Abstract
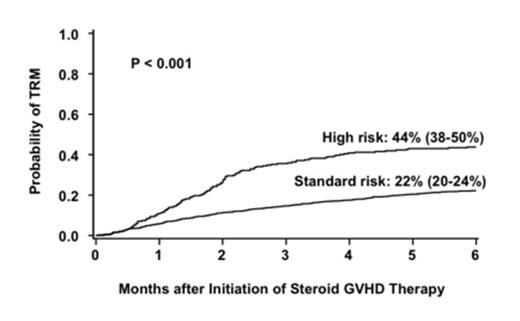
Corticosteroids are the standard initial therapy for acute graft-versus-host disease (aGVHD) but are effective in only half of the cases. Methods to identify patients who are unlikely to respond to conventional initial therapy and who warrant alternative, more effective initial therapy are needed. The Minnesota (MN) group recently defined high-risk (HR)-aGVHD at onset by a novel acute GVHD risk score (Br J Haem 157(6):732,2012). Patients with HR-aGVHD were less likely to respond to steroid therapy and had a 2-fold increased risk of treatment-related (non-relapse) mortality (TRM) compared to patients with standard-risk (SR)-aGVHD. To validate this novel aGVHD risk score, we examined a larger group of patients who received steroids as initial systemic therapy for aGVHD. A database of 1723 patients was created from 5 cohorts; the previously reported patients from Minnesota (n=864), Hopital Saint Louis, Paris (n=184), the University of Michigan (n=307) and the Blood and Marrow Clinical Trials Network studies 0302 (n=155), and 0802 (n=213). Using multiple regression analyses, we identified poorly responsive, HR-aGVHD by the number of involved organs and severity of aGVHD at onset observed in this cohort as outlined in Table 1. The overall response [(complete response/partial response (CR/PR)] rate 28 days after initiation of steroid therapy for aGVHD was lower in the 269 patients with HR-aGVHD than in the 1454 patients with SR-aGVHD [44% (95% CI 38-50%) vs. 68% (95% CI 66-70%), p<0.001. Similarly, the 6-month incidence of TRM was twice as high in patients with HR-aGVHD as in those with SR-aGVHD [44% (95% CI 38-50%) vs. 22% (95% CI 20-24%), p<0.001, Figure 1]. Finally, survival at 6 months after the onset of steroid treatment was lower in patients with HR-aGVHD than in those with SR-aGVHD [52% (95% CI 46-58%) vs 71% (95% CI 69-73%), p<0.001]. In multiple regression analysis, the probability of CR/PR at day 28 in patients with HR-aGVHD was lower than in those with SR-aGVHD [Odds ratio (OR) 0.3, 95% CI 0.2-0.4, p<0.001]. Donor type was the only other factor associated with response. Patients who received a graft from an HLA-matched (OR 0.7, 95% CI 0.6-0.9, p=0.01) or mismatched (OR 0.3, 95% CI 0.2-0.5 p<0.001) unrelated donor (URD) were less likely to respond than those who received either a related donor graft or an umbilical cord blood graft. Patients with HR-aGVHD had a 2-fold increase in risk of mortality (RR, 2.1, 95% CI, 1.7-2.6, p<0.001) and a 2.5-fold increased risk of TRM (RR 2.5, 95% CI, 2.0-3.2 p<0.001) compared to patients with SR-aGVHD. Risks of mortality and TRM were also significantly higher in older patients, recipients of HLA-matched or mismatched URD grafts and in those with onset of aGVHD treatment within 28 days after HCT. This refined definition of aGVHD risk is a better predictor of response, survival and TRM than the CIBMTR or MN grading systems (Table 2). Patients with HR-aGVHD warrant more targeted upfront therapy. A future prospective study to examine the aGVHD score and biomarkers in the same aGVHD population would help further refine the predictive ability of each and determine how or whether a combination of clinical score and biomarker levels could even better identify HR-aGVHD.
GVHD Risk Definition by Organ Number* and Stage
| GVHD Risk Score . | One Organ (n) . | Two Organs (n) . | Three Organs (n) . |
|---|---|---|---|
| Standard Risk N=1454 84% | Stage 1-3 Skin (901) Stage 1-2 GI (279) | Stage 1-3 Skin plus Stage 1 GI (223) Stage 1-3 Skin plus Stage 1-4 Liver (51) | |
| High Risk** N=269 16% | Stage 4 Skin (13) Stage 3-4 GI (74) Stage 1-4 Liver (25) | Stage 1-3 Skin plus Stage 2 GI (54) Stage 1-2 Lower GI plus Stage 1-3 Liver (12) Stage 3-4 GI plus Stage 1-3 Skin (45) Stage 3-4 GI plus Stage 1-4 Liver (10) | Stage 1-3 Skin plus Stage 1-2 GI plus Stage 1-3 Liver (23) Stage 1-3 Skin plus Stage 3-4 GI plus Stage 1-4 Liver (13) |
| GVHD Risk Score . | One Organ (n) . | Two Organs (n) . | Three Organs (n) . |
|---|---|---|---|
| Standard Risk N=1454 84% | Stage 1-3 Skin (901) Stage 1-2 GI (279) | Stage 1-3 Skin plus Stage 1 GI (223) Stage 1-3 Skin plus Stage 1-4 Liver (51) | |
| High Risk** N=269 16% | Stage 4 Skin (13) Stage 3-4 GI (74) Stage 1-4 Liver (25) | Stage 1-3 Skin plus Stage 2 GI (54) Stage 1-2 Lower GI plus Stage 1-3 Liver (12) Stage 3-4 GI plus Stage 1-3 Skin (45) Stage 3-4 GI plus Stage 1-4 Liver (10) | Stage 1-3 Skin plus Stage 1-2 GI plus Stage 1-3 Liver (23) Stage 1-3 Skin plus Stage 3-4 GI plus Stage 1-4 Liver (13) |
Outcomes Based on Published GVHD Grading Systems
| GVHD Grading System . | Severity . | N . | CR/PR at day 28 . | P . | 6 month survival (95% CI) . | P . | 6 month TRM (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|
| CIBMTR | A-B C-D | 930 793 | 626 (67%) 485 (61%) | 0.008 | 660 (71%) 515 (65%) | 0.008 | 213 (23%) 228 (29%) | 0.006 |
| MN | I-II III-IV | 1379 344 | 938 (68%) 173 (50%) | <0.001 | 984 (71%) 192 (56%) | <0.001 | 304 (22%) 137 (40%) | <0.001 |
| GVHD Risk Score | SR HR | 1454 269 | 993 (68%) 118 (44%) | <0.001 | 1039 (71%) 137 (53%) | <0.001 | 326 (22%) 115 (42%) | <0.001 |
| GVHD Grading System . | Severity . | N . | CR/PR at day 28 . | P . | 6 month survival (95% CI) . | P . | 6 month TRM (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|
| CIBMTR | A-B C-D | 930 793 | 626 (67%) 485 (61%) | 0.008 | 660 (71%) 515 (65%) | 0.008 | 213 (23%) 228 (29%) | 0.006 |
| MN | I-II III-IV | 1379 344 | 938 (68%) 173 (50%) | <0.001 | 984 (71%) 192 (56%) | <0.001 | 304 (22%) 137 (40%) | <0.001 |
| GVHD Risk Score | SR HR | 1454 269 | 993 (68%) 118 (44%) | <0.001 | 1039 (71%) 137 (53%) | <0.001 | 326 (22%) 115 (42%) | <0.001 |
* UGI plus Lower GI considered as one organ disease
Cumulative incidence of transplant related mortality at 6 months after initiation of steroid therapy by risk group
Cumulative incidence of transplant related mortality at 6 months after initiation of steroid therapy by risk group
Levine:University of Michigan: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.