Abstract
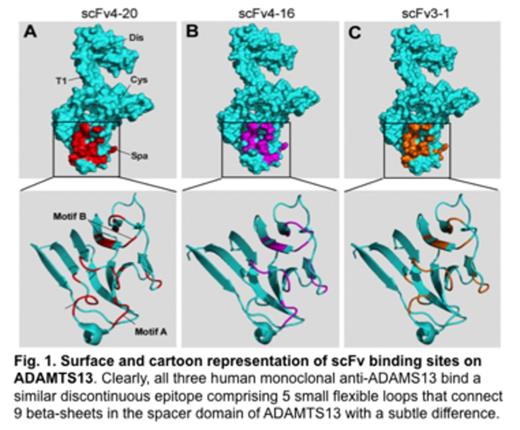
Acquired thrombotic thrombocytopenic purpura (TTP), a potentially fatal arterial thrombotic disorder, is primarily caused by autoantibodies that bind and inhibit plasma von Willebrand factor (VWF)-cleaving metalloprotease (ADAMTS13) activity. However, the mechanisms underlying autoantibody-mediated inhibition of ADAMTS13 activity and acquired TTP are not fully understood. By a hydrogen-deuterium exchange coupled with mass spectrometry (HX-MS) technique, we found that human monoclonal anti-ADAMTS13 antibodies, the single chain variable region fragments (scFvs)4-20, 4-16, and 3-1 that were, isolated by phage display from two patients with acquired TTP, predominantly bound to a discontinuous and conformational epitope in the spacer domain of ADAMTS13 with a subtle difference. The epitope for scFvs4-20 and 4-16 comprises five small flexible loops, including a previously described motif A (or exosite 3, R659-E664), motif B (exosite 4, L632-R639), and several other outlying residues (F592, Y658, and Y665), while scFv3-1 bound all other residues except for those in motif A. Site-directed mutagenesis and biochemical analysis demonstrated that both motifs A and B were found to be critical for recognition and proteolysis of VWF73 and multimeric VWF. Deletion of motif A or motif B in full-length ADAMTS13 abolished the binding of scFvs4-20 and 4-16 but not 3-1 (which did not bind motif A). Our findings demonstrate the powerful use of HX-MS for mapping antibody epitopes at nearly single amino acid resolution. This provides a new way to reveal mechanisms of autoantibody-mediated inhibition of plasma ADAMTS13 activity and acquired TTP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.