak, and early YS. We found that early YS blood and endothelial lineages mostly derive from independent epiblast populations, specified before gastrulation. Additionally, a subpopulation of the YS endothelium has hemogenic activity and displays characteristics similar to those found later in the embryonic hemogenic endothelium. Our results show that the earliest blood and endothelial cell populations in the mouse embryo are specified independently, and that hemogenic endothelium first appears in the YS and produces blood precursors with markers related to definitive hematopoiesis.
Introduction
Establishment of the extra-embryonic blood circulation after gastrulation is essential for embryonic survival. The first blood cells form in the blood islands of the yolk sac (YS) mesoderm (as reviewed in Ferkowicz and Yoder1 ). Precursors of the extra-embryonic mesoderm are located in the proximal epiblast and are the earliest to migrate though the streak.2 As early as 1874, histologic analysis led Ranvier (as cited by Maximow3 ) to propose that hemangioblasts in the early extra-embryonic mesoderm generate both blood and endothelial cells in the mammalian YS blood islands. Studies in chicks also suggested direct lineage relationships between blood and endothelial cells.4 Single cells from the mouse primitive streak (PS) and embryonic stem cells (ESC) cultured in vitro generate cells expressing markers of endothelium, blood, and smooth muscle,providing functional evidence for the presence of multipotent cells in the early mesoderm.5,6 In addition, single-cell fate mapping of zebrafish embryos shows that a fraction of the endothelium- and blood-committed larval-cell populations indeed derive from single blastula precursors.7
In contrast with these results, in vivo lineage analyses have not supported the presence of hemangioblasts in the early mouse embryo. Fate mapping studies have suggested that prospective YS endothelium and blood cell populations are already specified in adjacent, independent regions of the pregastrula epiblast.8,9 Additional studies using ESC injections in blastocysts also failed to find strong evidence of hemangioblasts as progenitors of both endothelial and hematopoietic lineages in YS blood islands.10 These studies, however, presented limitations, as they were not based on epiblast single-cell labeling and both involved embryo culture and manipulation.
In parallel with the hemangioblast hypothesis, other pioneering histologic analyses instigated the idea of the hemogenic endothelium,3 which has been demonstrated in vitro11,12 and in the large embryonic vessels of a chick, mouse, and fish.13-15 In addition, endothelial cells with hematopoietic potential have also been described in the YS of the mouse embryo.16-21 Although a lineage relationship between endothelial and blood cells is thus well established, the relative importance of the hemangioblast bifurcation pathway vs a linear endothelial hemogenic pathway in early embryogenesis has not been addressed.
Materials and methods
Mouse strains, embryo handling, and recombination induction
Embryonic development was estimated by defining the time of vaginal plug observation as 0.5 dpc. Induction of recombination, resulting in activation of LacZ or EYFP, was achieved as described previously22 by a single injection of 4-hydroxy-tamoxifen (4OHT) into pregnant females (from 0.15 mg to 0.2 mg per pregnant female) at E5, E5.5 (pregastrulation stage), E6, or E6.5 days of gestation. The 4OHT did not compromise embryonic development or induce abortion. Embryos were dissected and analyzed at E6.5, E8-8.5, or E9.5. β-Galactosidase activity assay was performed as previously described23 and was followed by antigreen fluorescent protein (GFP) immunohistochemistry. Embryos were postfixed in 4% paraformaldehyde (PFA) and stored in 80% glycerol. To identify the presence of hematopoietic and endothelial cells in early mouse embryos, we bred R26REYFP reporter mice with a constitutive Tie2Cre mouse line.24 To study the lineage of Vav- and Tie2-expressing cells, we crossed the inducible Tie2CreERT2 line25 or the constitutive VavCre line26 with the R26REYFP reporter line.27 All animal procedures were reviewed and approved by the Centro Nacional de Investigaciones Cardiovasculares (CNIC) or the National Centre for Cardiovascular Research Animal Experimentation Ethics Committee, according to national and European regulations.
Cluster characterization and analysis
To normalize the definition of cell clusters, we used a computerized approach. The steps of the procedure were as follows: 1) Detection of cells was performed using the threshold tool in ImageJ to produce a binary map containing the distribution of labeled cells; 2) A Matlab algorithm was run that determined the distance of every cell to its nearest neighbor and assigned cells to clusters according to a threshold distance established by the researcher. In the extreme case of a threshold distance of zero, each cell would generate a cluster. At the opposite extreme, a threshold distance extending the whole YS would assign all cells to a single cluster; and 3) Different threshold distances were then empirically applied and the resulting collection of clusters was analyzed. The experimental threshold distance was chosen big enough for maximization of color mismatch between neighboring clusters (to avoid clone fragmentation) and small enough for minimization of the frequency of bicolor clusters (to minimize clustering of neighboring independent clones). The optimal threshold distance depends on each experimental setting, as it is affected by the clonal dispersion behavior and the time allowed for clone growth. The use of 2 markers allows the estimation of polyclonality; in the set of clusters in which 2 independent clones map together, the frequency of color mismatch between the 2 clones is equal to the frequency of color match. Therefore, the frequency of polyclonal monocolor clusters can be directly estimated from the frequency of bicolor clusters. Importantly, the estimation of polyclonality is effective irrespective of the distance threshold chosen. The possible interferences from the occurrence of more than 2 clones in a single cluster were not considered due to the low expected frequency.
Immunohistochemistry
Primary antibodies were rat anti-mouse CD41 (#553847, BD Pharmingen) diluted 1:20 and CD45 (#553076, 1:50), full-length AvGFP rabbit polyclonal antibody (#632460, Living Colors) diluted 1:200, and rabbit anti-mouse Lyve1 (#103-PA50S, ReliaTech) diluted 1:50. Secondary antibodies were biotin-conjugated goat polyclonal anti-rat or anti-rabbit IgG (Abcam), both at 1:500. Processed samples were either mounted in phosphate-buffered saline:glycerol (1:1) for YS flat-mounts, or glycerol jelly (10 g gelatin, 60 ml H2O, and 70 ml glycerol) for sections. Images were taken with a Nikon 90i microscope.
Immunofluorescence
Primary antibodies were as detailed above, with the addition of rabbit monoclonal anti-Runx1 (#ab92336, Abcam) at 1:50. Secondary antibodies were Alexa Fluor-conjugated goat polyclonal anti-rat or anti-rabbit IgG (Life Technologies) at 1:500. Embryos were imaged at 405, 488, 594, or 633 nm with a Leica SP5 multiline inverted confocal microscope fitted with a 40×/1.25 oil objective, and a Nikon A1R confocal microscope using 405, 458, 488, 568, and 633 nm wavelengths and 20×/0.75 dry and 40×/1.30 oil objectives.
Whole-mount in situ hybridization
Embryos were fixed overnight at 4°C with 4% PFA in phosphate-buffered saline and in situ hybridization was performed following standard protocols. Briefly, overnight-fixed embryos were dehydrated through a series of methanol concentrations. Embryos were rehydrated and hydrogen peroxide cleared for 1 hour. They were then treated for 1 minute at room temperature with proteinase K diluted to 10 μg/mL in phosphate-buffered saline with 0.1% Tween-20 and fixed for 20 minutes with 4% PFA - 0.2% glutaraldehyde. Embryos were treated for 1 hour at 66°C in standard prehybridization buffer, and then hybridized overnight. Embryos were then washed with decreasing concentrations (5× and then 2×) of saline sodium citrate in 50% formamide (at 66°C), and then blocked at room temperature with 10% sheep serum and 1% blocking reagent (Roche) in 1× Tris buffered saline and 1/1000 Tween (TBT). Anti-DIG Fab fragment antibody (Roche) was diluted 1:2000 in blocking reagent overnight at 4°C. Excess antibody was thoroughly washed out with TBT. After treatment with TBT, the staining reaction with BM purple (Roche) was developed for varying times.
Three-dimensional image rendering
Reconstructions were generated from z-stacks of E8.5 YS using Imaris or NIS-elements software.
Results
In this study, we studied the lineage relationships between blood and endothelial lineages in the early mouse embryo, using a random retrospective clonal analysis approach that allowed tracking of the progeny of single cells without interfering in normal development. The cell labeling strategy was based on conditional activation of a ubiquitous Knockin CreERT2 (RERT28 ) using low-dose 4OHT induction22 combined with ROSA26R Cre-reporter alleles. For our analysis, we introduced the simultaneous use of 2 independent Rosa26R reporters (ROSA26R-LacZ and ROSA26R-EYFP27,29 ) (Figure 1A). The 4OHT administration to pregnant females activated either reporter in a cell-to-cell and allele-to-allele stochastic manner.22 Given that the reporter and inducer alleles were expressed ubiquitously, this approach allowed random recombination of any cell in the developing embryo. In addition, the simultaneous use of 2 reporters allowed the experimental estimation of polyclonality in a series of inductions (see “Materials and methods” above).
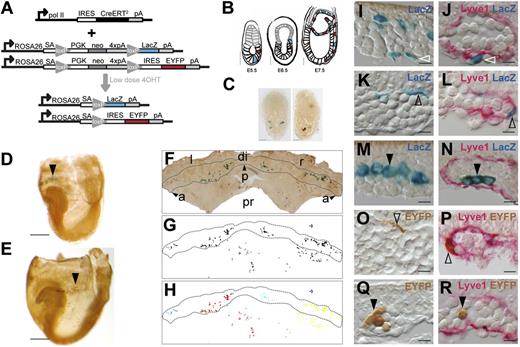
Random retrospective clonal analysis strategy. (A) Transgenic strategy with 2 independent reporters; ROSA26R-LacZ and ROSA26R-EYFP combined with the RERT transgene in which CreERT2 is expressed from the RNApol II gene. (B) Depiction of the intended strategy. After induction with low-dose 4OHT at E5.5, individual LacZ+ (blue) or EYFP+ (red) epiblast cells eventually migrate through the PS (E6.5) and colonize the extra-embryonic region (E7.5). (C) Examples of E6-6.5 embryos containing cells labeled with LacZ+ (X-Gal-stained) or GFP+ (antibody-detected) after 4OHT induction at E5.5. Scale bars, 500 µm. (D-E) E8.5 embryos retrieved after low dose 4OHT induction at E5.5 and containing either LacZ+ (D, arrowhead) or EYFP+ (E, arrowhead) cell clusters in the blood island region of the YS. Scale bars, 500 µm. (F-H) A flat-mounted E8.5 YS (F), its mask showing labeled cells (G), and the cell cluster segmentation revealed by color coding (H). (I-R) Histologic sections of the blood island region showing LacZ+ mesothelial (I-J), endothelial (K-L), and blood cells (M-N), and EYFP+ endothelial (O-P) and blood cells (Q-R). (J,L,N,P,R) Show co-localization with Lyve1+ immunostaining. Scale bars, 10 µm. Images in panels (C-E) were obtained with a Leica MZ-16 FA bionocular using a Plan Apo 1,0X objective and a Leica DFC310 FX camera. Images in (F) and (I-R) were obtained with a Nikon Eclipse 90i microscope using a Nikon DXM 1200c digital camera and 4X/0.2 NA objective (F) or 40X/0.95 NA objective and Nomarski contrast (I-R) (see also supplemental Figure 1). a and arrowhead, anterior; di, distal; IRES, internal ribosome entry site; l, left; neo, neomycin resistance gene; p and arrowhead, posterior; pA, polyadenylation site; PGK, phosphoglycerate kinase-1 promoter; pr, proximal; r, right; SA, splice acceptor.
Random retrospective clonal analysis strategy. (A) Transgenic strategy with 2 independent reporters; ROSA26R-LacZ and ROSA26R-EYFP combined with the RERT transgene in which CreERT2 is expressed from the RNApol II gene. (B) Depiction of the intended strategy. After induction with low-dose 4OHT at E5.5, individual LacZ+ (blue) or EYFP+ (red) epiblast cells eventually migrate through the PS (E6.5) and colonize the extra-embryonic region (E7.5). (C) Examples of E6-6.5 embryos containing cells labeled with LacZ+ (X-Gal-stained) or GFP+ (antibody-detected) after 4OHT induction at E5.5. Scale bars, 500 µm. (D-E) E8.5 embryos retrieved after low dose 4OHT induction at E5.5 and containing either LacZ+ (D, arrowhead) or EYFP+ (E, arrowhead) cell clusters in the blood island region of the YS. Scale bars, 500 µm. (F-H) A flat-mounted E8.5 YS (F), its mask showing labeled cells (G), and the cell cluster segmentation revealed by color coding (H). (I-R) Histologic sections of the blood island region showing LacZ+ mesothelial (I-J), endothelial (K-L), and blood cells (M-N), and EYFP+ endothelial (O-P) and blood cells (Q-R). (J,L,N,P,R) Show co-localization with Lyve1+ immunostaining. Scale bars, 10 µm. Images in panels (C-E) were obtained with a Leica MZ-16 FA bionocular using a Plan Apo 1,0X objective and a Leica DFC310 FX camera. Images in (F) and (I-R) were obtained with a Nikon Eclipse 90i microscope using a Nikon DXM 1200c digital camera and 4X/0.2 NA objective (F) or 40X/0.95 NA objective and Nomarski contrast (I-R) (see also supplemental Figure 1). a and arrowhead, anterior; di, distal; IRES, internal ribosome entry site; l, left; neo, neomycin resistance gene; p and arrowhead, posterior; pA, polyadenylation site; PGK, phosphoglycerate kinase-1 promoter; pr, proximal; r, right; SA, splice acceptor.
We first estimated the time window for induction after injection of a low dose of 4OHT during early embryogenesis. After induction at E5.5, 10 of 23 embryos (43%) contained either LacZ+ or EYFP+ cells at E6.5 (Figure 1B-C). When similarly induced embryos were allowed to progress to E8.5, 25 of 35 embryos were labeled (70%). Based on these results, ∼60% of the total final recombination events were already observable at E6.5 by the detection of the reporter genes. Given that the delay between recombination and reporter detection is at least 6 hours,30 we estimated that most recombination events took place within 24 hours of 4OHT injection. These estimates coincide with previous values reported for later developmental stages.22
Using a similar procedure, we generated a collection of 57 embryos induced with a low 4OHT dose at E5.5 and retrieved at E8.5 (Figure 1D-E). From these embryos, 33 contained labeled cells in blood islands and were further analyzed. To examine the distribution of labeled cell groups, we made YS flat-mounts (Figure 1F). Mask images were generated depicting the YS contour, the position of labeled cells, and the limits of the blood island region (Figure 1G-H). Labeled cells appeared in discrete coherent groups (“clusters”) where they intermingled with unlabeled cells (Figure 1F-H). A proportion of the embryos contained more than 1 cluster and some embryos presented clusters in which 2 cell groups expressing different reporters overlapped. To objectively assign cells to clusters, we developed a proximity-based clustering algorithm and identified cell clusters by their proximity, regardless of which reporter was activated (see “Materials and methods”), and selected clusters overlapping the blood island belt for further analysis (Figure 1F-H). Subsequently, we classified clusters by reporter expression: LacZ+, EYFP+, or Double+ (LacZ+ and EYFP+). We detected 49 mono-labeled groups and 7 Double+ groups (see supplemental Table 1, available on the Blood Web site). For further analysis of Double+ clusters, we considered the groups of cells expressing each reporter as independent clusters. We, therefore, studied 63 cell groups, of which an estimated 56 were monoclonal and 7 (11%) were polyclonal (see “Materials and methods”) (supplemental Table 1).
Clusters were individually examined in flat-mounts to determine the presence and abundance of endothelial and blood cells. Cells were first assigned to endothelial or hematopoietic lineages by their morphologic appearance and position in the blood islands. Cell morphology was assessed both in flat-mounts and in histologic sections (Figure 1I-R). Flat and extended cells wrapping blood cells in the blood island region were tentatively assigned to the endothelial type (Figure 1K,O). Rounded cells enclosed by the endothelial cells within blood islands were tentatively assigned to the hematopoietic type (Figure 1M,Q). The identity of the labeled cells was further determined by co-localization with Lyve1, a marker of YS endothelium31 (supplemental Figure 1). Endothelial staining also permits the identification of mesothelial cells (external to the endothelial layer, Figure 1I-J) and hematopoietic cells (internal to the endothelial layer, Figure 1I-R). Schemes summarizing the distribution of the labeled cells in the YS and in the rest of embryonic and extra-embryonic areas are presented in supplemental Figure 2.
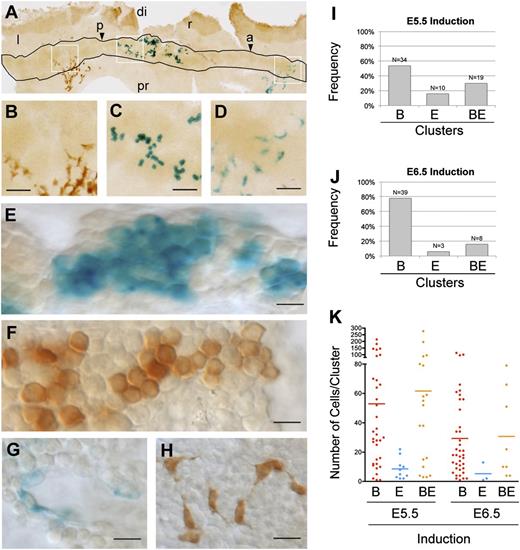
The distribution of cell clusters according to their contribution to the endothelial and blood cell populations indicated that 70% of clusters induced at E5.5 contribute to only one population, with a prevalence of blood cell clusters (B-clusters) over endothelial cell clusters (E-clusters) (Figure 2A-I and supplemental Table 1). B-clusters were larger than E-clusters (Figure 2K); they contained an average of 53 cells, which corresponds to 5.7 cell divisions since recombination, whereas the endothelial clusters contained an average of 9 cells, corresponding to 3.2 cell postrecombination divisions. Several B-clusters contained more than 100 cells (6.6 divisions) and one contained 215 cells (7.7 divisions). These results indicate that most YS precursors in the E6-6.5 epiblast are specified to produce either blood or endothelium, despite undergoing several rounds of cell division. Given the early stage of the recombination and the fast cycling in the epiblast, PS, and YS blood progenitors (4-6 cell cycles/day32-34 ), we estimate that by the time the labeled cells colonize the PS (E7), the average clone is composed of 5 cells, and therefore, that the number of PS cells interrogated for differentiation potential is considerably larger than the actual number of cell clusters examined. These results indicate that most epiblast cells can contribute only to blood or to endothelial precursors. Since epiblast cells are pluripotent and their fate depends on their position in the early embryo,35,36 these results agree with the previously observed early specification of the blood- and endothelial-forming regions in the epiblast.9
Random clonal analysis of early YS blood and endothelial precursors. (A-D) An example flat-mounted E8.5 YS with the blood island region outlined and the simultaneous detection of LacZ+ and EYFP+ cell clusters. Boxed areas are enlarged in (B-D). Scale bars, 50 µm. (E-H) High magnification from flat-mounts showing details of representative LacZ+ (E) and EYFP+ (F) B-clusters, and of LacZ+ (G) and EYFP+ (H) E-clusters. Scale bars, 10 µm. (I-J) Frequencies observed of B-, E-, and BE-clusters in E8.5 YS after 4OHT induction at E5.5 (I) or E6.5 (J). (K) Single-cluster size distribution (dots) and average size (bars) of B-, E-, and BE-clusters in E8.5 YS after 4OHT induction at E5.5 and E6.5. Images in (A-F) were obtained with a Nikon Eclipse 90i microscope using a Nikon DXM 1200c digital camera and 4X/0.2 NA objective (A) or 20X/0.75 NA (B-D), or 40X/0.95 NA objective and Nomarski contrast (E-H). (See also supplemental Figure 2 and supplemental Table 1.) a and arrowhead, anterior; di, distal; p and arrowhead, posterior; pr, proximal; l, left; r, right.
Random clonal analysis of early YS blood and endothelial precursors. (A-D) An example flat-mounted E8.5 YS with the blood island region outlined and the simultaneous detection of LacZ+ and EYFP+ cell clusters. Boxed areas are enlarged in (B-D). Scale bars, 50 µm. (E-H) High magnification from flat-mounts showing details of representative LacZ+ (E) and EYFP+ (F) B-clusters, and of LacZ+ (G) and EYFP+ (H) E-clusters. Scale bars, 10 µm. (I-J) Frequencies observed of B-, E-, and BE-clusters in E8.5 YS after 4OHT induction at E5.5 (I) or E6.5 (J). (K) Single-cluster size distribution (dots) and average size (bars) of B-, E-, and BE-clusters in E8.5 YS after 4OHT induction at E5.5 and E6.5. Images in (A-F) were obtained with a Nikon Eclipse 90i microscope using a Nikon DXM 1200c digital camera and 4X/0.2 NA objective (A) or 20X/0.75 NA (B-D), or 40X/0.95 NA objective and Nomarski contrast (E-H). (See also supplemental Figure 2 and supplemental Table 1.) a and arrowhead, anterior; di, distal; p and arrowhead, posterior; pr, proximal; l, left; r, right.
A proportion (30%) of the clusters characterized, however, contributes to both blood and endothelium (BE) clusters and cannot be explained by polyclonality generated from independent recombinations (11%) (Figure 2I and supplemental Table 1). These clusters could derive either from bipotent BE mesodermal precursors, or, given the early induction window, from recombinations in the early epiblast before full specification of the blood- and endothelial-forming regions. Supporting this second idea, BE-clusters are 15% larger than B-clusters and 6.7 times larger than E-clusters (Figure 2K), suggesting an average earlier induction. To test this, we performed inductions at E6.5 (21 embryos positive in blood islands from a total of 59 embryos), aiming to target cells mainly in the PS. The BE-cluster frequency dropped to 16%, approaching that of experimental polyclonality (14%) (Figure 2J, supplemental Figure 2, and supplemental Table 1). In addition, in E6.5-induced embryos, BE-clusters were of similar size to B-clusters (Figure 2K and supplemental Table 1). These results indicate that hematopoietic-committed precursors that contribute massively to blood cells can be targeted very early in development. To determine the stability of this contribution, we examined E5.5-induced embryos at E9.5, after circulation was established. We observed that 8 of 45 embryos showed widespread blood labeling without endothelial labeling. Some of these clones were massive and contributed more than 10% of total blood cells at E9.5 (see example in supplemental Figure 3).
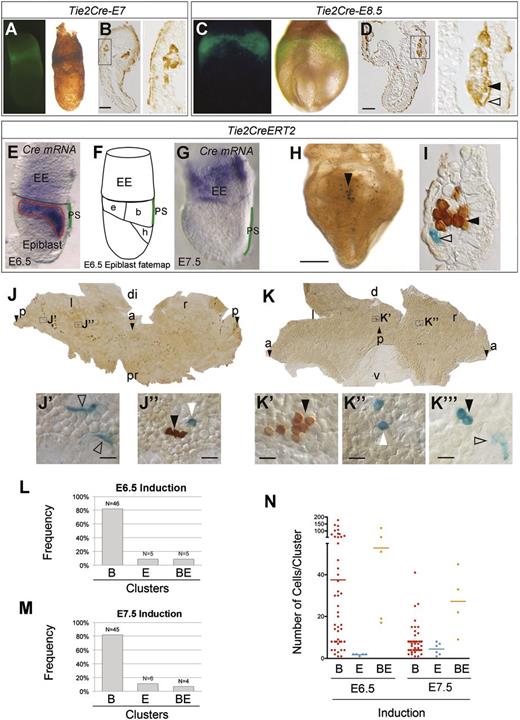
Therefore, the results from random induction at E5.5 and E6.5 suggest that the earliest blood and endothelial YS cells mostly derive from independent pools of epiblast cells specified before gastrulation. These experiments do not, however, exclude the presence of small populations of mesodermal precursors with dual BE potential, which would be difficult to trace with a random recombination strategy. To get around this, we induced recombination driven by the regulatory sequences of Tie2, which is expressed in blood and endothelial precursors and is thought to form part of the hemangioblast signature.6,17 We first determined the contribution of the whole Tie2 lineage using a constitutive Tie2-Cre line,24 finding that Tie2Cre specifically labels all endothelial and blood cells in blood islands at E7.0 (Figure 3A-B) and E8.5 (Figure 3C-D). We next used the inducible Tie2-CreERT225 driver for clonal analysis in the double-reporter assay (Figure 3E-I). Given that induction in Tie2-CreERT2 embryos is not random but targeted to the Cre-expressing cell population, we studied the expression of CreERT2 messenger RNA (Figure 3E-G). Unexpectedly, CreERT2 expression in pregastrulation embryos coincided completely with the blood- and endothelial-fated epiblast regions and partially with the heart-fated region (Figure 3E-F). By E7.5, CreERT2 expression had completely relocated to the extra-embryonic region and was no longer detected in the epiblast or PS (Figure 3G). After induction of a collection of 56 embryos with the Tie2-CreERT2 driver at E6.5, 91% of clusters were B- or E-clusters, with BE-clusters at 9%, below the polyclonality frequency (14%) (Figure 3H-J’’,L). These results indicate that the shared contribution of Tie2+ cells to YS blood and endothelial lineages does not result from Tie2 expression in a common precursor but from its independent activation in both lineages. B-clusters were larger than E-clusters but smaller than BE-clusters, again suggesting that these originated earlier (Figure 3N). We next performed induction of 55 embryos at E7.5, to target the YS CreERT2+ cells. B-clusters and E-clusters again predominated (93%), but there was a persistent presence of BE-clusters (7%), which in this case was above the polyclonality rate (3.5%), suggesting a persistent endothelial-blood lineage relationship within the CreERT2+ cells in the YS (Figure 3K-K’’’,M). Cluster size was considerably smaller than in earlier experiments, reflecting the short time between induction and observation (Figure 3N).
Tie2-driven clonal analysis of early YS blood and endothelial precursors. (A-D) Tie2Cre-induced ROSA26R-EYFP detection by immunofluorescence in whole-mounts (A,C) and by immunohistochemistry in histologic sections (B,D) at E7 (A-B) and E8.5 (C-D). Boxed areas (B,D) are magnified in the panels to the right and show expression in endothelial (open arrowhead) and blood lineages (filled arrowhead). Scale bars, 100 µm. (E-G) In situ hybridization with a Cre probe at E6.5 (E) and E7.5 (G). The scheme in (F) depicts the epiblast fate map (modified from Kinder et al8 ). PS is highlighted in green in (E,G) and the blood-endothelium-heart forming region is outlined in red in (E). (H) Whole-mount E8.5 embryo showing Tie2CreERT2-induced labeling of cell clusters using the double reporter strategy (ROSA26R-LacZ and -EYFP). Scale bar, 500 µm. (I) Cross section of an E8.5 YS blood island showing a LacZ-labeled endothelial cell (open arrowhead) and EYFP-labeled hematopoietic cells (filled arrowhead). (J-K) Flat-mounted E8.5 YS showing general cell distribution of Tie2CreERT2-induced labeling and magnified details (J’-K’’). (K’’’) Detail of a BE cluster from a different embryo (LT26-E2). LacZ+ blood cells are marked with white arrowheads, LacZ+ endothelial cells with open arrowheads, and EYFP+ with black arrowheads. Scale bars, 20 µm. (L-M) Frequencies observed of B-, E-, and BE-clusters in the E8.5 YS after 4OHT induction at E6.5 (L) or E7.5 (M). (N) Single-cluster size distribution (dots) and average size (bars) of B-, E-, and BE-clusters in E8.5 YS after 4OHT induction at E6.5 and E7.5. Images in (A,C,E,G-H) were obtained with a Leica MZ-16 FA bionocular using a Plan Apo 1,0X objective and a Leica DFC310 FX camera. Images in (B,D,I-K’’) were obtained with a Nikon Eclipse 90i microscope using a Nikon DXM 1200c digital camera and 4X/0.2 NA objective (J) or 40X/0.95 NA objective, and Nomarski contrast (I-K’’). (See also supplemental Table 1.) a and arrowhead, anterior; b, blood; di, distal; e, endothelium; EE, extra-embryonic; h, heart; l, left; p and arrowhead, posterior; pr, proximal; r, right.
Tie2-driven clonal analysis of early YS blood and endothelial precursors. (A-D) Tie2Cre-induced ROSA26R-EYFP detection by immunofluorescence in whole-mounts (A,C) and by immunohistochemistry in histologic sections (B,D) at E7 (A-B) and E8.5 (C-D). Boxed areas (B,D) are magnified in the panels to the right and show expression in endothelial (open arrowhead) and blood lineages (filled arrowhead). Scale bars, 100 µm. (E-G) In situ hybridization with a Cre probe at E6.5 (E) and E7.5 (G). The scheme in (F) depicts the epiblast fate map (modified from Kinder et al8 ). PS is highlighted in green in (E,G) and the blood-endothelium-heart forming region is outlined in red in (E). (H) Whole-mount E8.5 embryo showing Tie2CreERT2-induced labeling of cell clusters using the double reporter strategy (ROSA26R-LacZ and -EYFP). Scale bar, 500 µm. (I) Cross section of an E8.5 YS blood island showing a LacZ-labeled endothelial cell (open arrowhead) and EYFP-labeled hematopoietic cells (filled arrowhead). (J-K) Flat-mounted E8.5 YS showing general cell distribution of Tie2CreERT2-induced labeling and magnified details (J’-K’’). (K’’’) Detail of a BE cluster from a different embryo (LT26-E2). LacZ+ blood cells are marked with white arrowheads, LacZ+ endothelial cells with open arrowheads, and EYFP+ with black arrowheads. Scale bars, 20 µm. (L-M) Frequencies observed of B-, E-, and BE-clusters in the E8.5 YS after 4OHT induction at E6.5 (L) or E7.5 (M). (N) Single-cluster size distribution (dots) and average size (bars) of B-, E-, and BE-clusters in E8.5 YS after 4OHT induction at E6.5 and E7.5. Images in (A,C,E,G-H) were obtained with a Leica MZ-16 FA bionocular using a Plan Apo 1,0X objective and a Leica DFC310 FX camera. Images in (B,D,I-K’’) were obtained with a Nikon Eclipse 90i microscope using a Nikon DXM 1200c digital camera and 4X/0.2 NA objective (J) or 40X/0.95 NA objective, and Nomarski contrast (I-K’’). (See also supplemental Table 1.) a and arrowhead, anterior; b, blood; di, distal; e, endothelium; EE, extra-embryonic; h, heart; l, left; p and arrowhead, posterior; pr, proximal; r, right.
The results of the random and Tie2-targeted recombination strategies suggest an early segregation of the first blood and endothelial lineages, which would be specified in uncommitted regions of the pregastrulation epiblast. However, a small subpopulation of blood and endothelial cells indeed seems to retain a lineage relationship related in the YS. To determine the nature of this relationship, we studied the arrangement of endothelial and blood cells in BE-clusters. Labeled endothelial and blood cells occur close together in both the Tie2-CreERT2 and the random inductions (Figure 3K’’’ and supplemental Figure 4A-B). This is consistent with hemogenic activity of the endothelium, in which the progenitor endothelium and the hematopoietic cluster would be in close association; whereas it would be difficult to reconcile with hemangioblast activity in the PS, which would generate a looser association after the extensive cell migration through the YS. Previous in vitro studies and marker analyses have in fact suggested the presence of hemogenic endothelium in the mouse YS17,37 before circulation is established.
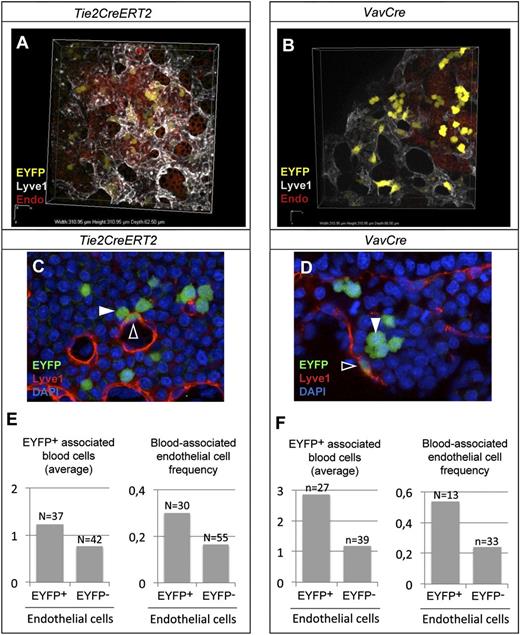
To directly study whether the YS endothelium presents hemogenic activity, we performed nonclonal lineage tracing studies to determine direct short-range interactions between Tie2CreERT2+ endothelial and blood cells. We induced recombination in Tie2-CreERT2 E7.5 embryos at relatively high frequency to generate a salt-and-pepper distribution of recombined cells and studied the distribution of the labeled cells at high resolution in confocal 3D reconstructions at E8.5 (Figure 4A and supplemental Movie 1). We then scored the association between recombined cells in the endothelium and hematopoietic cells in their vicinity. We found that EYFP+ endothelial cells were twice as likely than randomly chosen EYFP− to be associated with EYFP+ blood cells in their immediate vicinity and that they associate with a larger number of EYFP+ blood cells (Figure 4C,E).
Hemogenic activity of E8.5 YS endothelium. (A-B) Examples of high-resolution 3D confocal reconstruction of the YS blood island region at E8.5, showing recombined endothelial and hematopoietic cells (EYFP) after (A) Tie2CreERT2-induced recombination (polyclonal frequency) or (B) VavCre-induced recombination. (C-D) EYFP+ Lyve1+ endothelial cells (open arrowhead) in close association with EYFP+ blood cells (filled arrowhead). (E-F) Average number of EYFP+ blood cells within 1 to 2 cell diameters of EYFP+ or EYFP− endothelial cells (left), and the frequency of EYFP+ or EYFP− endothelial with EYFP+ blood cells within 1 to 2 cell diameters. (See also supplemental Figure 3 and supplemental Movies 1-2.) Fluorescence from EYFP, Cy3, Cy5, and DAPI in (A-D) was acquired with a Nikon confocal A1-R equipment using a 40X/1.30 NA oil objective at room temperature. All 3D reconstructions and videos were done using the NIS Elements AR 3.22.08 software. Endo, visceral endoderm.
Hemogenic activity of E8.5 YS endothelium. (A-B) Examples of high-resolution 3D confocal reconstruction of the YS blood island region at E8.5, showing recombined endothelial and hematopoietic cells (EYFP) after (A) Tie2CreERT2-induced recombination (polyclonal frequency) or (B) VavCre-induced recombination. (C-D) EYFP+ Lyve1+ endothelial cells (open arrowhead) in close association with EYFP+ blood cells (filled arrowhead). (E-F) Average number of EYFP+ blood cells within 1 to 2 cell diameters of EYFP+ or EYFP− endothelial cells (left), and the frequency of EYFP+ or EYFP− endothelial with EYFP+ blood cells within 1 to 2 cell diameters. (See also supplemental Figure 3 and supplemental Movies 1-2.) Fluorescence from EYFP, Cy3, Cy5, and DAPI in (A-D) was acquired with a Nikon confocal A1-R equipment using a 40X/1.30 NA oil objective at room temperature. All 3D reconstructions and videos were done using the NIS Elements AR 3.22.08 software. Endo, visceral endoderm.
To further explore the lineage relationship between YS endothelial cells and blood formation, we used the VavCre driver line26 (Figure 4B), where recombination occurs in definitive blood progenitors but not in definitive endothelium26,38 (supplemental Figure 4C-G). In contrast, in the YS endothelium, VavCre+ cells were found dispersed not only in the blood compartment but also in the endothelial population at E8.5 and E11.5 (Figure 4B, supplemental Figure 4C, and supplemental Movie 2). In the embryo, the VavCre+ lineage was detected in the subaortic mesenchyme and intra-aortic hematopoietic clusters but not in the aorta endothelium of the aorta-gonad-mesonephros (AGM) (supplemental Figure 4F-G). These observations suggested that YS endothelial VavCre+ cells could represent endothelial cells with hematopoietic potential. Association analysis indeed indicated that VavCre-recombined EYFP+ endothelial cells are 2.4 times more likely than randomly chosen EYFP− to be associated with EYFP+ blood cells in their immediate vicinity and that they associate with a larger number of blood cells (Figure 4D,F).
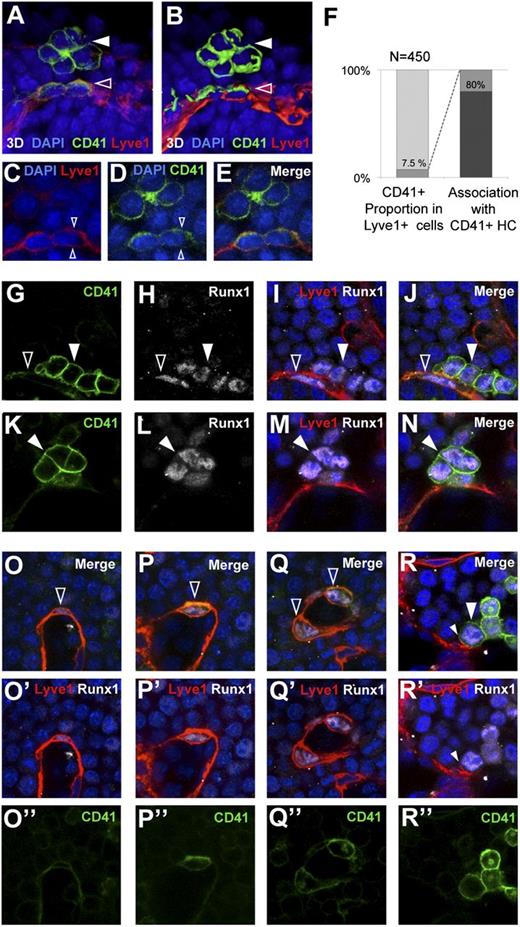
These studies confirm the existence of a short-range lineage relationship between endothelial and blood cells compatible with endothelial hemogenic activity. Additionally, the finding that VavCre-recombined endothelial cells are related to hematopoietic activity in the E8.5 YS suggests that this endothelial hemogenic activity has similarities with the generation of definitive hematopoietic progenitors in the AGM. We, therefore, studied in the E8.5 YS the detailed expression pattern of CD41 and Runx1, both of which are expressed in the AGM hemogenic endothelium and indicate commitment to the blood lineage.39,40 We found that 7.5% of the YS endothelial cells (Lyve1+) co-express CD41 (Figure 5A-F). Interestingly, 80% of the CD41+ endothelial cells presented a CD41hi blood cell cluster in their vicinity (Figure 5F and supplemental Movie 3). CD41hi blood cells in these clusters clearly differed from other blood cells in the high compactness of their association. In addition, CD41+ endothelial cells were rounder than the general endothelial cell population, and in most of them, CD41 was asymmetrically distributed toward the blood island luminal side (Figure 5A-E and supplemental Movie 3). CD41hi cells were invariably Runx1+, both in the endothelium and in the blood cell clusters (Figure 5G-N). The degree of cell roundness correlated with marker expression; flatter cells integrated in the endothelial layer showed low Runx1/CD41 and strong Lyve1 expression, whereas rounder cells showed higher levels of CD41/Runx1 and lower levels of Lyve1 (Figure 5O-R’’).
Hemogenic endothelium markers in E8.5 YS endothelium and associated hematopoietic clusters. (A) CD41 expression in the YS endothelium and an associated hematopoietic cluster. (B) 3D surface rendering of the image in (A). Solid arrowheads indicate the CD41+ hematopoietic cluster and open arrowheads indicate CD41+ endothelial cells. (C-E) Confocal optical sections of the specimen in (A), showing symmetric Lyve1 and asymmetric CD41 signals (arrowheads). (F) Frequency of CD41hi cells in the Lyve1+ endothelial cell population (left) and the frequency of Lyve1+ endothelial cell association with a CD41+ hematopoietic cluster (right). (G-N) Confocal microscopy images of CD41+Runx1+ hematopoietic clusters in close contact with the endothelium, and associated (G-J) or nonassociated (K-N) with a Lyve1+Runx1+ endothelial cell. Solid arrowheads indicate CD41+Runx1+ hematopoietic clusters and open arrowheads indicate CD41+Runx1+ endothelial cells. (O-R) Images showing different degrees of integration of Lyve1+CD41+Runx1+ cells in the YS endothelium. Flat cells highly integrated in the endothelium show low Runx1 and low CD41 (O-O’’), whereas increasingly round cells show progressively higher levels of Runx1 and CD41 (P-P’’,Q-Q’’), and completely round cells show high CD41 and Runx1 (R-R’’) and residual Lyve1 staining (small arrowheads in R,R’). Fluorescence from EYFP, Cy5, Cy3, and DAPI was obtained with a Leica SP5 confocal microscope using a 40X/1.25 NA objective. All 3D reconstructions in (A-B) were done with the Imaris software package (see also supplemental Movie 3).
Hemogenic endothelium markers in E8.5 YS endothelium and associated hematopoietic clusters. (A) CD41 expression in the YS endothelium and an associated hematopoietic cluster. (B) 3D surface rendering of the image in (A). Solid arrowheads indicate the CD41+ hematopoietic cluster and open arrowheads indicate CD41+ endothelial cells. (C-E) Confocal optical sections of the specimen in (A), showing symmetric Lyve1 and asymmetric CD41 signals (arrowheads). (F) Frequency of CD41hi cells in the Lyve1+ endothelial cell population (left) and the frequency of Lyve1+ endothelial cell association with a CD41+ hematopoietic cluster (right). (G-N) Confocal microscopy images of CD41+Runx1+ hematopoietic clusters in close contact with the endothelium, and associated (G-J) or nonassociated (K-N) with a Lyve1+Runx1+ endothelial cell. Solid arrowheads indicate CD41+Runx1+ hematopoietic clusters and open arrowheads indicate CD41+Runx1+ endothelial cells. (O-R) Images showing different degrees of integration of Lyve1+CD41+Runx1+ cells in the YS endothelium. Flat cells highly integrated in the endothelium show low Runx1 and low CD41 (O-O’’), whereas increasingly round cells show progressively higher levels of Runx1 and CD41 (P-P’’,Q-Q’’), and completely round cells show high CD41 and Runx1 (R-R’’) and residual Lyve1 staining (small arrowheads in R,R’). Fluorescence from EYFP, Cy5, Cy3, and DAPI was obtained with a Leica SP5 confocal microscope using a 40X/1.25 NA objective. All 3D reconstructions in (A-B) were done with the Imaris software package (see also supplemental Movie 3).
Discussion
In this study, we presented a new approach for the random retrospective analysis of single cell contributions to blood islands. Our approach allows, for the first time, the study of clonal contributions including a description of the qualitative and quantitative contribution of single precursors, as well as the spreading behavior of their progeny. This approach differs importantly from previous approaches in this area of study. The previous prospective studies8,9 were based on the transplantation of groups of cells, and therefore, were not effective in the complete separation of the blood-contributing and endothelium-contributing cells. Similarly, the studies by Ueno et al10 were based on the injection of labeled ESC into blastocysts, and therefore, were not clonal either at gastrulation or at the time of analysis. Because the limits of the contributions of single cells could not be determined in this study, the observational unit in which lineage coincidences were determined was the “blood island.” The clonal approach has allowed us to determine that single precursors can colonize extensive regions of the YS, indicating that analysis of the contribution to single “blood islands” does not account for the whole progeny of single PS progenitors. In addition, our study is based on a genetic method and does not involve direct embryo manipulation, whereas the previous studies involved extensive ex-utero manipulation and cell transplantation or injection,8-10 so that possible developmental alteration deriving from embryo manipulations could be avoided.
The results obtained indicate that the specification of blood and endothelial cells in the YS does not involve an intermediate hemangioblast. Instead, we found that the hemogenic activity of the YS endothelium is a main source of the lineage relationship between endothelial and blood cells. This lineage relationship would be established in the YS and not derive from early mesodermal precursors in the PS. Previous studies identified hemogenic activity of the YS endothelium in transplantation or colony assay experiments.41 Here, we added the evidence for the in situ occurrence of this activity in the precirculation YS. Interestingly, the YS endothelial hemogenic activity is associated with the expression of CD41-high, Runx1-high, and Vav, all markers of definitive hematopoietic precursors in the embryo. In the YS, Runx1, and CD41, high expression is linked to the appearance of definitive hematopoietic progenitors,17,37,42 therefore, suggesting that YS hemogenic endothelial activity might correspond to the appearance of these multipotential precursors.
Previous studies showed that the Runx1+ hemogenic endothelium lineage is committed to blood fate, making no contribution to definitive endothelium40,43 and CD41 is found to be associated with hemogenic endothelium in the YS in both previous studies41 and ours. In this study, we found that VavCre+ endothelial cells appear in the YS, however, the definitive fate of this lineage is hematopoietic, indicating the hemogenic nature of these cells. The common endothelial-blood lineage detected during embryogenesis thus results predominantly from a linear specification pathway in which mesodermal precursors give rise to blood-committed hemogenic endothelium, rather than being generated through a bifurcating pathway in which mesodermal precursors (hemangioblasts) generate both fates. The previous studies on the origin of blood island cells8-10 and hemogenic endothelium,17 together with the present work, therefore suggests the early independent specification of primitive endothelium/blood and the subsequent establishment of hemogenic endothelium in the YS (Figure 6). This view would reconcile in vivo fate map and chimeric embryo studies8-10 with in vitro analysis of cultured ESC and PS precursors.5,6 The identification of bipotential endothelial- and blood-contributing precursors in the zebrafish blastula and early gastrula can also be explained by either hemangioblast or hemogenic endothelium origins, or both. Interestingly, in zebrafish, the endothelial contribution from the bipotential clones is restricted to the axial vessels, which specifically contain the definitive hemogenic endothelium in the fish.7
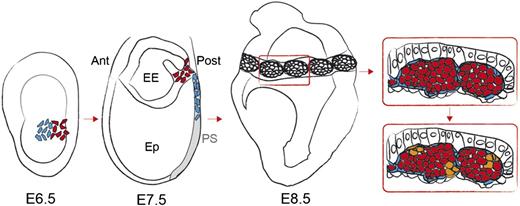
Model of the emergence of hemogenic endothelium in the YS blood islands from mesodermal precursors. A model is proposed incorporating the concepts established in previous studies and in the present work.8-10,17 Precursors of endothelium (blue) and blood (red) derive from adjacent regions of the E6.5 mouse epiblast.8 Blood precursors would be the first to ingress the PS, followed by endothelial precursors. Both lineages colonize the YS and form the blood islands through cell-cell interactions between the 2 lineages. Interspersed endothelial cells in the blood islands become hemogenic and give rise to CD41+ Runx1+ hematopoietic clusters (orange). Ant, anterior; EE, extra-embryonic; Ep, epiblast; Post, posterior.
Model of the emergence of hemogenic endothelium in the YS blood islands from mesodermal precursors. A model is proposed incorporating the concepts established in previous studies and in the present work.8-10,17 Precursors of endothelium (blue) and blood (red) derive from adjacent regions of the E6.5 mouse epiblast.8 Blood precursors would be the first to ingress the PS, followed by endothelial precursors. Both lineages colonize the YS and form the blood islands through cell-cell interactions between the 2 lineages. Interspersed endothelial cells in the blood islands become hemogenic and give rise to CD41+ Runx1+ hematopoietic clusters (orange). Ant, anterior; EE, extra-embryonic; Ep, epiblast; Post, posterior.
Our results show that the earliest blood and endothelial lineages in the mouse embryo derive from independent epiblast populations, and not from a PS bipotential precursor. In addition, we show that hemogenic endothelium first appears in the YS, before it is active in the floor of the dorsal aorta, suggesting that the hematopoietic precursors originating from the YS hemogenic endothelium are related to definitive hematopoietic precursors.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Doohan for immunostaining and advice; T. Córdoba, V. García, E. Escudero, and S. Vela for mouse work; E. Arza and A. M. Santos from the Centro Nacional de Investigaciones Cardiovasculares (CNIC) Microscopy Unit for help with microscopy; P. López-Romero for statistics; H. Azegrouz from CNIC Celomics Unit for algorithm generation; and S. Bartlett for text editing.
This study was supported by grants from the Spanish Ministry of Economy and Competition (BFU2012-31086 and RD06/0010/0008). The CNIC is supported by the Ministry of Economy and Competition and the Pro-CNIC Foundation.
Authorship
Contribution: L.P.-B., S.T., C.V., J.I., and L.C. performed experiments; and L.P.-B. and M.T. analyzed results, created the figures, designed the research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for L.P.-B. is Instituto de Investigación Sanitaria Puerta de Hierro, Majadahonda, Spain.
Correspondence: Miguel Torres, Departamento de Desarrollo y Reparación Cardiovascular, Centro Nacional de Investigaciones Cardiovasculares, Madrid E-28029, Spain; e-mail: mtorres@cnic.es.