Abstract
Comorbidities could provide important prognostic information about the treatment of a primary disease. The hematopoietic cell transplantation comorbidity index (HCT-CI) has been extensively evaluated and validated in the settings of allogeneic HCT from HLA-matched donors. Transplants from HLA-MM grafts have more risks for graft-rejection and graft-versus-host disease (GVHD), hence more non-relapse mortality (NRM) compared to HLA-matched grafts. Likewise, cord blood HCT carry relatively higher risks for delayed engraftment and infection. Whether comorbidities convey the same prognostic influence on outcomes after HLA-MM and UCB compared to HLA-matched HCT is unknown.
To this end, we evaluated the prognostic capacity of HCT-CI among a retrospective cohort of 325 patients (pts), who were consecutively treated between 2000 and 2010 with allo-HCT using HLA-MM (n=184) or UCB (n=141) grafts. Pts receiving HLA-haploidentical grafts were not included in this analysis. Comorbidities were graded per the newly established guidelines for scoring the HCT-CI (Sorror ML, Blood. 2013). A sample of 30 patients was coded by two evaluators to assess the level of inter-rater reliability for scoring comorbidities. Cumulative incidences and Kaplan Meier estimates were used to evaluate NRM and survival, respectively. Relapse or progression of the primary disease was treated as a competing risk for NRM. Proportional hazards models were used to estimate the hazard ratio (HR) for NRM and survival associated with HCT-CI scores in each of the two groups. Performance of the model in predicting outcomes after each graft source was validated using c-statistic estimates for continuous prediction. IRR was evaluated using weighted kappa statistic estimates.
Results showed an excellent level of agreement on comorbidity coding between two evaluators with kappa statistic estimate of 0.89 (SE 0.06) and weighted kappa estimate of 0.95 (SE 0.03).
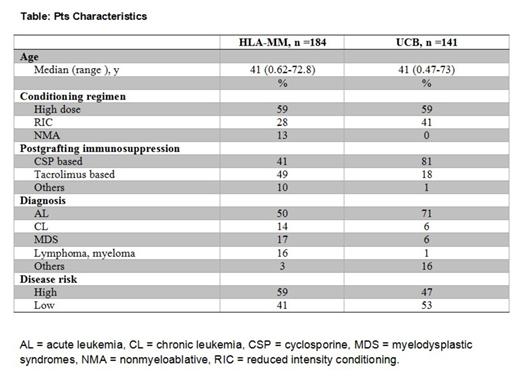
Overall, pts received high-dose (n=192), reduced–intensity (n=81), or nonmyeloablative (n=52) conditioning regimens. Diagnoses were acute (n=192) or chronic (n=34) leukemia, myelodysplastic syndromes (n=40), lymphoma (n=32), and others (n=27). HCT-CI scores of 0-2 and ≥3 were found in 148 and 177 pts, respectively. Pts characteristics among each group of donor source are described (Table).
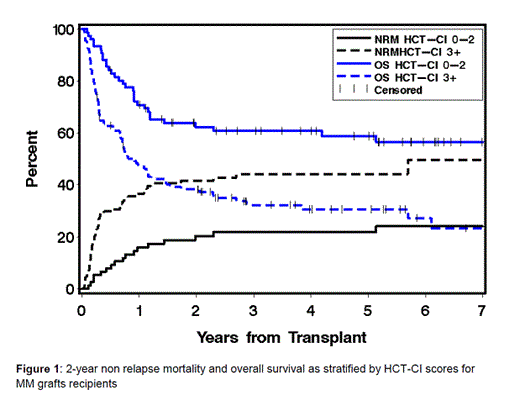
Recipients of HLA-MM grafts, who had HCT-CI scores of 0-2 experienced NRM incidences of 20% at 2-year compared to 40% among those with scores of ≥3. The figures for 2-year overall survival (OS) rates were 62% vs. 38%, respectively. Higher HCT-CI scores (≥3 vs. 0-2) were associated with increased HR [95% confidence interval (CI)] for NRM [2.96 (1.71-5.26), p< 0.0001] and OS [2.35 (1.54-3.59), p< 0.0001]. Additionally, the prognostic significance of the model was validated by calculating c-statistics estimates of 0.64 and 0.61 for NRM and OS, respectively.
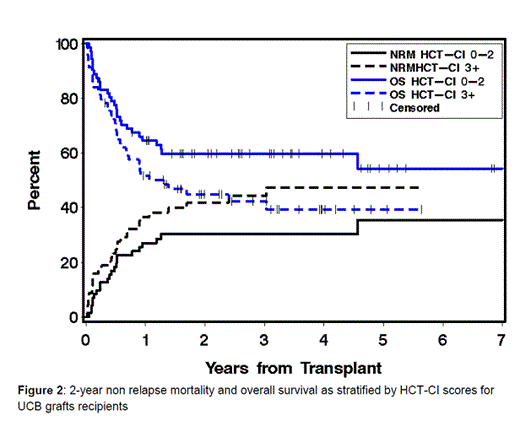
Likewise, recipients of UCD grafts, who had scores of ≥3 experienced higher incidences of NRM at 2-year (42%) compared to those with scores of 0-2 (30%) and lower rates of OS (45% vs. 60%), respectively. Pts with higher comorbidity scores had higher HRs for NRM [1.62 (0.93-2.81)] and OS [1.57 (0.97-2.54)] but with borderline statistical significance (p=0.09 and p=0.07, respectively). C-statistic estimates were 0.59 for NRM and 0.59 for survival.
The HCT-CI can clearly risk-stratify the outcomes after HLA-MM grafts. While a trend for discriminating outcomes after UCB HCT by comorbidity scores was noted, the magnitude of statistical significance might has been affected by the relatively smaller number of those patients. Future studies are warranted to explore the use of the HCT-CI in optimizing the decision-making process about the most appropriate graft source for pts who lack a suitably HLA-matched graft. It could also be a key factor in stratifying randomized studies designed to compare outcomes after alternative graft sources.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.