Abstract
Background: Light chain (AL) amyloidosis is a rare plasma cell neoplasm associated with systemic amyloid deposition leading to organ dysfunction and death without treatment. The use of AHCT in AL amyloidosis remains controversial as a prospective randomized control trial suggested inferior outcomes when compared with standard chemotherapy, driven primarily by high peri-transplant mortality (TRM) up to 24%. Improved patient selection criteria, supportive care and risk-adapted therapy have reduced TRM in recent single center studies. We analyzed trends and prognostic factors associated with AHCT outcomes in AL amyloidosis patients.
Methods: We identified 1532 AL amyloidosis patients who underwent AHCT following high dose melphalan (MEL) within 24 months of diagnosis between 1995 and 2012 from the CIBMTR database. A subset of patients with more complete level of research data reported between 2001 and 2012 was analyzed for multivariate analysis (n=354). The primary endpoints were day30 and day100 mortality, hematologic progression free survival (PFS), hematologic relapse/progression and overall survival (OS). Data regarding cardiac, renal, hepatic and neurologic amyloid involvement was collected. Hematologic and organ response and progression were defined based on the 2004 uniform consensus criteria.
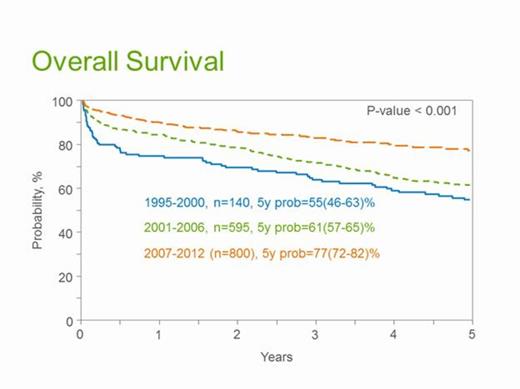
Results: The median age at transplant was 57 years, with 61% males. AHCT was performed within 6 months of diagnosis in 66% patients. Karnofsky performance score (KPS) was <80 in 14%, HCT-CI was ≥ 3 in 20% and 69% had a lambda isotype. Organ involvement included renal, cardiac, hepatic and autonomic nervous system involvement in 74%, 38%, 16% and 11% respectively. Coexistent myeloma (>10% bone marrow plasma cells) was seen in 14%. Progressively higher numbers of patients received AHCT from 1995-2000 (n=140) to 2001-2006 (n=595) and 2007-2012 (n=800). The majority were untreated pre-transplant (77%) while 8% received melphalan, 6% thalidomide and 4% each received lenalidomide and bortezomib based pre-AHCT therapy. The median CD34 cell dose infused was 4.4 X 106/kg cells (IQR 3.3-6.2). MEL dose reduction was common (60% received < MEL 180 mg/m2 and 38% < MEL 140 mg/m2). The median length of hospital stay was 17 days (IQR 13-23). The median follow-up of patients from the time of transplant was 61 months (range 3-145). Day100 response included hematologic complete response, CR (12%), partial response, PR (26%), stable disease, SD (23%), and renal response (12%) with an ultimate best response of hematologic CR (33%), PR (28%), SD (19%) and renal response in 31%. Table 1 shows day30 and day100 mortality and OS at 1, 3 and 5 years showing steady declines in mortality rates and improvements in survival in successive time cohorts. Figure 1 shows the 5 year OS in each of the time cohorts. On multivariate analysis, albumin ≥ 3 g/dl at diagnosis, KPS ≥80, pre-transplant anti-plasma cell therapy and MEL ≥180 mg/m2 were associated with lower hematologic relapse/progression. KPS ≥80 and predominant renal involvement were associated with superior hematologic PFS while KPS ≥80 and < 2 organ involvement correlated with OS.
Outcomes of AHCT in AL amyloidosis. Values are expressed as probabilities with 95% confidence intervals.
| . | 1995-2000 N=140 . | 2001-2006 N=595 . | 2007-2012 N=800 . |
|---|---|---|---|
| Day 30 mortality | 11 (7-17) | 5 (4-7) | 3 (2-4) |
| Day 100 mortality | 20 (14-27) | 11 (8-13) | 5 (4-7) |
| 1 year survival | 75 (67-82) | 85 (81-87) | 90 (88-92) |
| 3 year survival | 64 (56-72) | 72 (68-75) | 83 (80-86) |
| 5 year survival | 55 (46-63) | 61 (57-65) | 77 (72-82) |
| . | 1995-2000 N=140 . | 2001-2006 N=595 . | 2007-2012 N=800 . |
|---|---|---|---|
| Day 30 mortality | 11 (7-17) | 5 (4-7) | 3 (2-4) |
| Day 100 mortality | 20 (14-27) | 11 (8-13) | 5 (4-7) |
| 1 year survival | 75 (67-82) | 85 (81-87) | 90 (88-92) |
| 3 year survival | 64 (56-72) | 72 (68-75) | 83 (80-86) |
| 5 year survival | 55 (46-63) | 61 (57-65) | 77 (72-82) |
Conclusions: There has been a significant improvement in survival of AL patients after AHCT driven primarily by a reduction in early peri-transplant mortality. Limited organ involvement, higher KPS, use of pre-transplant therapy and higher dose melphalan conditioning contributed to superior outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.