Abstract
Introduction: We have previously shown that blocking CD28:CD80/86 costimulation with CTLA4-Ig can be a successful strategy to inhibit GVHD. However, since CTLA4-Ig targets CD80/86, there remain concerns of off-target, immune activating effects, given that it can inhibit both positive signaling through CD28 and negative signaling through CTLA4. In order to specifically target CD28, we have developed a humanized anti-CD28 monovalent Fab antibody (FR104) and have tested its ability to protect against GVHD.
Methods: NHP underwent MHC-mismatched transplantation after myeloablative radiation-based pre-transplant conditioning. They were transplanted with GCSF-mobilized PBSCs (4.2x10^8 +/- 1.1x10^8 TNC/kg and 1.9 x 10^7 +/- 0.5 x 10^7 CD3+ T cells/kg. GVHD prophylaxis consisted of either monotherapy with FR104 (5mg/kg given weekly) or with combination FR104 and rapamycin (rapamycin trough 5-15 ng/ml). These were compared to three control groups: no GVHD prophylaxis, rapamycin monotherapy and autologous HSCT. Clinical and histologic GVHD was monitored using our NHP grading scale, and longitudinal immunologic analysis was performed. Treatment was continued as tolerated with planned terminal analysis at 35 days in all survivors.
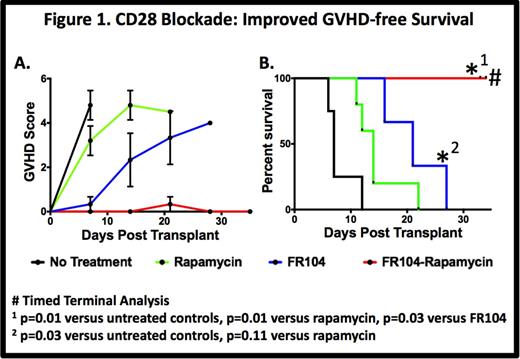
Results: Untreated controls (n = 5) developed rapid, severe multi-organ GVHD (MST=7 days). Rapamycin monotherapy (n=6) partially protected against GVHD (MST =14 days). Monotherapy with FR104 showed a significant prolongation in survival compared to untreated animals (MST = 21 days, p=0.02 n = 3), with breakthrough GVHD (liver, skin predominant) ultimately occurring. In contrast to all other groups, combination therapy with FR104 and rapamycin (n=4) resulted in striking protection from GVHD with all animals reaching planned terminal analysis with minimal clinical disease (p=0.01 vs. untreated controls, p=0.01 vs. rapamycin monotherapy, Fig. 1).
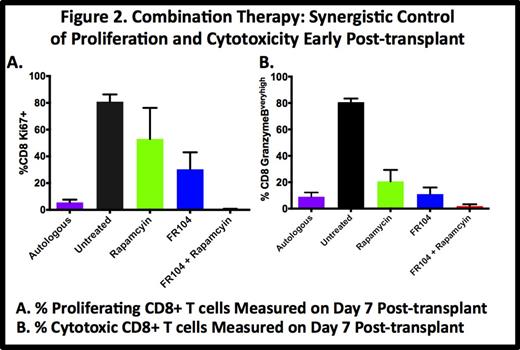
We have documented a major role for both T cell proliferation (Ki-67) and Granzyme B (Gran-B) overexpression in GVHD, with untreated controls demonstrating rampant CD8+ proliferation (81% + 5.4% Ki-67+ at day 7 vs. 4% pre-transplant) and Gran-B overexpression (81% + 2.7% Gran-Bvery high at day 7 vs. 0.5% + 0.5% pre-transplant). Monotherapy with either rapamycin or FR104 partially controlled proliferation (53% + 23% and 30% + 21.7%, respectively) and Gran-B overexpression (21% + 8.7% and 11% + 5%, respectively) on day 7. Combination therapy with FR104 + rapamycin resulted in synergistic control of both proliferation and Gran-B overexpression (0.6% + 0.2% Ki67+ and 2.3% + 1.3% Gran-B overexpression on Day 7), reverting the CD8+ activation phenotype to that observed in auto-HSCT controls (5.6% + 2.1% Ki67+, 9% + 3.2% Gran-B overexpression, Fig. 2). This reversion to the auto-HSCT phenotype was maintained until the terminal analysis, and the control of Day 7 proliferation and Gran-B overexpression correlated closely with GVHD-free survival.
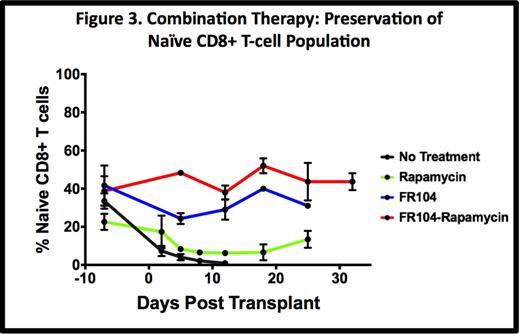
The control of CD28-mediated T cell activation in FR104-treated recipients resulted in significant preservation of the naïve CD8+ T cell (CD8 Tn) phenotype in these animals. Thus, in untreated controls and rapamycin monotherapy groups, the CD8 Tn population virtually disappeared post-transplant (2.1% + 0.7% and 6.6% + 1.5% at day 7, respectively compared to 33.6%+ 4.1% pre-transplant), consistent with widespread allo-stimulation and costimulation. In contrast, FR104 monotherapy resulted in partial preservation of CD8 Tn (24.3% + 2.8% at Day 7) and FR104 + rapamycin combination therapy promoted nearly complete preservation of CD8 Tn (48.3% + 0.9% at day +7), with maintenance of this naïve population for the duration of therapy (Fig. 3).
Discussion: These results show, for the first time, that specific blockade of CD28 can inhibit NHP GVHD, and when coupled with rapamycin it can effectively control both the clinical and immunologic sequelae of this disease. CD28 blockade with FR104 + rapamycin significantly inhibited both CD8+ T cell proliferation and Granzyme B overexpression, and lead to robust preservation of the naïve T cell phenotype, consistent with efficient inhibition of allo-activation. Our results suggest that targeting the CD28:CD80/86 costimulation axis by selective CD28 blockade may be effective in inhibiting the T cell activation associated with GVHD, and is deserving of clinical evaluation for safety and efficacy in patients undergoing HCT.
Vanhove:Effimune: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract