Abstract
Background. Niche-derived signals are essential for maintenance and expansion of stem/progenitor cells in the bone marrow. Recently published studies used elegant genetic tools to dissect predominant cellular sources of known HSPC regulators, such as CXCL12 and kit-ligand (Ding et al, Nature 2012, Greenbaum et al, Nature 2013). However, strategies enabling identification of novel niche-derived factors are lacking. Here, we describe an approach which utilizes spatial proximity between osteolineage cells (OLC) and HSPC in the post-transplant bone marrow niche as a guide to niche factor discovery and reveals the role of Interleukin-18 (IL18) as a quiescence regulator of early hematopoietic progenitors.
Results. We established a neonatal bone marrow (BM) transplantation model, in which adult BM-derived LKS CD34-Flk2- HSPCs fluorescently labeled with DiI were transplanted into irradiated newborn col2.3GFP recipients (in this mouse strain, the majority of OLCs are labeled with GFP). Forty-eight hours later, we obtained sections of femoral trabecular bone from transplanted mice and located rare OLCs harboring DiI-positive HSPCs in their immediate proximity. We harvested individual OLCs located within two cell diameters (proximal OLCs) and greater than five cell diameters (distal OLCs) from DiI-labeled HSPCs and performed comparative transcriptome analysis by single cell RNA-Seq. Remarkably, we found that proximal OLCs were distinct from their distal counterparts and showed a higher expression levels of known niche-associated molecules, as well as secreted factors not previously linked to regulatory niche function, including a pro-inflammatory cytokine IL18.
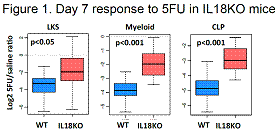
Quantification of primitive subsets in the BM of IL18 knock-out (IL18KO) mice showed no abnormalities. However, cell cycle studies revealed that while long-term HSCs (which did not express the IL18 receptor) were unaffected by IL18 deletion, early progenitors - short-term HSC, multi-potent progenitor and common lymphoid progenitor – were more actively cycling. Accordingly, following sublethal exposure to 5-fluorouracil (5FU), IL18KO animals displayed a 2-fold increase in frequency of lin-kit+Sca1+ (LKS) cells, lin-kit+ myeloid progenitors and CLPs, as compared wild-type (WT) controls (Figure 1). On the other hand, exogenous administration of recombinant IL18 to 5FU-treated WT animals was associated with 25% mortality, while no deaths were observed in the vehicle-treated group. Taken together, these data illustrate that IL18 constrains the ability of the progenitor pool to respond to a genotoxic stress.
Next, we tested if IL18 acts in a non cell-autonomous fashion. We transplanted progenitor-enriched bone marrow fraction (LKS cells) into IL18KO or WT hosts and observed an approximately 2-fold increase in both myeloid and lymphoid cells in peripheral blood of IL18KO animals. This finding was recapitulated in a reciprocal experiment, when LKS cells from IL18 receptor knock-out animals were transplanted into WT hosts, supporting the possibility that the effect of IL18 on progenitor proliferation is direct.
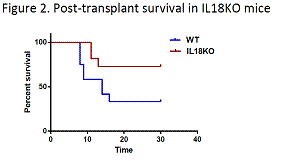
Finally, we investigated if the loss of IL18-mediated quiescence can be therapeutically exploited in the transplant setting. To this end, we assessed survival of lethally irradiated WT and IL18KO mice following transplantation with a limiting BM dose, i.e. predicted to confer survival to 50% or less of WT animals. Notably, we observed reduced 30-day post-transplant mortality (33% versus 72%, p value = 0.05) in IL18KO animals, which likely resulted from accelerated progenitor expansion in the absence of IL18 in the host microenvironment (Figure 2).
Conclusions.Our study demonstrates the capability of proximity-based single cell analysis of the post-transplant bone marrow microenvironment to identify a novel niche factor – IL 18. We show that IL18 specifically regulates quiescence of early hematopoietic progenitors, particularly under the conditions of stress hematopoiesis, such as exposure to 5FU or post-transplant hematopoietic expansion. The results presented here provide an insight into poorly understood molecular mechanisms of progenitor regulation by the niche. Moreover, improved post-transplant survival of animals in the absence of IL18 suggests that pharmacological inhibition of IL18 has the potential of improving clinical outcomes in transplant recipients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.