In this issue of Blood, Travers et al report that a number of synthetic polyphosphate (polyP) inhibitors are able to reduce thrombosis in mice without increasing bleeding as much as heparin.1
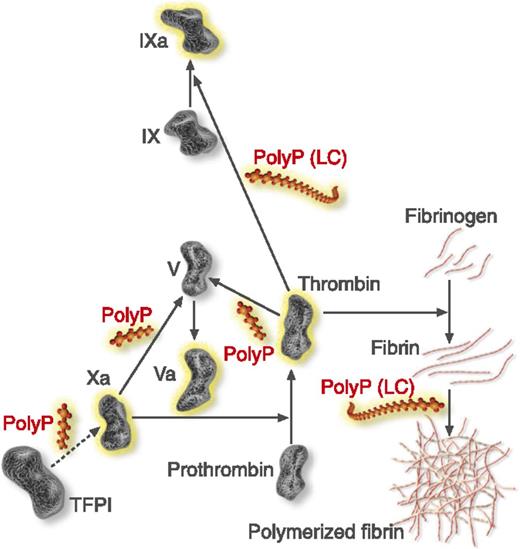
Steps of the coagulation cascade affected by polyphosphate (polyP). PolyP (red) accelerates factor V activation by factors Xa and thrombin, accelerates factor IX back-activation by thrombin, abrogates the ability of TFPI to inhibit factor Xa, and enhances fibrin polymerization. Fibrin polymerization and contact pathway activation are preferentially stimulated by long-chain (LC) polyP, like that present in microorganisms. Modified from Smith and Morrissey.8 Professional illustration by Luk Cox, Somersault18:24.
Steps of the coagulation cascade affected by polyphosphate (polyP). PolyP (red) accelerates factor V activation by factors Xa and thrombin, accelerates factor IX back-activation by thrombin, abrogates the ability of TFPI to inhibit factor Xa, and enhances fibrin polymerization. Fibrin polymerization and contact pathway activation are preferentially stimulated by long-chain (LC) polyP, like that present in microorganisms. Modified from Smith and Morrissey.8 Professional illustration by Luk Cox, Somersault18:24.
Collectively, arterial thrombosis and venous thromboembolism are leading causes of death in the developed world, and a better understanding of their pathogenic mechanisms will contribute to the development of safe and effective drugs. A case in point is understanding the role of anionic compounds such as polyP in their pathogenesis.
PolyP is a linear polymer of from 3 to hundreds of orthophosphates linked by high-energy phosphoanhydride bonds and is found in every organism that has been investigated, from bacteria to humans.2 In bacteria and eukaryotes, this polymer accumulates in acidic organelles rich in calcium and other cations known as acidocalcisomes.3 Recent studies have demonstrated that polyphosphate is abundant in platelet-dense granules4 and in mast cell granules,5 which are therefore considered acidocalcisomes.
In general, polyP present in blood cells is smaller than that present in microbial cells, with approximately 60 to 100 orthophosphate monomers, whereas microbial polyP could be as large as thousands of monomers. PolyP has a variety of structural, metabolic, and regulatory roles that have been the subject of recent reviews.2,6 Several functions of polyP are relevant to hematology.
The report that polyP is present in human platelet-dense granules and is secreted upon platelet activation4 suggested that this polymer could have a role in blood coagulation, and this is indeed the case.7 Initial studies revealed that polyP accelerates blood clotting by activating the contact pathway, promoting the activation of factor V, and abrogating the function of tissue factor pathway inhibitor (TFPI). PolyP also delays clot lysis by enhancing the thrombin-activatable fibrinolysis inhibitor (TAFI).7 Subsequent studies8 revealed that polyP enhances thrombin generation through multiple points of the coagulation cascade. In addition to accelerating factor V activation by factors Xa and thrombin, it accelerates factor XI back-activation by thrombin, abrogates the ability of TFPI to inhibit factor Xa, and enhances fibrin polymerization (see figure). Contact pathway activation and fibrin polymerization are preferentially stimulated by long-chain polyP, similar to that present in microbes.8
Another important discovery was that polyP is a potent proinflammatory agent.9 Stimulation of the contact pathway, which is dispensable for coagulation in vivo, results in kallikrein-mediated release of bradikinin from high-molecular-weight kininogen resulting in proinflammatory reactions.9 The finding that polyP is also present in mast cell granules and secreted upon mast cell activation5 can also explain the proinflammatory and procoagulant activities of these cells. Long-chain polyP is also able to suppress complement via the terminal pathway by destabilizing C5b,6, reducing the lytic capacity of the membrane attack complex.8
Contact pathway activation also contributes to thrombosis.8 Previous studies had demonstrated that cationic polymers, by inhibiting anionic compounds such as polyP or nucleic acids, attenuate thrombosis but their usefulness is limited because of their toxicity.1 In contrast, several dendritic polymer-based universal heparin reversal agents (UHRAs) containing multiple cationic groups with a protective layer of polyethylene glycol (PEG) have very low toxicity and still inhibit anionic compounds.1
By measuring the ability of these novel UHRA compounds to inhibit polyP-thrombin binding and polyP-initiated plasma clotting, Travers et al were able to select 4 inhibitors for further testing.1 The compounds did not activate complement, showed low levels of platelet activation, did not cause fibrinogen activation as did other polybasic compounds, and had antithrombotic activity in 2 mouse models of arterial thrombosis causing less bleeding than heparin.1 Surprisingly, the best polyP inhibitor in vitro (UHRA 8) did not perform as well as others tested in vivo, and the authors cannot conclusively rule out that polyP binding is the only basis for their ability to inhibit thrombus formation in vivo.1
Interestingly, the mode of action of these compounds differs from that of conventional antithrombotics. Because long-chain polyP is more effective in triggering the contact pathway of blood coagulation and is abundant in microbes, a potential clinical use of these compounds in sepsis and disseminated intravascular coagulation is envisaged.1
Overall, this study dramatically changes our insights regarding the role of polyP in thrombosis and sets the stage for the pursuit of new clinically useful compounds.
Conflict-of-interest disclosure: The author declares no competing financial interests.