Abstract
The survival rate of childhood acute lymphoblastic leukemia (ALL) is now close to 90 %. However, 15-20 % of patients still experience relapse, which is caused by persistence of minimal residual disease (MRD) in the bone marrow during chemotherapy. Thus, a method for identification of drugs that are capable of eliminating this cell population is necessary, but not currently available. Indeed, current and past ALL in vitro sensitivity assays demand high cell numbers (100,000 ALL cells per well in a 96-well plate), which is beyond what can routinely be harvested from the MRD population in the BM at the end of induction therapy.
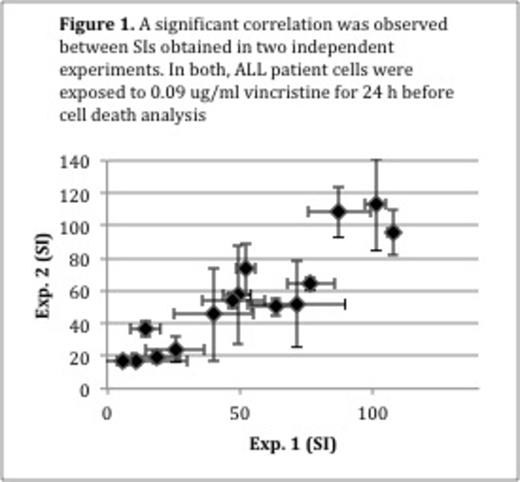
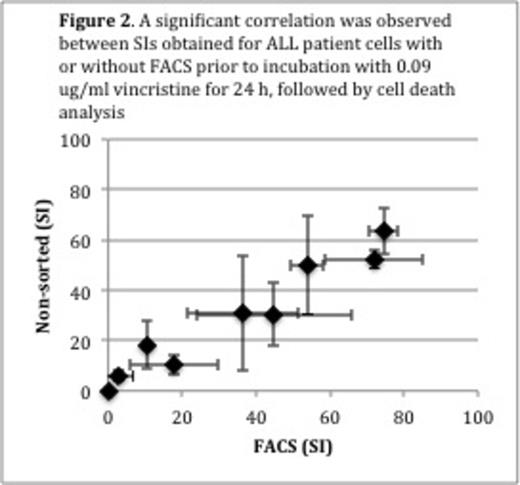
Accordingly, we developed a cell death assay that is far more sensitive and needs only 1,000 cells per well in a 96-well plate. It is based on stainings with annexinV-V450, a marker for early apoptosis, and Live/Dead Yellow, a marker for loss of cell membrane integrity, which can be quantified by flow cytometry. In order to compare patient samples, the viable fraction of treated cells were normalized to the viable fraction of untreated cells and classified as the survival index (SI). Assay time was set to 24 hours. When developing the assay we included the drugs that are currently used in the NOPHO ALL2008 induction therapy, namely vincristine, doxorubicin, dexamethasone and prednisolone. We determined one empirically derived cut-off concentration per drug and per cell density that produced a large scatter of SIs. Thereby only one drug concentration per cell density was needed for in vitro sensitivity testing (Vincristine: 0.09 and 9.23 mg/ml; doxorubicin: 0.5 and 2 mg/ml; prednisolone: 50 and 200 mg/ml; dexamethasone 2 and 100 mg/ml). The cell death assay showed only minor day-to-day variation (e.g. 0.09 mg/ml vincristine: N=15; rS= 0.92, p < 0.001) (Figure 1). In order to simulate a situation where FACS would be used for isolation of ALL subpopulations or MRD prior to in vitro sensitivity testing, ALL cells were mixed with peripheral blood mononuclear cells (MNCs) from healthy individuals to a final leukemic cell concentration of 10-2 and thereafter Fluorescence-activated cell sorted (FACSed) directly into a 96-well plate for exposure to chemotherapeutic drugs. The SI obtained was highly correlated to that of ALL cells that were not sorted, but directly and manually pipetted into the 96-well plate (e.g. 0.09 mg/ml vincristine: N=9; rS= 0.90, p = 0.001) (Figure 2). Next, we tested whether the in vitro sensitivity results obtained using 1,000 cells per well would resemble results obtained using 100,000 cells per well (i.e. the current standard). We exposed 1,000 and 100,000 cells per well in a 96-well plate from 36 ALL patients and three healthy individuals to the drugs listed above. For all drugs the SI for 1,000 and 100,000 cells per well were significantly correlated (vincristine: rs=0.65, p < 0.001; doxorubicin: rs= 0.48, p = 0.003; dexamethasone: rs= 0.55, p < 0.001; prednisolone: rs=0.35, p=0.04).
Thus, we have developed a sensitive, yet robust in vitro sensitivity testing platform that includes FACS prior to cell death analysis. It demands only 3,000 ALL cells per drug to be tested in triplicates, and 3,000 cells that serve as untreated controls. Since a standard follow-up BM sample at day 28 after diagnosis contains at least 20 million MNCs in total, it enables in vitro sensitivity profiling of patients with MRD value > 0.1 %, potentially allowing future tailored chemotherapy according to the chemosensitivity profile of residual leukemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.